Abstract
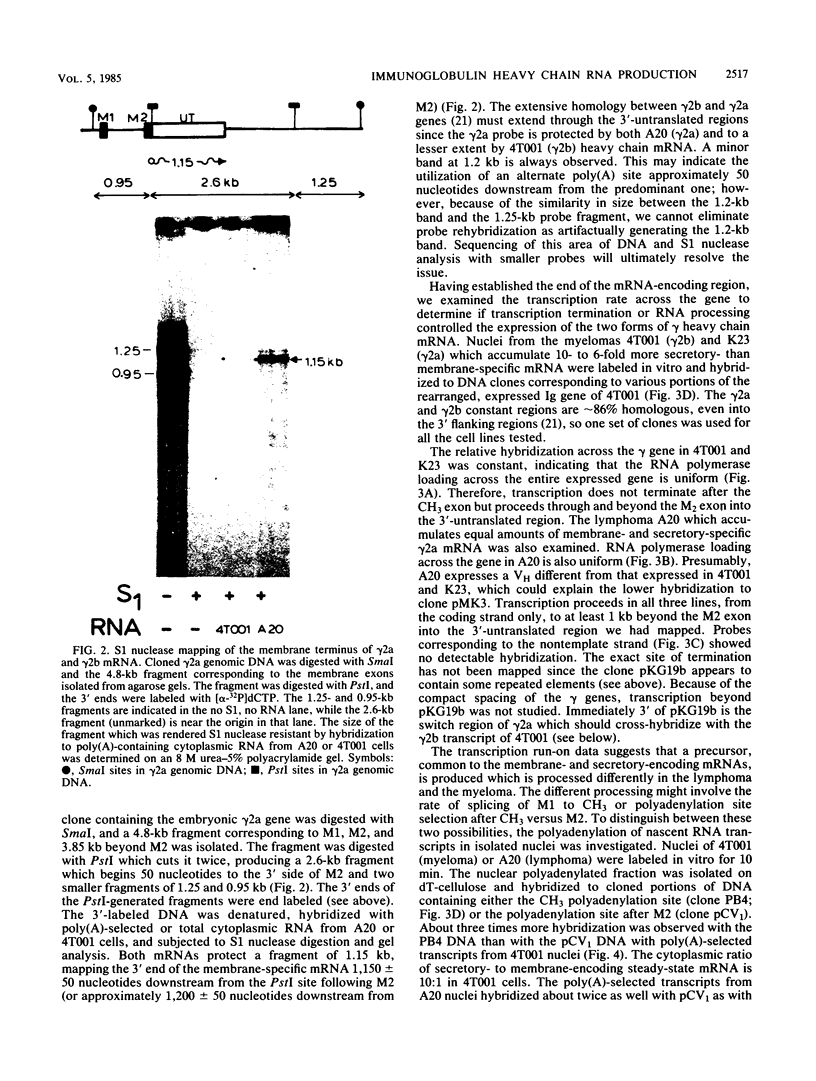
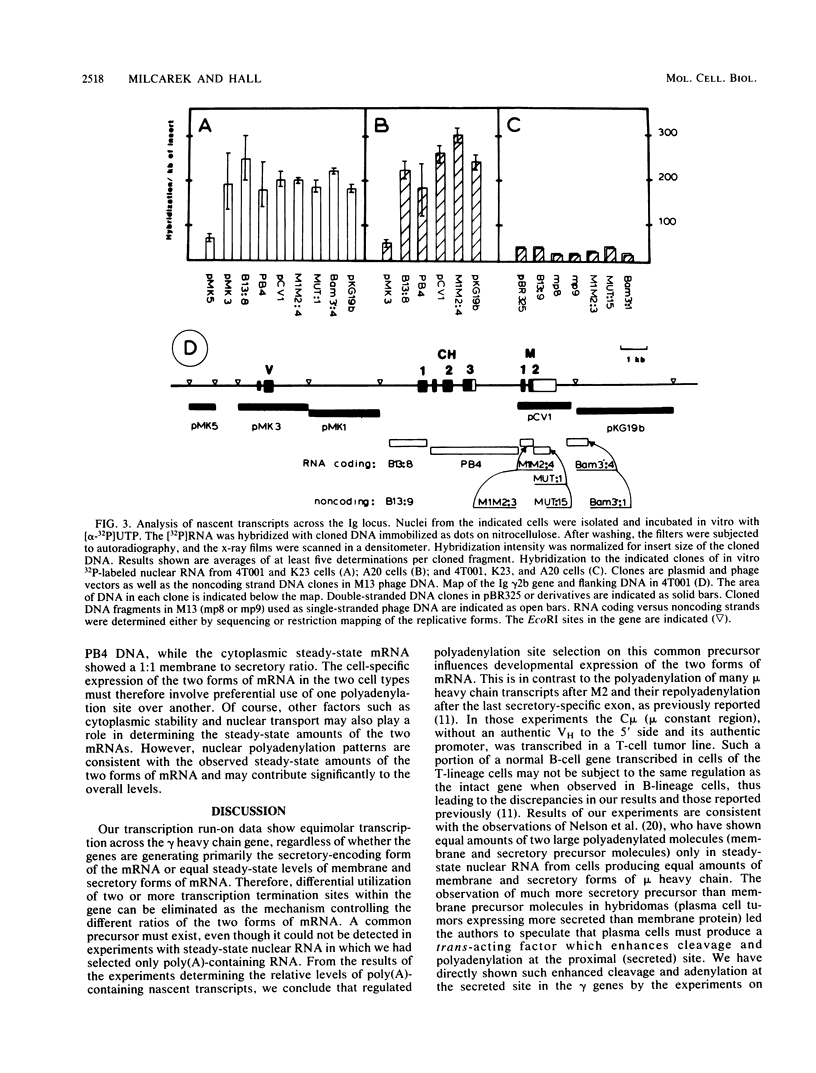
Immunoglobulin heavy chain genes encode at least two forms of mRNA, secretory- and membrane-specific. In less mature B cells and tumors arising from them, lymphomas, the membrane form of the protein and mRNA are in high abundance, while in more mature stages, plasma cells, and myeloma tumor cells, the secreted forms of protein and mRNA predominate. In myeloma cells producing approximately 8:1 ratios of secretory- to membrane-encoding forms of gamma-heavy chain mRNA, we observed equimolar transcription of the secretory- and membrane-encoding exons of the gene. In isolated nuclei from 4T001 (gamma 2b) and K23 (gamma 2a) myeloma cells, the secretory-encoding mRNA polyadenylation site was used at least three times as often as the membrane-encoding mRNA polyadenylation site. In the A20 (gamma 2a) lymphoma, which produces equal amounts of mature secretory- and membrane-encoding heavy chain mRNAs, results of experiments with isolated nuclei showed that the membrane mRNA polyadenylation site was used about two times as often as the secretory mRNA polyadenylation site. Selective use of alternate polyadenylation and cleavage sites, therefore, can modulate the production of the two mRNAs from a single gene during B cell differentiation.
Full text
PDF


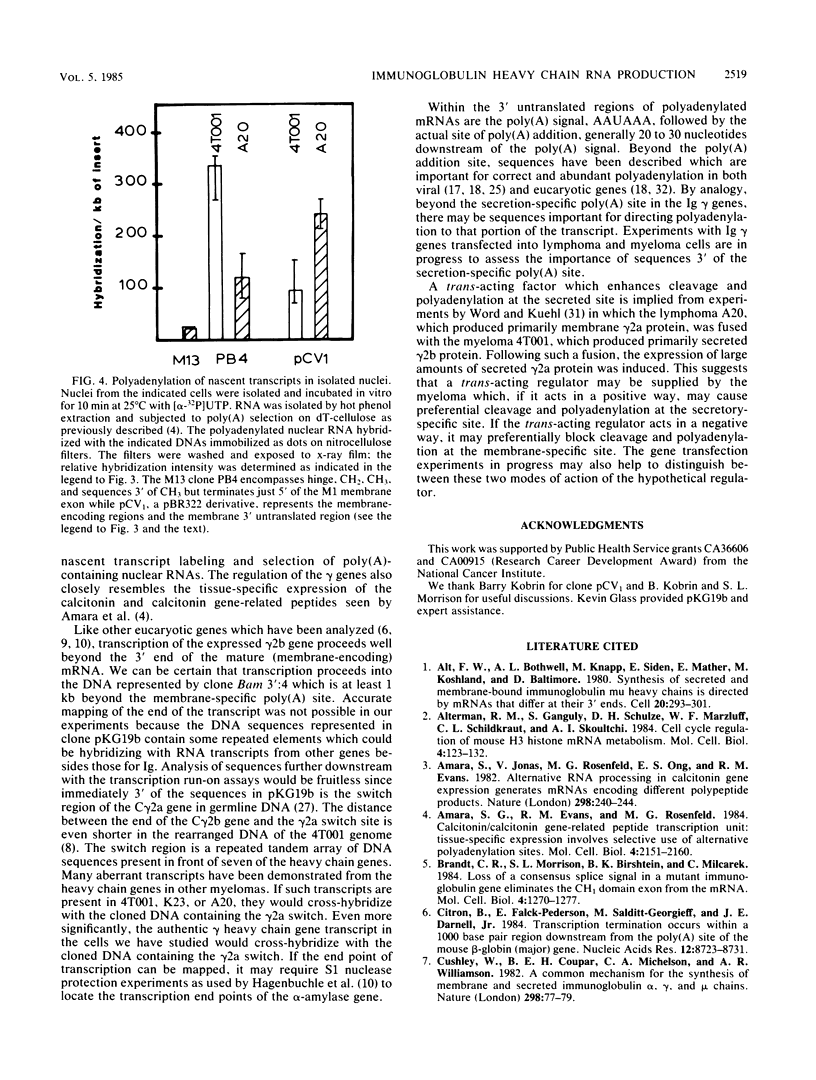

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Bothwell A. L., Knapp M., Siden E., Mather E., Koshland M., Baltimore D. Synthesis of secreted and membrane-bound immunoglobulin mu heavy chains is directed by mRNAs that differ at their 3' ends. Cell. 1980 Jun;20(2):293–301. doi: 10.1016/0092-8674(80)90615-7. [DOI] [PubMed] [Google Scholar]
- Alterman R. B., Ganguly S., Schulze D. H., Marzluff W. F., Schildkraut C. L., Skoultchi A. I. Cell cycle regulation of mouse H3 histone mRNA metabolism. Mol Cell Biol. 1984 Jan;4(1):123–132. doi: 10.1128/mcb.4.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara S. G., Evans R. M., Rosenfeld M. G. Calcitonin/calcitonin gene-related peptide transcription unit: tissue-specific expression involves selective use of alternative polyadenylation sites. Mol Cell Biol. 1984 Oct;4(10):2151–2160. doi: 10.1128/mcb.4.10.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Brandt C. R., Morrison S. L., Birshtein B. K., Milcarek C. Loss of a consensus splice signal in a mutant immunoglobulin gene eliminates the CH1 domain exon from the mRNA. Mol Cell Biol. 1984 Jul;4(7):1270–1277. doi: 10.1128/mcb.4.7.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron B., Falck-Pedersen E., Salditt-Georgieff M., Darnell J. E., Jr Transcription termination occurs within a 1000 base pair region downstream from the poly(A) site of the mouse beta-globin (major) gene. Nucleic Acids Res. 1984 Nov 26;12(22):8723–8731. doi: 10.1093/nar/12.22.8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushley W., Coupar B. E., Mickelson C. A., Williamson A. R. A common mechanism for the synthesis of membrane and secreted immunoglobulin alpha, gamma and mu chains. Nature. 1982 Jul 1;298(5869):77–79. doi: 10.1038/298077a0. [DOI] [PubMed] [Google Scholar]
- Eckhardt L. A., Birshtein B. K. Independent immunoglobulin class-switch events occurring in a single myeloma cell line. Mol Cell Biol. 1985 Apr;5(4):856–868. doi: 10.1128/mcb.5.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayne E. G., Leys E. J., Crouse G. F., Hook A. G., Kellems R. E. Transcription of the mouse dihydrofolate reductase gene proceeds unabated through seven polyadenylation sites and terminates near a region of repeated DNA. Mol Cell Biol. 1984 Dec;4(12):2921–2924. doi: 10.1128/mcb.4.12.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbüchle O., Wellauer P. K., Cribbs D. L., Schibler U. Termination of transcription in the mouse alpha-amylase gene Amy-2a occurs at multiple sites downstream of the polyadenylation site. Cell. 1984 Oct;38(3):737–744. doi: 10.1016/0092-8674(84)90269-1. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Morahan G., Cowman A. F., Harris A. W. Production of RNA for secreted immunoglobulin mu chains does not require transcriptional termination 5' to the microM exons. Nature. 1983 Jan 6;301(5895):84–86. doi: 10.1038/301084a0. [DOI] [PubMed] [Google Scholar]
- Kikutani H., Sitia R., Good R. A., Stavnezer J. Synthesis and processing of the alpha heavy chains of secreted and membrane-bound IgA. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6436–6440. doi: 10.1073/pnas.78.10.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. J., Kanellopoulos-Langevin C., Merwin R. M., Sachs D. H., Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979 Feb;122(2):549–554. [PubMed] [Google Scholar]
- Lang R. B., Stanton L. W., Marcu K. B. On immunoglobulin heavy chain gene switching: two gamma 2b genes are rearranged via switch sequences in MPC-11 cells but only one is expressed. Nucleic Acids Res. 1982 Jan 22;10(2):611–630. doi: 10.1093/nar/10.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskov R., Scharff M. D. Synthesis, assembly, and secretion of gamma globulin by mouse myeloma cells. I. Adaptation of the Merwin plasma cell tumor-11 to culture, cloning, and characterization of gamma globulin subunits. J Exp Med. 1970 Mar 1;131(3):515–541. doi: 10.1084/jem.131.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather E. L., Nelson K. J., Haimovich J., Perry R. P. Mode of regulation of immunoglobulin mu- and delta-chain expression varies during B-lymphocyte maturation. Cell. 1984 Feb;36(2):329–338. doi: 10.1016/0092-8674(84)90226-5. [DOI] [PubMed] [Google Scholar]
- McDevitt M. A., Imperiale M. J., Ali H., Nevins J. R. Requirement of a downstream sequence for generation of a poly(A) addition site. Cell. 1984 Jul;37(3):993–999. doi: 10.1016/0092-8674(84)90433-1. [DOI] [PubMed] [Google Scholar]
- McLauchlan J., Gaffney D., Whitton J. L., Clements J. B. The consensus sequence YGTGTTYY located downstream from the AATAAA signal is required for efficient formation of mRNA 3' termini. Nucleic Acids Res. 1985 Feb 25;13(4):1347–1368. doi: 10.1093/nar/13.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. L. Sequentially derived mutants of the constant region of the heavy chain of murine immunoglobulins. J Immunol. 1979 Aug;123(2):793–800. [PubMed] [Google Scholar]
- Nelson K. J., Haimovich J., Perry R. P. Characterization of productive and sterile transcripts from the immunoglobulin heavy-chain locus: processing of micron and muS mRNA. Mol Cell Biol. 1983 Jul;3(7):1317–1332. doi: 10.1128/mcb.3.7.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollo R., Auffray C., Morchamps C., Rougeon F. Comparison of mouse immunoglobulin gamma 2a and gamma 2b chain genes suggests that exons can be exchanged between genes in a multigenic family. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2442–2446. doi: 10.1073/pnas.78.4.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Choi E., Souza L., Carter C., Word C., Kuehl M., Eisenberg D., Wall R. Gene segments encoding transmembrane carboxyl termini of immunoglobulin gamma chains. Cell. 1981 Oct;26(1 Pt 1):19–27. doi: 10.1016/0092-8674(81)90029-5. [DOI] [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Mermod J. J., Amara S. G., Swanson L. W., Sawchenko P. E., Rivier J., Vale W. W., Evans R. M. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983 Jul 14;304(5922):129–135. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- Sadofsky M., Alwine J. C. Sequences on the 3' side of hexanucleotide AAUAAA affect efficiency of cleavage at the polyadenylation site. Mol Cell Biol. 1984 Aug;4(8):1460–1468. doi: 10.1128/mcb.4.8.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Marcu K. B., Perry R. P. The synthesis and processing of the messenger RNAs specifying heavy and light chain immunoglobulins in MPC-11 cells. Cell. 1978 Dec;15(4):1495–1509. doi: 10.1016/0092-8674(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Schwinghamer M. W., Shepherd R. J. Formaldehyde-containing slab gels for analysis of denatured, tritium-labeled RNA. Anal Biochem. 1980 Apr;103(2):426–434. doi: 10.1016/0003-2697(80)90634-x. [DOI] [PubMed] [Google Scholar]
- Shimizu A., Takahashi N., Yamawaki-Kataoka Y., Nishida Y., Kataoka T., Honjo T. Ordering of mouse immunoglobulin heavy chain genes by molecular cloning. Nature. 1981 Jan 15;289(5794):149–153. doi: 10.1038/289149a0. [DOI] [PubMed] [Google Scholar]
- Sidman C. B lymphocyte differentiation and the control of IgM mu chain expression. Cell. 1981 Feb;23(2):379–389. doi: 10.1016/0092-8674(81)90133-1. [DOI] [PubMed] [Google Scholar]
- Tyler B. M., Cowman A. F., Adams J. M., Harris A. W. Generation of long mRNA for membrane immunoglobulin gamma 2a chains by differential splicing. Nature. 1981 Oct 1;293(5831):406–408. doi: 10.1038/293406a0. [DOI] [PubMed] [Google Scholar]
- Word C. J., Kuehl W. M. Expression of surface and secreted IgG2a by a murine B-lymphoma before and after hybridization to myeloma cells. Mol Immunol. 1981 Apr;18(4):311–322. doi: 10.1016/0161-5890(81)90055-9. [DOI] [PubMed] [Google Scholar]
- Woychik R. P., Lyons R. H., Post L., Rottman F. M. Requirement for the 3' flanking region of the bovine growth hormone gene for accurate polyadenylylation. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3944–3948. doi: 10.1073/pnas.81.13.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]