Abstract
We present a case of a 71-year-old man, with a history of hypertension and dyslipidaemia, who presented with typical cardiac chest pain and palpitations of 2 h duration. The examination revealed irregular pulse of 138 bpm, blood pressure 115/75 mm Hg, variable first and normal second heart sounds. The lungs were clear to auscultation. The ECG showed atrial fibrillation with a rapid ventricular rate. His heart rate was controlled with β blockers and the acute coronary syndrome treatment protocol was initiated. His baseline blood reports were within normal limits and two serial troponin I tests were negative. Coronary angiogram showed dissection in the left coronary system extending into the branch vessels and 30–40% stenosis in the right coronary artery. The patient underwent coronary artery bypass graft as an emergent case. He suffered a mild stroke postsurgery with complete functional recovery. He is being followed up in the clinic and has performed well.
Background
Spontaneous coronary artery dissection (SCAD) is an uncommon and a rare cause of acute coronary syndrome (ACS) and sudden cardiac death.1 A number of conditions and diseases are associated with SCAD. Various risk factors for SCAD include pregnancy, intensive exercise, cocaine abuse and connective tissue disorders like Ehlers-Danlos disease and the Marfan’s syndrome.1–4 We present here a case of SCAD in a 71-year-old man admitted with a new onset of paroxysmal atrial fibrillation and SCAD. Our patient did not have the common risk factors for SCAD and the presentation was unusual for a man in the seventh decade of life.
Case presentation
A 71-year-old man, with a history of hypertension and dyslipidaemia presented to the emergency department (ED) with chest pain and palpitations of 2 h duration. He had typical central chest pain of moderate intensity radiating to the neck. The pain improved with nitrates and β blockers that he received in the ED. He did not have a history of coronary artery disease. He neither used tobacco nor had any other addiction. He was on aspirin 75 mg once daily, losartan 50 mg once daily and omeprazole 20 mg once daily.
On examination he had irregular pulse of 138 bpm, blood pressure 115/75 mm Hg, jugular venous pulse was not raised and he had no pedal oedema. His first heart sound was variable with a normal second heart sound; no murmurs or added sounds were audible. The lungs were clear to auscultation. The rest of the examination was unremarkable. The ECG showed atrial fibrillation (AF) with rapid ventricular rate and no ST changes significant of ischaemia.
Investigations
The patient's baseline blood reports were within normal limits and two serial troponin I tests were negative. For further workup of ischaemia a coronary angiogram was undertaken. The aorta was tortuous and a Judkins left (JL4) catheter could not engage the left main (LM) ostium. A non-selective contrast injection was given in the cusp, which raised suspicion of dissection in the left anterior descending artery (LAD, figures 1–3). An Amplatz catheter was then used to selectively engage the LM ostium. The LM ostium was cannulated in the first attempt with ease. A selective careful injection confirmed dissection in the LAD from the ostium to mid-LAD extending into the diagonal branch and proximal left circumflex extending into a large obtuse marginal branch. There was sluggish flow (TIMI II) in LAD. The right coronary artery had 30–40% stenosis in the midsegment. The left ventricular function was assessed on echocardiogram and was preserved.
Figure 1.
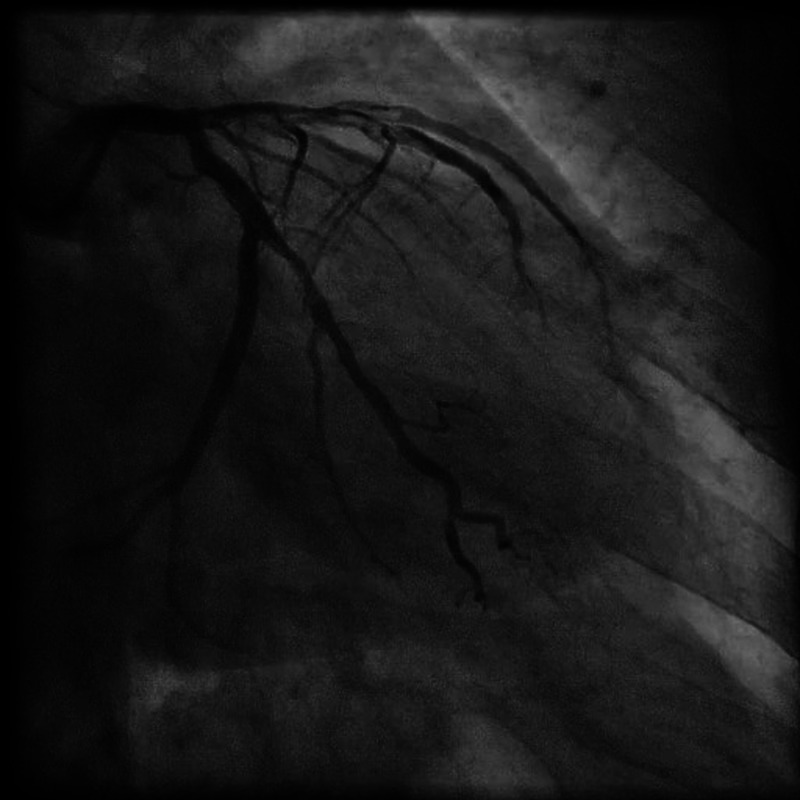
Coronary angiogram RAO 35 CAUD 27 view, showing dissection in left anterior descending artery extending to first diagonal branch and proximal left circumflex extending to obtuse marginal branch.
Figure 2.
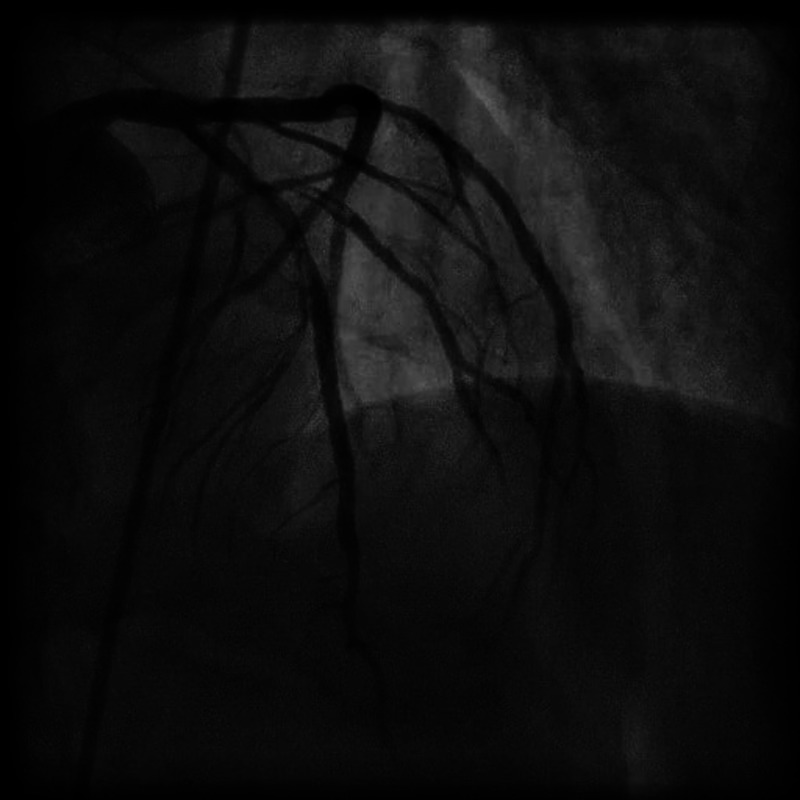
Coronary angiogram LAO 02 CRAN 29 view, showing dissection in left anterior descending artery and first diagonal branch.
Figure 3.
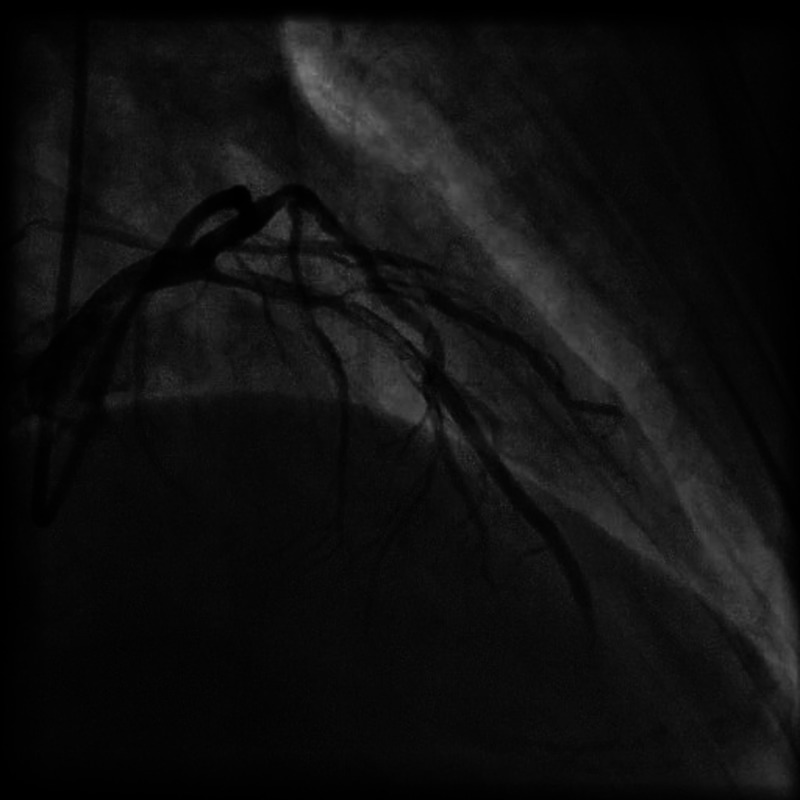
Coronary angiogram RAO 39 CRAN 33 view, showing dissection in left anterior descending artery extending to first diagonal branch and proximal left circumflex extending to obtuse marginal branch.
Treatment
The patient's heart rate was controlled with β blockers and the ACS protocol was initiated including the use of low molecular weight heparin. In view of the extensive dissection in the left coronary system extending into the branch vessels, the patient was advised coronary artery bypass graft surgery (CABG) on an emergent basis.
Outcome and follow-up
The patient underwent CABG on the same day. The postoperative course was eventful with a small ischaemic stroke. He was discharged home after successfully recovering from the functional deficit. He is being followed up in the clinic and had performed well on the last clinic visit without recurrence of chest pain or AF.
Discussion
SCAD is a rare cause of ACS and was first reported in 1931.5 Females are more commonly affected than males and the female to male ratio is 2:1. SCAD is more commonly noted in the left coronary system as was the case in our patient.6 Dissection in the coronary artery is characterised by a partition of the layers of the arterial wall. This leads to a false lumen or an intramural haematoma in the area of the tunica media.2 Coronary angiogram is the main modality for diagnosis of coronary artery dissection. Imaging tools for coronary artery like optical coherence tomography (OCT) and intravascular ultrasound (IVUS) have enabled a more detailed clinical assessment of SCAD.2 OCT and IVUS give comprehensive morphological information on the coronary lesions and on the site of dissection planes involving the different layers of the arterial wall. In addition, non-invasive cardiac CT coronary angiogram has been utilised for longitudinal follow-up assessment of patients with SCAD. We did not use IVUS in our patient for the reason that angiographic assessment revealed high diagnostic accuracy. We did not expect additional imaging information to alter the clinical decision for an emergent CABG. SCAD occurs in 26.1% of cases during pregnancy and most commonly during the postpartum period.7 8 SCAD may be associated with intensive exercise, cocaine abuse, use of oral contraceptives and connective tissue disorders like the Marfan’s syndrome and the Ehlers-Danlos disease.1–4A genetic factor has also been reported in the literature.9 There is paucity of randomised trial data on the best modality of treatment of SCAD. The currently available data are based on case reports and case series. Various strategies of management have been discussed. Conservative treatment is a possible management strategy in stable patients with SCAD.10 Antiplatelet agents can be used because of platelet aggregation and thrombi formation leading to flow limitation.1 Intravenous antiplatelet agents like glycoprotein (GP) IIb/IIIa inhibitors have been effectively used in patients with SCAD.2 We did not use GP IIb/IIIa inhibitor in our patient because the patient was moved emergently for CABG. However, use of a GP IIb/IIIa inhibitor would have been a possible bail-out strategy. Coronary artery angioplasty with stenting can be performed in select cases after recognition of the true and false lumen.1,1 In patients with multivessel dissection, CABG is a reasonable choice.1,2 Our patient underwent CABG owing to the complex long dissection on the left side involving the branch vessels.
Learning points.
Spontaneous coronary artery dissection (SCAD) is a rare disease, more commonly seen in women.1
The clinical manifestation of SCAD depends on the grade and the flow obstructing severity of the coronary artery dissection. Clinical presentation varies from asymptomatic to unstable angina, acute myocardial infarction, ventricular arrhythmias and to sudden cardiac death.
As coronary angiogram is commonly used for the assessment of patients with acute coronary syndrome, therefore, SCAD is mostly diagnosed on coronary angiograms.
The modes of treatment include medical, surgical or percutaneous intervention.
The management strategy is based upon clinical presentation, supplementary findings and morphological information during invasive assessment of coronary artery dissection.
Footnotes
Contributors: AHL wrote the manuscript, collected images and took consent from the patient. AHK gave suggestions about writing the manuscript and was also directly involved in patient care as well. KAK gave suggestions about writing the manuscript. He proof read the manuscript and gave final approval for submission of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kilic ID, Tanriverdi H, Evrengul H, et al. Spontaneous dissection of native coronary arteries. Heart 2005;2013:223–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auer J, Punzengruber C, Berent R, et al. Spontaneous coronary artery dissection involving the left main stem: assessment by intravascular ultrasound. Heart 2004;2013:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hering D, Piper C, Hohmann C, et al. Prospective study of the incidence, pathogenesis and therapy of spontaneous, by coronary angiography diagnosed coronary artery dissection. Z Kardiol 1998;2013:961–70 [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen MB, Aharonian V, Mansukhani P, et al. Spontaneous coronary dissection: a cluster of cases with this rare finding. Am Heart J 1994;2013:1382–7 [DOI] [PubMed] [Google Scholar]
- 5.Pretty HC. Dissecting aneurysm of coronary artery in a woman aged 42. BMJ 1931;2013:667 [Google Scholar]
- 6.Verma PK, Sandhu MS, Mittal BR, et al. Large spontaneous coronary artery dissections: a study of three cases, literature review, and possible therapeutic strategies. Angiology 2004;2013:309–18 [DOI] [PubMed] [Google Scholar]
- 7.Shamloo BK, Chintala RS, Nasur A, et al. Spontaneous coronary artery dissection: aggressive vs. conservative therapy. J Invasive Cardiol 2010;2013:222–8 [PubMed] [Google Scholar]
- 8.Wisecarver J, Jones J, Goaley T. Spontaneous coronary artery dissection. The challenge of detection, the enigma of the cause. Am J Forensic Med Pathol 1989;2013:60–2 [PubMed] [Google Scholar]
- 9.Missouris CG, Ring A, Ward D. A young woman with chest pain. Heart 2000;2013:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung S, Mithani V, Watson RM. Healing of spontaneous coronary dissection in the context of glycoprotein IIb/IIIa inhibitor therapy: a case report. Catheter Cardiovasc Interv 2000;2013:95–100 [DOI] [PubMed] [Google Scholar]
- 11.Roig S, Gomez JA, Fiol M, et al. Spontaneous coronary artery dissection causing acute coronary syndrome: an early diagnosis implies a good prognosis. Am J Emerg Med 2003;2013:549–51 [DOI] [PubMed] [Google Scholar]
- 12.Ooi A, Lavrsen M, Monro J, et al. Successful emergency surgery on triple-vessel spontaneous coronary artery dissection. Eur J Cardiothorac Surg 2004;2013:447–9 [DOI] [PubMed] [Google Scholar]


