Abstract
Stress proteins HSP90 (Heat shock proteins) are essential molecular chaperones involved in signal transduction, cell cycle control, stress management, folding and degradation of proteins. HSP90 have been found in a variety of organisms including pathogens suggesting that they are ancient and conserved proteins. Here, using molecular modeling and docking protocols, antibiotic Geldenamycin and its analog are targeted to the HSP90 homolog proteins of pathogenic protozoans Plasmodium falciparum, Leishmania donovani, Trypanosoma brucei and Entamoeba Histolytica. The designed analogs of geldenamycin have shown drug like property with improved binding affinity to their targets. A decrease in insilico affinity of the analogs for the Human HSP90 target indicates that they can be used as potential drug candidates.
Background
The molecular chaperones, heat-shock Proteins (HSP) are the stress proteins involved in a multitude of proteomemaintenance functions, including de novo folding, refolding of stress-denatured proteins, oligomeric assembly, protein trafficking and assistance in photolytic degradation [1–4]. Chaperones are classified according to their molecular weight for example HSP40, HSP60, HSP70, HSP90, and HSP100 [5, 6]. The sequence and structure of HSPs from different organisms show a high degree of similarity. Tuberculosis, malaria, leishmaniasis, and trypanosomiasis affect millions of people globally. The HSP90 of intracellular protozoan parasites like Trypanosoma, Leishmania, Toxoplasma, and Plasmodium affect important cellular process [5–8]. HSP90 triggers important stage transitions during their life cycles of protozoan pathogens [7, 8]. The primary structures of Hsp90 share a significant homology across different organisms [9]. The antibiotic, geldanamycin (GA), inhibits the HSP90-mediated conformational maturation/refolding reaction, and results in the degradation of HSP90 substrates [10]. The structure of the geldanamycin-binding domain of HSP90 (residues 9-232) reveals a ligand binding pocket, that is highly conserved across diverse species [11]. Geldanamycin binds and adopts a compact structure similar to that of a polypeptide chain in a turn conformation. Here, we have generated homology models of HSP90 protein sequences of Trypanosoma, Leishmania, Toxoplasma, and Plasmodium. Using chemoinformatic approaches we have designed the analogs of geldanamycin and checked their drug like properties. We find an increase in the affinity of the geldanamycin-analogs for the protozoan HSP90 proteins. However a decrease in the affinity for the human HSP90 during insilico docking experiments, suggests that these molecules may be used as drug candidate.
Methodology
Homology models of HSP90 homlogs:
Homologs of HSP90 of gi|510182|emb|CAA82765.1| heatshock Protein [Plasmodium falciparum], gi|322502095|emb|CBZ37178.1| heat shock Protein 83-1, partial [Leishmania donovani], gi|320900|pir||A44983 heat shock Protein 83 [Trypanosoma brucei] and gi|67474857|ref|XP_653162.1| heat shock Protein 90 [Entamoeba histolytica HM-1: IMSS], gi|153792590|ref|NP_001017963.2| heat shock Protein HSP 90- alpha isoform 1 [Homo sapiens], were retrieved from NCBI Protein database. The homology model structures of homolog of HSP90 were generated using CPHmodels 3.2 of CPH Server (http://www.cbs.dtu.dk/Svices/CPHmodels/). The template (PDBID: 1YET) was selected. The template recognition was based on Profile-Profile alignment guided by secondary structure and exposure predictions. Modeled structures were submitted to SAVES Server (Structural Analysis and Verification Server (http://nihServer .mbi.ucla.edu/SAVES/) for stereochemical quality check. The server checks modeled structure using PROCHECK, WHAT_CHECK, ERRAT, VERIFY_3D and PROVE Tools. Modeled structures were refined by utilizing ModRefiner TOOL (http://zhanglab.ccmb.med.umich.edu/ModRefiner/).
ModRefiner is High-Resolution Protein structure refinement tool, which can start from C-alpha trace, main-chain model or full-atomic model. Modeled structures were compared using Matras (MArkovian TRAnsition of Protein Structure) at http://biunit.aist-nara.ac.jp/matras.
Analogs of Geldanamycin:
Geldenamycin structure was retrieved using the PubChem Structure Search Tool (http://pubchem.ncbi.nlm.nih.gov/search). SMILES notation of ligand geldenamiycin was submitted to CORINA server (http: //www.molecularnetworks. com/online demos/corina_demo). CORINA is a fast and powerful 3D structure generator for small and medium sized, typically drug-like molecules. The ligand was further modified by using 3D conformor tool of PubChem. SMILES format of prepared analogs were converted to PDB format by using corina tool. Interaction studies between Geldanamycin and HSP90 were performed using AutoDock tool (http://autodock.scripps.edu/). The energy scoring function of Auto Dock was used to infer the affinity of each of the prepared analog with their HSP90 targets.
Discussion
The chaperones, heat shock Proteins (HSP) are ubiquitous in almost all cells. A multiple sequence alignment of HSP90 proteins of human and protozoan (Figure 1) using MATRAS revealed high similarity (62-80%) Table 1 (see supplementary material). HSP of Human showed ~ 65% of identity with all other HSP sequences. Homology models of Protozoan and human HSP were generated by using CPH server and validated at SAVES server (see methodology). As expected, HSP90 model structures were well superimposed onto each other and on the template (PDBID: 1YET) as well (Figure 2). Similar RMSD pattern of the aligned structures showed that the homologs are closely related Table 2 (see supplementary material). High sequence and structural similarity prompted us to target the ligand binding site with one common ligand, geldenamycin. Geldanamycin has inhibitory role on Protozoa diseases but it has also affinity for human HSP90 [4].
Figure 1.
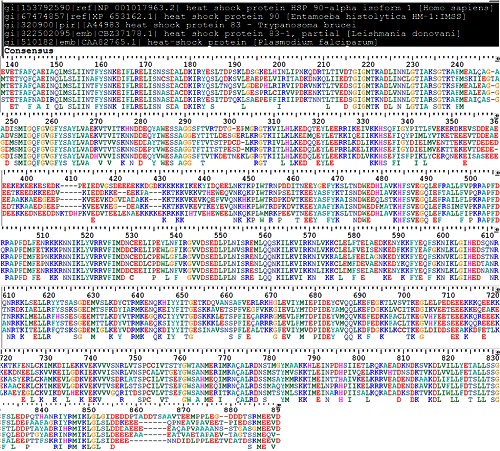
Multiple sequence alignment of homologs of HSP90
Figure 2.
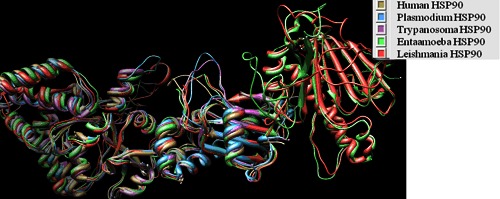
Structure superimposition of HSP90 homologs.
The ligand Geldanamycin showed a binding energy of ‘- 5.09 kcal/mole’, ‘-4.57 kcal/mole’, ‘-5.42’ and ‘-4.76’ for Plasmodium, Trypanosomes, Leishmania and Entamoeba respectively. The ligand geldanamycin showed an affinity for human HSP90 with a binding energy of -4.68 kcal/mole. In order to decrease the affinity of geldenamycin for the Human HSP90 and increase affinity for pathogens, we generated the analogs of Geldanamycin. Various changes were incorporated in the structure of geldanamycin and finally we selected two analogs to target the Hsps Table 3 (see supplementary material). We find that both analogs of GA showed an increased affinity for protozoans, however a decreased affinity for human HSP90. Table 4 (see supplementary material) was encouraging. An inhibitor must follow ADME properties (absorption, intracellular penetration, binding by plasma proteins, half -life, metabolism and toxicity). The ADME (absorption, distribution, metabolism, and excretion) properties were checked using Lipinski filter. Lipinski rule of 5 helps in distinguishing between drug like and non drug like molecules. The analogs showed positive result for their drug like properties Table 5 (see supplementary material). Moreover, the higher affinity of GA and HSPs of pathogens compared to GA- Hsp of human showed that drug may have a selective affinity in targeting the pathogen HSP90. Though the GA binding site of the HSP homolgs were conserved, the interaction analysis of binding site of HSP90 of targets with Geldanamycin and its analog showed that diverse set of residues were involved in the interaction with the drug molecule Table 6 (see supplementary material). The GA-analog2 showed increased number of interaction compared to GA and GA-analog1 in Trypanosoma and Leishmania HSP90. The GA and its analog1 showed increased number of interaction compared to GA-analog2 in plasmodium HSP90. However, both of the analogs showed a decrease in their affinity with human HSP90. These results suggest that high throughput screening of diverse and potent variants of GA can be further explored to target the HSPs of pathogens.
Supplementary material
Acknowledgments
We thank department of Bioinformatics, MMV, Banaras Hindu University for providing the computational facility.
Footnotes
Citation:Singh & Atri, Bioinformation 9(7): 329-333 (2013)
References
- 1.Chiosis G, et al. Drug Discov Today. 2004;9:881. doi: 10.1016/S1359-6446(04)03245-3. [DOI] [PubMed] [Google Scholar]
- 2.Bagatell R, et al. Mol Cancer Ther. 2004;3:1021. [PubMed] [Google Scholar]
- 3.Bartlett AI, Radford SE. Nat Struct Mol Biol. 2009;16:582. doi: 10.1038/nsmb.1592. [DOI] [PubMed] [Google Scholar]
- 4.Pallavi R, et al. J Biol Chem. 2010;285:37964. doi: 10.1074/jbc.M110.155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, et al. Curr Protoc Immunol. 2004 doi: 10.1002/0471142735.ima01ts58. DOI: 10.1002/0471142735.ima01ts58. [DOI] [PubMed] [Google Scholar]
- 6.Wegele H, et al. Rev Physiol Biochem Pharmacol. 2004;151:1. doi: 10.1007/s10254-003-0021-1. [DOI] [PubMed] [Google Scholar]
- 7.Echeverria PC, et al. J Mol Biol. 2005;350:723. doi: 10.1016/j.jmb.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 8.Banumathy G, et al. J Biol Chem. 2003;278:18336. doi: 10.1074/jbc.M211309200. [DOI] [PubMed] [Google Scholar]
- 9.Chen B, et al. BMC Genomics. 2006;7:156. doi: 10.1186/1471-2164-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sittler A, et al. Hum Mol Genet. 2001;10:1307. doi: 10.1093/hmg/10.12.1307. [DOI] [PubMed] [Google Scholar]
- 11.Stebbins CE, et al. Cell. 1997;89:239. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


