Abstract
The cystic dilation of ventriculus terminalis (CDVT) is a rare anatomical variant in adulthood. In this report we describe a new case of an adult with multilobed CDVT, causing low-back pain and subjective disturbances in walking. A myelotomy with fenestration of the cyst was performed with a good clinical and radiological outcome.
Background
The ventriculus terminalis (VT), also known as the ‘fifth ventricle’, it is a small ependyma-lined cavity within the conus medullaris which is usually in continuity with the central canal of the spinal cord. This ventricle is formed during embryogenesis as a result of canalisation and retrogressive differentiation.1 While the VT has been described as a common developmental phenomenon in newborns, this anatomical condition is less frequent in children (about 2.9% of cases) and even lesser in adults.2 In fact cystic dilation of ventriculus terminalis (CDVT) is a rare condition in this population and only 45 cases have been reported to date.3
In this report we describe a new case of VT multilobed dilation and review the relevant literature about clinical presentation and management.
Case presentation
A 61-year-old woman presented with 1-year history of progressive low-back pain (visual analogue pain scale—VAS: 8) and right coxalgia refractory to analgesic therapy associated with subjective disturbances while walking. No disease was reported in medical history. A previous orthopaedic evaluation excluded pathology at the right hip to justify the symptoms. Neurological examination was normal, except for brisk deep-tendon reflexes in the legs. The patient performed a lumbosacral MRI that showed a multilobed cystic dilation inside the conus medullaris with marked mass effect and without enhancement. The cyst extended from T11 to L1 and measured 60×13 mm. No herniated lumbar disc was observed and no other cystic lesions were found in the spinal cord (figure 1A). Somatosensory and motor evoked potentials were normal. Under general anaesthesia, a standard T11–L1 laminectomy was performed. At this stage, the conus medullaris appeared swollen. A mediolongitudinal caudal myelotomy was then performed: the lesion was fenestrated into the subarachnoideal space of the cauda. A 1 cm2 window of cyst wall was excised using sharp microscissors in order to prevent closure of this fenestration. Under microscopic illumination a suture (8.0) was finally put (figure 2). Histology of the fenestred fragment showed a cyst wall lined by ependimal cells and it was positive to epethelial membrane antigen (EMA) and glial fibral acid protein (GFAP) in immunochemistry.
Figure 1.
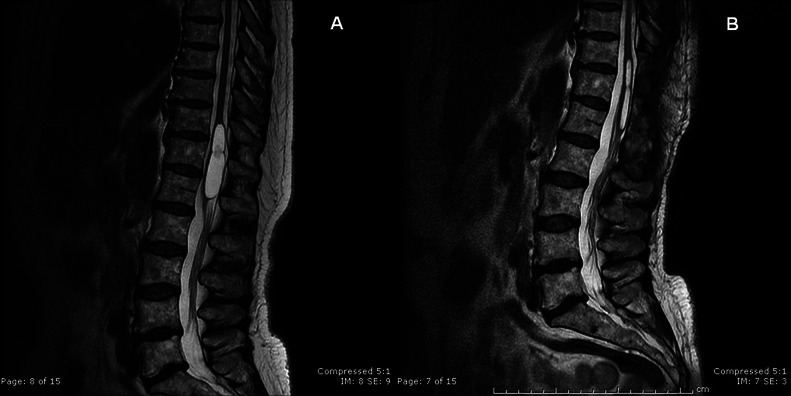
(A) T2-weighted sagittal lumbar MRI of multilobed cystic dilation of conus medullaris; (B) postoperative T2-weighted sagittal lumbar MRI showing significant reduction in the cyst size.
Figure 2.
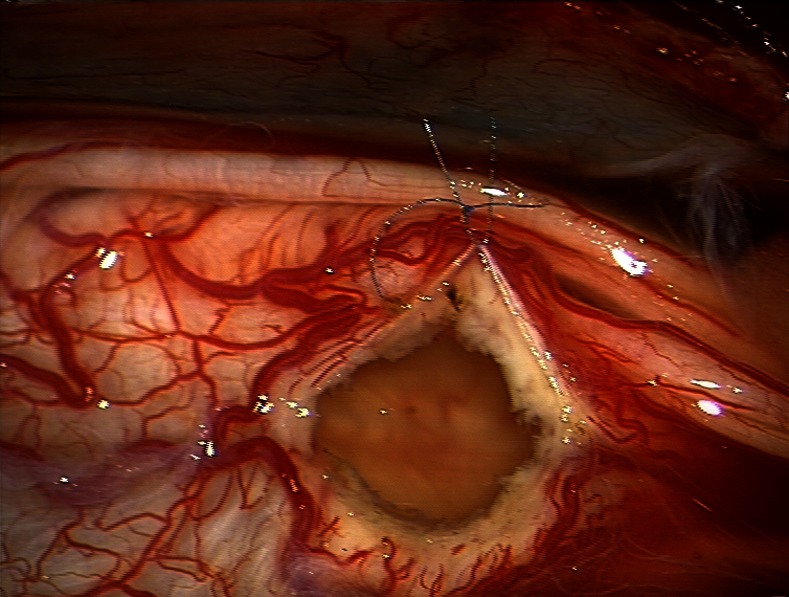
Intraoperative image of the window of cyst wall with a suture (8.0).
Outcome and follow-up
Eight months after the neurosurgical intervention, the pain improved significantly (VAS: 2) without therapy and lumbar MRI showed a reduction in dimensions of cystic dilation without any mass effect on conus medullaris (figure 1B).
Discussion
The CDVT or fifth ventricle is a rare condition in adulthood. Only 45 cases have been reported to date and 35 patients suffered from specific symptoms and were surgically treated3 4. Stilling was the first to describe this condition in 1859, while in 1875 Krause discovered the VT was an actual cavity lined by ciliated ependymal cells and named it ‘the fifth ventricle’.5 The VT forms between the fourth and sixth week of gestation but it reaches its maximum dimension only after 2.5 years of age. The spinal cord is formed embriologically in two stages: neurolation and canalisation followed by retrogressive differentiation. The first step involves flexion and closure of the neural plate to form the neural tube and in this stage most of the spinal cord is formed. After neurolation the caudal end of the neural tube and notochord merge to become an aggregate of undifferentiated cells called ‘caudal cell mass’. Small vacuoles develop within this cell mass and they form an ependyma-lined tube that usually fuses with the more rostral central canal, creating the VT. During the last stage, or ‘retrogressive differentiation’, the filum terminale originates from the involution of the distal cord in a fibrous glioependymal strand.6 Through all these developmental processes there is potential for anatomical variation. The causes of communication failure between VT and ependymal tube are still unknown, although inflammatory processes, trauma and ischaemic events have been postulated.4 In literature some clinical classifications of patients with CDVT have been suggested and indications to surgical treatment are not yet clear. In 2008 de Moura Batista et al7 proposed a clinical classification in three groups: type I—patients with non-specific symptoms (low-back pain, lumbosciatic pain, leg pain); type II—patients with focal neurological deficits (paresis and muscolar atrophy, sensitivity alteration, walking disturbances); Type III. Patients with sphinteric disorders, confirmed by morphofunctional examinations. According to this classification, patients in groups II and III could benefit from surgical treatment, while patients in group I do not have potential for clinical improvement and only a clinical and radiological follow-up is indicated. The patient described in this report could be included in Type I, because of the presence of non-specific symptoms (low-back pain with right coxalgia and subjective walking disturbances). After some time all symptoms worsened and analgesic response was totally absent. In this respect time course represents an important variable when taking into account conservative or invasive therapeutic interventions. In literature some authors described patients with acute worsening of symptoms, while others reported a stable neurological condition over years.3 8 Ganau et al3 suggested a subdivision of Type I symptoms in two subgroups: Type Ia with stable clinical symptoms after a couple of months of observation and without clear connections to VT; Type Ib with non-specific but worsening symptoms requiring surgical intervention. According to this analysis our case is to be included in the Type Ib subdivision. Good clinical follow-up results confirm the possible surgical indications for these patients, after exclusion of other causes. In literature some methods of intervention have been suggested: dorsolumbar laminectomy followed by longitudinal myelotomy and cystosubarachnoid shunt. Such intervention is not widely applied because of poor long-term results.3 9 Today the fenestration of the cyst and excision of a window from the cyst wall to prevent its recurrence is the preferred treatment. In recent years some authors performed percutaneous aspiration of CDVT using real-time MRI, with good early results.10 In our case, we decided to perform myelotomy even if multilobed dilation was present. The fenestration followed by arachnoideal fixing by 8.0 suture helped establish a communication between the CDVT and the subarachnoideal space of the cauda; although the dilation was multilobed, a unique window of cyst wall was needed into the caudal portion of conus medullaris, without cranial extension of the myelotomy. This approach helped reducing the risk of sensitive post-treatment complications overall.
Learning points.
Cystic dilation of ventriculus terminalis (CDVT) is a rare condition in adults with a small number of surgically treated patients.
Patient with Type Ib CDVT could benefit from surgical intervention.
Caudal myelotomy with fenestration of the cyst is the gold standard approach, in spite of the presence of a multilobed cystic dilation of the ventriculus terminalis.
Acknowledgments
The authors are grateful to Dr Irene Pozzi for language revision of the manuscript.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sigal R, Denys A, Halimi P, et al. Ventriculus terminalis of the conus medullaris: MR imaging in four patients with congenital dilatatiom. Am J Neuroradiol 1991;2013:733–7 [PMC free article] [PubMed] [Google Scholar]
- 2.Liccardo G, Ruggeri F, De Cerchio L, et al. Fifth ventricle: an unusual cystic lesion of the conus medullaris. Spinal Cord 2005;2013:507–10 [DOI] [PubMed] [Google Scholar]
- 3.Ganau M, Talacchi A, Cecchi P, et al. Cystic dilatation of the ventriculus terminalis. J Neurosurg Spine 2012;2013:86–92 [DOI] [PubMed] [Google Scholar]
- 4.Dhillon RS, McKelvie PA, Wang YY, et al. Cystic lesion of the ventriculus terminalis in an adult. J Clin Neurosc 2010;2013:1601–3 [DOI] [PubMed] [Google Scholar]
- 5.Kernohan JW. The ventriculus terminalis: its growth and development. J Com Neurol 1924;2013:10–125 [Google Scholar]
- 6.Naidich T, McLone DG. Growth and development. In: Kricun ME, ed. Imaging of the spine and spinal cord. Philadelphia: Saunders, 1988:1–19 [Google Scholar]
- 7.de Moura Batista L, Acioly M, Carvalho C, et al. Cystic lesion of the ventriculus terminalis: proposal for a new clinical classification. J Neurosurg Spine 2008;2013:163–8 [DOI] [PubMed] [Google Scholar]
- 8.Nassar SI, Correll JW, Housepian EM. Intramedullary cystic lesions of the conus medullaris. J Neurol Neurosurg Psychiatry 1968;2013:106–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batzdorf U. Primary spinal syringomyelia. Invited submission from the joint section meeting on disorders of the spine and peripheral nerves. J Neurosurg Spine 2005;2013:429–35 [DOI] [PubMed] [Google Scholar]
- 10.Takahashi S, Saruhashi Y, Odate S, et al. Percutaneous aspiration of spinal terminal ventricle cyst using real-time magnetic resonance imaging and navigation. Spine 2009;2013:629–34 [DOI] [PubMed] [Google Scholar]


