Abstract
Biofilm formation by Candida species is a major contribute to their pathogenic potential.The aim of this study was to determine in vitro effects of EDTA, cycloheximide, and heparin-benzyl alcohol preservative on C. albicans (126) and non-albicans (31)vaginal yeast isolates biofilm formations and their susceptibility against three antifungal Etest strips. Results of the crystal violet-assay, indicated that biofilms formation were most commonly observed [100%] for C. kefyr, C. utilis, C. famata, and Rhodotorula mucilaginosa, followed by C. glabrata [70%], C. tropicalis [50%], C. albicans [29%], Saccharomyces cerevisiae [0.0%]. EDTA (0.3mg/ml) significantly inhibited biofilm formation in both C. albicans and non-albicans isolates (P=0.0001) presumably due to chelation of necessary metal cations for the process-completion. In contrast, heparin (-benzyl alcohol preservative) stimulated biofilm formation in all tested isolates, but not at significant level (P=0.567). Conversely, cycloheximide significantly (P=0.0001) inhibited biofilm formation in all C. albicans strains(126) and its effect was even 3 fold more pronounced than EDTA inhibition, probably due to its attenuation of proteins (enzymes) and/or complex molecules necessary for biofilm formation. Results also showed that all nonalbicans yeasts isolates were susceptible to 5-flucytosine (MIC50, 0.016 µg/ml; MIC90, 0.064 µg/ml), but 14% of C. albicans isolates were resistant (MIC50, 0.064 µg/ml; MIC90 >32 µg/ml). The MIC50 value of amphotricin B for all C. albicans and non-albicans isolates was at a narrow range of 0.023 µg /ml, and the MIC90 values were 0.047 µg/ml and 0.064 µg/ml respectively, thereby confirming its efficacy as a first line empiric- treatment of Candida spp infections.
Keywords: Candida spp, Biofilm formation, EDTA, Heparin-benzyl alcohol, Cycloheximide, Antifungal agents
Background
Species of Candida are considered important opportunistic pathogens. Thus, C. albicans is the most common cause of genital tract infections, in approximately 85-95% of cases, while other species such as C. glabrata, C. krusei, C. tropicalis, C. parasilosis, Saccharomyces cerevisiae, and Rhodutorula spp., have also been increased lately [1]. Positive vaginal cultures for Candida spp. can be found in almost 30% of pregnant and 15% of non-pregnant women [2]. Candida spp. is now the fourth most common cause of hosp ital-acquired blood stream infections. Despite advances in antifungal therapy, the high attributable mortality rate (~40-60%), due to Candida infections has not clearly improved in the last 2 decades [3, 4]. Many of these infections are implant-associated infections, where the micro-organisms form adherent biofilms on the surfaces of catheters, joint replacements, prosthetic heart valves, and other medical devices [5]. Biofilm formation on biotic and abiotic surfaces is a key pathogenic attribute of Candida spp. that enhances its ability to adhere to surfaces and thereby maintain colonization and/or cause diseases in humans. Therefore, biofilm formation represents a major challenge for the treatment of biomaterial-related Candida infections; and in most cases removal and/or replacement of the infected device is difficult or high risk. Policy-management of biomaterial-related complications includes the use of antibiotic lock therapy, heparin locks, and heparin-coated catheters. Ironically, the use of systemic heparin has been identified as a risk factor for catheter-related sepsis in dialysis patients [6]. Heparin has also been found to stimulate Staphylococcus aureus biofilm formation in vitro [7]. Also, [8] found, that benzyl alcohol, frequently associated with heparin as preservative, induced the most significant increase in biofilm production in Staphylococcus epidermidis. In contrast, Miceli et al., demonstrated the inhibitory effects of heparin, though at high concentration (10,000 IU/ml), with or without methyl paraben and/or propyl paraben as preservatives on C. albicans (5 strains) mature-biofilm formation in vitro. EDTA (Ethylene di-amine tetra-acetic acid) has also been reported to inhibit in vitro biofilm formation by Candida spp. [9]. Additionally, several studies indicated that tetracycline [10]; inhibited the prokaryotic Staph. epidermidis biofilm formation and also affects Candida-colonization and/or virulence factors [11]. Although the toxicity of cycloheximide, a potent protein synthesis inhibitor in eukaryotic cells, precluded its clinical use, the drug is being extensively used in mycological culture media to inhibit saprophytic fungi including yeasts. However, as a valuable research tool in laboratory, its effects on the biofilm formation in Candida albicans strains have not been previously explored. Therefore, the present study deals with the investigation of in vitro effects of EDTA, cycloheximide, and heparin (-benzyl alcohol preservative) on biofilms formation by C. albicans (126 strains) and non-albicans (31) yeast isolates previously isolated from vaginal specimens of pregnant and non-pregnant Saudi women and recently reported from out laboratory, as well as their susceptibility patterns against amphotericin B, 5-flucytosine and itraconazole using Etest strips method.
Methodology
Microorganisms:
One hundred and fifty seven isolates belonged to eight species of yeasts were recently recovered from vaginal specimens (707 HVS) of pregnant and non-pregnant Saudi women, and used in this study. These comprises C. albicans (126); C. glabrata (20); C. tropicalis (4); C. kefyr (2); S. cerevisiae (2); and one strain of C. famata; C. utilis ; or R. mucilaginosa. The collected Candida species strains were stored in 10% glycerinated water at −20°C until experiments were performed. The reference strain C. albicans (ATCC 10231) was used as control in biofilm assay, and antifungal susceptibility tests [12].
Inoculum-preparation and Biofilm quantification:
Biofim formation was assisted as previously described [13, 14]. Briefly, fresh yeast-inoculum-pellets were collected by centrifugation (5 minutes at 4000 rpm) from static logarithmic growth in Sabouraud Dextrose Broth (SDB) at 37°C for 6 h, washed and re-suspended in sterile saline to match 3 McFarland turbidity scale. To each well of the microtiter plates (flat bottom) 180µl of SDB (supplemented with extra 8% glucose) were added, then inoculated with 20µl of the above yeast cell suspension. C.albicans (ATCC 10231) reference strain and yeast-free medium controls were also included. Four wells for each yeast isolate were performed: i-saline (50µl), ii- EDTA 50µl (to give final concentration 0.3 mg/ml; iii- heparin from its appropriate dilution in sterile saline, 50µl (to give final concentration of heparin 1000 IU/ml and 0.4 mg/ml benzyl alcohol; and cyclohexamide 50µl (only for C. albicans strains), to give final concentration of 100µM. The plates were then incubated for 48 h at 37°C [15]. After incubation, plate growth was assessed by measuring the absorbance at 490nm (A490). Then for biofilm quantification, the planktonic cells were aspirated, and the wells were washed thrice with sterile saline. The plates were then inverted on filter paper and were allowed to dry for 1 h at room temperature, 200µl of 1% crystal violet (Sigma, USA) was added to each well, and the plate was allowed to stand for 20 min at room temperature. The wells were subsequently washed thrice with sterile saline to wash off the excess crystal violet. A volume of 200µl of ethanol was added to each well and absorbance was measured at 630nm (A630). Testing of these isolates was performed thrice for each yeast strain. As control the background crystal violet binding was measured for wells exposed only to the medium with no Candida spp. Biofilm positive strains were considered those wells where the A630 of which was higher than mean A630 of negative control plus 3X SD.
Antifungal susceptibility testing:
Antifungal susceptibility testing was performed using Etest strips (ETS). Amphotricin B, 5-flucytosine, and itraconazole, ETS were provided by AB BOIDISK (Solna, Sweden), with a concentration gradient range from 0.002µg/ml to 32µg/ml. The strips were stored at –20°C until use. The medium used was RPMI-1640 agar (Sigma Aldrich Ltd., st. Quentin Fallavires, France) at 10.4g/l supplemented with MOPS (3-[Nmorpholino]- propanesulfonic acid at 165mM), glucose at 20g/l, agar at 15g/l, and L-glutamine at 200mM (added after autoclaving). The tested Candida spp. isolate colony previously grown on Sabouraud Dextrose Agar (SDA) plate for 24 h at 37°C, was picked up, suspended in 0.85% sterile saline, and adjusted to match (0.5) McFarland turbidity. A swab of the cell suspension was then spread in three directions on entire surface of a RPMI plate, and left for 15 min to allow moisture absorption at room temperature before applying the ETS on the agar. The agar plates were then incubated with bottom up at 37°C for 24 h except for C. glabrata, R. mucilaginosa, or S. cerevisiae the incubation time was 48 h. After incubation, the MICs of the tested antifungal agents were determined as the lowest concentration at which the border of the elliptical inhibition zone intercepted the scale on the ETS [16]. The interpretive susceptibility criteria were performed according to Negri et al., (2009) breakpoints: amphotericin B (≤1µg/ml, susceptible; ≥2µg/ml, resistant); 5-flucytosine (≤4µg/ml, susceptible; 8-16µg/ml, intermediate; ≥64µg/ml, resistant); itraconazole (≤0.125µg/ml, susceptible; 0.25-0.5µg/ml, susceptible-dose dependent; ≥1.0µg/ml, resistant).
Statistical methods:
The results were analyzed using SPSS 19 (Statistical Package for Social Science; release 19.0). Chi-square test and Fishers exact test were used to compare association between proportions and a P-value < 0.05 was considered as significant.
Result
Results revealed that biofilm formation varied from one strain to another within the same species as well as from species to species. As presented in Table 1 (see supplementary material), only 29 % of C. albicans strains formed biofilms, while as much as 68 % of non-albicans spp. strains were capable of biofilm formation. The rank order of biofilm formation was C. kefyr, C. utilis, C. famata, and R. mucilaginosa (100%), followed by C. glabrata (70%), C. tropicalis (50%), C. albicans (29%), and S. cerevisiae (0%). As illustrated in Figure 1 & Figure 2 there was no correlation between intensity of growth and capacity of biofilm formation. EDTA proved efficient in reducing the biofilm formation by non-albicans spp. (P<0.0001) almost completely Table 2 (see supplementary material), and with considerable reduction in the biofilm formation capacity of most C. albicans strains (P<0.0001). Likewise, cycloheximide (100µM) significantly (P<0.0001) inhibited (Table 2) the biofilm formation capacity of all C. albicans strains, and this effect was even three fold more pronounced than that of EDTA inhibition. Heparin at the concentration used (800U/ml, benzyl alcohol preservative) unanimously enhanced the biofilm formation in all tested non-albicans spp. (P=0.567) and C. albicans (P= 0.414) strains, yet this enhancement, as compared to respective salinecontrol was not significant Table 3 (see supplementarymaterial) & Figure 3. Whether this observed biofilmenhancement is due to heparin as such and/or the associated benzyl alcohol preservative is not clear and warranted further investigation.
Figure 1.
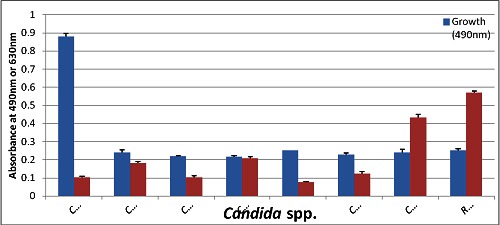
Distribution of Candida spp. isolates on the basis of their capability for growth and biofilm-formation at 37 c for 48h.
Figure 2.
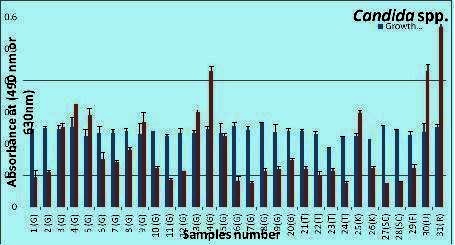
The blue line represent the absorbance of the growth, wells read at (490nm); and the red represent the absorbance of bioflim-formation crystal viloet ethanol extracted stained-wells read at (630).
Figure 3.
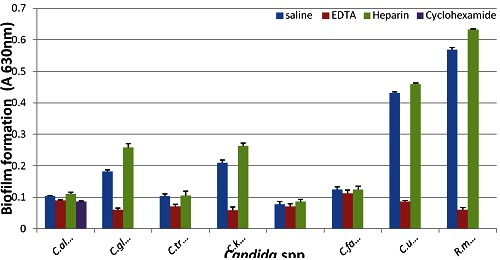
Effets of EDTA, cycloheximide and heparin on tested candida spp. capability to form biofilms at 37°C for 48h.
Additionally, (Table 3) summarizes the in vitro susceptibility testing (MICs) of 126 C. albicans and 31 non-albicans spp. strains against amphotericin B, 5-flucytosine and itraconazole by Etest method. The data were computed as the concentrations of antifungal agent necessary to inhibit 50% of the isolates (MIC50) and concentration to inhibit 90% (MIC90) of the isolates. C. albicans isolates taken together gave (MIC90) value for amphotericin B, 5-flucytosine and itraconazole of 0.047, >32 and 2 µg/ml, respectively. Whereas, non-albicans spp. isolates taken together gave MIC90 value for amphotericin B, 5-flucytosine and itraconazole of 0.064, 0.064 and >32 µg/ml, respectively (Table 3). Amphotericin B MICs showed a very narrow range for the species tested. In contrast, 5-flucytosine and itraconazole MICs showed a broad range for all the species. As expected, most of C. glabrata strains were susceptible to amphotericin B and 5- flucytosine but resistant to itraconazole (Table 3). Results also showed that all Candida spp. including C. albicans were susceptible to amphotericin B and also to 5-flucytosine with the exception 14% of the C. albicans strains. Mean while as much as 32 % of non-albicans isolates (mostly C. glabrata) were resistant to itraconazole, but only 14% of C.albicans isolates where resistant this drug.
Discussion
The phenomenon of in vitro biofilm formation by microbes on various abiotic surfaces has been extensively studied in bacteria and to a lesser extent in fungi, and there appears to be a direct relationship between the capability of organisms to form a biofilm and their pathogenicity. Many Candida spp. infections involve the biofilm formation on implanted devices such as intrauterine devices and urinary catheter. In this study, SDB medium supplemented with extra glucose (8%) was used to induce biofilm formation by examined Candida isolates. The ability of our yeast-isolates to form biofilm varied among different Candida species and even within the same species. The non-albicans spp. exhibited greater capacity in biofilm formation (68%) than that of C. albicans (29%). These findings are consistent with those previously reported by Shin et al [17], Silva et al [18], Parthak et al [19]. However, Kuhn et al [20] reported, that isolates of C. albicans consistently form higher biofilm formation in vitro than non-albicans spp. EDTA disrupts microbial biofilm through its chelation of calcium, iron, and magnesium with established anticoagulant activity and some inhibitory activity against Staphylococci and Candida spp. [21] ..These findings were confirmed by ours, where EDTA significantly inhibited the capacity of biofilm formations by non-albicans spp. As well as C. albicans strains. According to previous studies, EDTA inhibits C. albicans biofilm formation through its inhibitory effect on filamentation. Whereas Percival et al [22] reported that EDTA is a more appropriate anticoagulant and antimicrobial catheter lock than heparin. EDTA has also been shown to inhibit biofilm formation in S. aureus and in Pseudomonas aeruginosa [23].
In the present study heparin at the concentration used (1000U/ml, benzyl alcohol preservative) unanimously enhanced the biofilm formation in all non-albicans spp. as well as C. albicans. In contrast one of the latest studies reported, that heparin with or without, methyl paraben, and/or propyl paraben inhibited C. albicans (only five strains) biofilm formation up to 90%. These controversial findings regarding the effect of heparin on Candida spp biofilm formation, has also been reported for its effect on biofilm formation by Staphylococcus spp. Thus in agreement with our findings on yeast isolates. Likewise, the use of systemic heparin has been identified as a risk factor for catheter-related sepsis in dialysis patients. In contrast, Sauer et al [24] reported that, heparin reduced biofilm formation in Staphylococcus aureus. Further, heparin markedly reduced biofilm formation by 87% of Staphylococcus lugdunensis strains [25]. These discrepancies may be attributed to differences in heparin associated preservatives and /or used medium cation-contents, particularly calcium and magnesium which have been reported to restore the S. aureus heparin inhibitory effect.
Our finding that heparin enhances biofilm formation by all tested Candida spp. including albicans is compatible with previous findings that the use of systemic heparin has been identified as a risk factor for catheter-related sepsis in dialysis. On the other hand cycloheximide is incorporated in fungal media to inhibit saprophytic fungi including yeasts. However, as a research tool in the elucidation of steps in biofilm formation, the effect of cycloheximide on C. albicans biofilms formation has not been previously explored. In the current study, cycloheximide strikingly inhibited biofilm formation by all C. albicans strains, presumably due its attenuation of de novo synthesis of proteins, enzymes [26, 27] and/or complex molecules necessary for the biofilm expression. In this context, several protein synthesis inhibitor antibiotics have been shown to reduce the formation of biofilm in several bacteria (McCool et al., 2008; Flemming et al., 2009). In contrast antibiotics which inhibit cell wall synthesis have been reported to stimulate biofilm formation in prokaryotic pathogens, apparently due to weakness of cell wall integrity which facilitate out diffusion of complex molecules for biofilm formation [28] (Bagge et al., 2004).
In this study, the susceptibility testing profiles indicated that amphotericin B exhibited a very narrow range MIC90 of (0.047 µg/ml and 0.064 µg/ml) for 126 C. albicans strains and 31 nonalbicans strains, respectively. In a similar study, Hanafy and Morsy [29], found that all the tested clinical isolates (39) were susceptible to amphotericin B. Whereas Kabli, [30] found that all the isolates (107) were also susceptible to the amphotericin B, tested by Etest strips. These findings confirm previous conclusion that amphotericin B was active against the majority of the tested yeast and filamentous fungi, including species known to cause rare and difficult to treat infections, and therefore this agent plays an important role in the management of invasive fungal infection [31, 32]. In contrast, in Taiwan [33] (Cheng et al., 2004), reported that three isolates 2% (out of 170 isolates) of non-albicans spp. were resistant to amphotericin B. Whereas in Brazil Negri et al., [34] reported, that two isolates, 2% (out of 100 isolates) of Candida spp., from Iran twenty isolates, 7% (out of 285 isolates) and from Iraq [35] 7 isolates, 47% (out of 15 isolates) of C. albicans strains were resistant to amphotericin B. This discrepancy confirms that Candida spp. susceptibility patterns varied from country to another, presumably due to frequent use of antifungal drugs per se.
Further our results indicated that with the exception of 14% of C. albicans (126 isolates) and all non-albicans Candida spp. were susceptible to 5-flocytosine. In comparison Mokaddas et al., [36] reported, that 9% out of (107 clinical isolates) and 1% (out of 240 isolates) were resistant to 5-flucytosine, respectively. On the other hand itraconazole is among oral antifungal agents currently used in the management of sever fungal infections. However, in this study as much as 32% of non-albicans strains (mostly C. glabrata) were resistant to itraconazole, but only 14% of C. albicans strains where resistant the same drug. Wheras Hanafy and Morsy, found that 12 out of 18 C. albicans strains and 3 out of 21 non-albicans strains were resistant to itraconazole. A lower rate of resistance to this drug was found in Brazil (10%, 5/52); an even higher rate of resistance (40%, 17/43) to this drug was reported from Taiwan. Again as stated above this discrapancy may reflect extensive use and/or rational use of the drug per se. The resistance observed in this study against conventional antifungal agents should be viewed with concern and necessitates continuous monitoring through surveillance studies.
Financial Disclosure
The authors state no conflict of interest.
Supplementary material
Acknowledgments
The authors would like to express their thanks and gratitude to King Abdulaziz City for Science and Technology (KACST), Riyadh, for funding this research project through a MSCstudent grant No: A-S-11-0608 to Mr Ziab Zakey Al-Ahmadey..
Footnotes
Citation:Al-akeel et al, Bioinformation 9(7): 357-362 (2013)
References
- 1.Consolaro MEL, et al. Rev Iberoam Micol. 2004;21:202. [PubMed] [Google Scholar]
- 2.Al-akeel RA, et al. Afri J Microbiol Res. 2003;7:56. [Google Scholar]
- 3.Huang G. Virulence. 2012;3:251. doi: 10.4161/viru.20010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miceli MH, et al. Antimicrob Agents Chemother. 2012;56:148. doi: 10.1128/AAC.05061-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas LJ. Trends Microbiol. 2003;11:30. doi: 10.1016/s0966-842x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 6.Diskin CJ, et al. Nephron Clin Pract. 2007;107:128. doi: 10.1159/000110032. [DOI] [PubMed] [Google Scholar]
- 7.Shanks RM, et al. Infect Immun. 2005;73:4596. doi: 10.1128/IAI.73.8.4596-4606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milisavljevic V, et al. Am J Infect Control. 2008;36:552. doi: 10.1016/j.ajic.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Ramage G, et al. Mycopathologia. 2007;164:301. doi: 10.1007/s11046-007-9068-x. [DOI] [PubMed] [Google Scholar]
- 10.Flemming K, et al. J Antimicrob Chemother. 2009;63:136. doi: 10.1093/jac/dkn464. [DOI] [PubMed] [Google Scholar]
- 11.McCool L, et al. Infect Dis Obstet Gynecol. 2008 doi: 10.1155/2008/493508. doi:10.1155/2008/493508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paiva LC, et al. Micron. 2012;43:497. doi: 10.1016/j.micron.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Shanks RM, et al. Nephrol Dial Transplant. 2006;21:2247. doi: 10.1093/ndt/gfl170. [DOI] [PubMed] [Google Scholar]
- 14.Ruzicka F, et al. Folia Microbiol (Praha) 2007;52:209. doi: 10.1007/BF02931300. [DOI] [PubMed] [Google Scholar]
- 15.Raad I, et al. Int J Antimicrob Agents. 2008;32:515. doi: 10.1016/j.ijantimicag.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Badiee P, et al. Iran J Microbiol. 2011;3:183. [PMC free article] [PubMed] [Google Scholar]
- 17.Shin JH, et al. J Clin Microbiol. 2002;40:1244. doi: 10.1128/JCM.40.4.1244-1248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva S, et al. Med Mycol. 2009;47:681. doi: 10.3109/13693780802549594. [DOI] [PubMed] [Google Scholar]
- 19.Pathak AK, et al. J Appl Oral Sci. 2010;70:75. doi: 10.1590/S1678-77572012000100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn DM, et al. Infect Immun. 2002;70:878. doi: 10.1128/iai.70.2.878-888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raad I, et al. Chemother. 2003;47:3580. doi: 10.1128/AAC.47.11.3580-3585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Percival SL, et al. Infect Control Hosp Epidemiol. 2005;26:515. doi: 10.1086/502577. [DOI] [PubMed] [Google Scholar]
- 23.Banin E, et al. App Env Microbiol. 2006;72:2064. doi: 10.1128/AEM.72.3.2064-2069.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sauer K, et al. J Antimicrobial Chemother. 2009;63:937. doi: 10.1093/jac/dkp060. [DOI] [PubMed] [Google Scholar]
- 25.Frank KL, et al. Diagn Microbiol Infect Dis. 2008;60:9. doi: 10.1016/j.diagmicrobio.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Yigit N, et al. Eurasian J Med. 2011;43:27. doi: 10.5152/eajm.2011.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva S, et al. Trends Microbiol. 2011;19:242. doi: 10.1016/j.tim.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Bagge N, et al. Antimicrob Agents Chemother. 2004;48:1168. doi: 10.1128/AAC.48.4.1168-1174.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanafy A, et al. Afr J Microbiol Res. 2012;6:6978. [Google Scholar]
- 30.Kabli SA. J Bio Sci. 2008;15:189. [Google Scholar]
- 31.Teseng YH, et al. JFDA. 2005;13:12. [Google Scholar]
- 32.Samra Z, et al. Eur J Clin Microbiol Infect Dis. 2005;24:592. doi: 10.1007/s10096-005-0005-y. [DOI] [PubMed] [Google Scholar]
- 33.Cheng MF, et al. Diagn Microbiol Infect Dis. 2004;48:33. doi: 10.1016/j.diagmicrobio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Negri M, et al. J Clin Lab Anal. 2009;23:24. doi: 10.1002/jcla.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jebur MS. Afr J Microbiol. 2011;5:3367. [Google Scholar]
- 36.Mokaddas EM, et al. J Med Microbiol. 2007;56:255. doi: 10.1099/jmm.0.46817-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


