Abstract
A man presented with multifocal pancreatic metastases 9 years after nephrectomy for renal cell carcinoma. He was managed with oral sunitinib. He had favourable response to treatment with excellent compliance.
Background
This is a rare case of pancreatic metastases in an operated case of renal cell carcinoma (RCC). The management paradigm has evolved with oral sunitinib as the first-line therapy in metastatic RCC.
Case presentation
A 56-year-old man underwent left radical nephrectomy in 2003 for left RCC. The size of the primary tumour was only 3×2 cm. Histopathologically, it was classified as Fuhrman grade I RCC with the involvement of renal vein after institutional pathological review by a team of experienced pathologists. It was staged as T3a N0 M0, Stage III RCC. Postoperatively, the patient has been on regular follow-up with 6 monthly blood investigations, contrast-enhanced CT (CECT) abdomen and x-ray chest. After 9 years of regular follow-up and disease-free status, he reported in August 2012 with vague abdominal pain with loss of weight and appetite.
Investigations
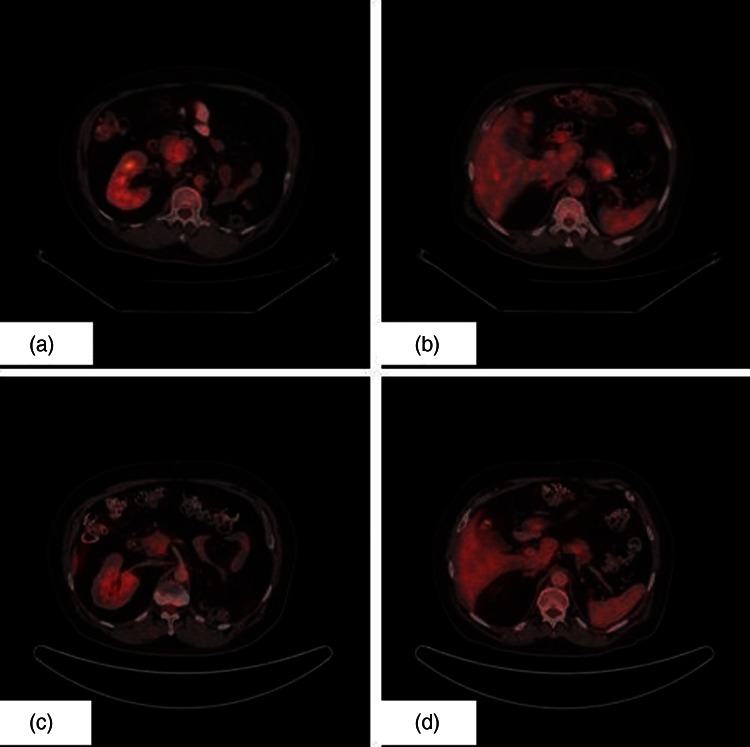
Ultrasonography of abdomen showed a lesion in the head of the pancreas. A CECT of the abdomen revealed a mass in the head of the pancreas of size 4.0 cm×3.0 cm. Biochemical investigations were within normal limits. Carbohydrate antigen 19-9 level was normal. Positron emission tomography (PET) scan revealed two soft tissue mass lesions in the pancreas: one in the head region (4.0 cm×3.0 cm) (standard uptake value (SUV) 4.1) and the other in the tail of pancreas (3.3 cm×3.1 cm) (SUV 4.8) (figure 1A,B). Fine needle aspiration showed clusters of malignant cells which are moderately pleomorphic having round to oval nuclei, coarse chromatin and abundant clear cytoplasm suggestive of metastatic RCC (figure 2).
Figure 1.
(A). Positron emission tomography (PET) image showing metastatic lesion in head of pancreas. (B). PET image showing metastatic lesion in tail of pancreas. (C). Postsunitinib PET image showing response in the metastatic lesion in head of pancreas. (D). Postsunitinib PET image showing response in the metastatic lesion in tail of pancreas.
Figure 2.

Fine needle aspiration image showing metastatic renal cell carcinoma (H&E).
Differential diagnosis
None.
Treatment
In view of metastatic RCC with multifocal disease in pancreas, the patient was started on oral sunitinib. Baseline cardiac evaluation was normal.
Outcome and follow-up
After three cycles of oral sunitinib, he again underwent interval PET scan for response assessment and the metastatic lesions had significant decrease in size and SUV uptake (figure 1C,D). He tolerated oral sunitinib well with no significant haematological and biochemical toxicity. He is presently on the fifth cycle of oral sunitinib and is doing well with clinically asymptomatic status.
Discussion
RCC comprises 2% of all cancers and is the most common malignant tumour of the kidneys in adults.1 Metastases occur in more than 25% of cases and the common sites of metastases are bones, lungs, brain, liver, adrenal glands and contralateral kidney.2 3 Metastases of the primary tumour occur even many years after its removal. Pancreatic metastases are very rare and only anecdotal reports exist in literature. In up to 50% of cases, the course is asymptomatic and the tumour is often diagnosed as part of follow-up examinations. Pancreatic metastases from RCC have been recorded over the course of 6 months to 27 years following nephrectomy and 11% of these metastases have been described in literature as occurring more than 10 years after the initial radical surgical procedure.4 The mode of spread of RCC to the pancreas is controversial. Haematogenous spread is along the draining collateral vein of a hypervascular renal tumour with or without associated renal vein thrombosis. Lymphatic spread is by retrograde lymph flow secondary to tumour infiltration of the retroperitoneal lymph nodes. Direct spread to the pancreas is unusual. Sellner et al hypothesised that the tumour cells had a high affinity for the parenchyma of the pancreas which is supported by the finding of late metachronous metastasis in the residual pancreas.5 The clinical course is asymptomatic in up to 50% of the cases and symptomatic patients have vague gastrointestinal symptoms like abdominal pain, biliary obstruction, abdominal mass, pancreatic exocrine/endocrine dysfunction and weight loss.6
Surgical resection of metastatic disease limited to the pancreas has a 5-year survival rate of 29–35%.7 In patients with isolated pancreatic metastasis, standardised pancreatic resection adapted to the location of the tumour, in terms of partial pancreaticoduodenectomy, distal pancreatectomy and total pancreatectomy is generally recommended.8 9 But, in patients with multiple pancreatic metastases, as in the index case, the role of surgery is controversial, because there is insignificant benefit of surgery on the assumption that multiple pancreatic metastases signal incipient fatal disseminated metastatic disease.10 In a recent retrospective series of 20 patients of RCC with pancreatic metastases, median survival was 8.7 years (range 1.2–12 years) after resection.11 In a review of 203 patients of RCC with pancreatic metastasis treated with pancreatic resection, the median survival was 3.3 years (range 1.0–10.0 years).12
RCC is not sensitive to standard chemotherapy. Currently available data of chemotherapy do not demonstrate reproducible antitumour activity or improvement in survival of patients treated for metastatic RCC.13 RCC is considered to be an immunogenic disease and durable responses can be seen with cytokine therapy. As such, immunotherapy has long been the principal treatment modality for managing advanced RCC, but it has also limited efficacy.14
Knowledge of biological basis of renal oncogenesis has facilitated the transition from a non-specific immune approach using cytokines (ie, interleukin-2, fine needle aspiration (IFN-α)) to a molecular approach that targets the specific pathways involved in RCC pathology. Some of these agents have been food and drug administration approved for the treatment of advanced RCC, whereas others are still considered investigational for this indication.15 There are two different approaches to block the vascular endothelial growth factor (VEGF) pathway. One approach is the use of tyrosine kinase inhibitors to block intracellular domain of VEGF receptor (sunitinib, sorafenib, axitinib and pazopanib). The other approach is the use of monoclonal antibodies to neutralise circulating VEGF and to prevent its activating the VEGF receptor (bevacizumab). All of these agents significantly prolong the time to disease progression in comparison with either placebo or IFN-α.16–18 As such, these agents represent the new standard of care for metastatic disease. As per the recent guidelines, oral sunitinib is the first line treatment option in metastatic RCC and it was followed in the index case.19–21
This is a rare case of Fuhrman grade I RCC presenting with multifocal pancreatic metastases 9 years after nephrectomy. The index case is currently on oral sunitinib and has shown favourable response to sunitinib without any significant toxicity.
Learning points.
Pancreatic metastases is a rare site of metastases in renal cell carcinoma (RCC).
Earlier management options focused on metastatectomy or pancreatic resection.
Sunitinib is now the first line therapy for metastatic RCC with favourable response and compliance.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med 1996;2013:865–75 [DOI] [PubMed] [Google Scholar]
- 2.Faure JP, Tuech JJ, Richer JP, et al. Pancreatic metastasis of renal cell carcinoma: presentation, treatment and survival. J Urol 2001;2013:20–2 [DOI] [PubMed] [Google Scholar]
- 3.Roland CF, van Heerden JA. Nonpancreatic primary tumors with metastasis to the pancreas. Surg Gynecol Obstet 1989;2013:345–7 [PubMed] [Google Scholar]
- 4.Wente MN, Kleeff J, Esposito I, et al. Renal cancer cell metastasis into the pancreas: a single-center experience and overview of the literature. Pancreas 2005;2013:218–22 [DOI] [PubMed] [Google Scholar]
- 5.Sellner F, Tykalsky N, De Santis M, et al. Solitary and multiple isolated metastases of clear cell renal carcinoma to the pancreas: an indication for pancreatic surgery. Ann Surg Oncol 2006. ;2013:75–85 [DOI] [PubMed] [Google Scholar]
- 6.Ballarin R, Spaggiari M, Cautero N, et al. Pancreatic metastases from renal cell carcinoma: the state of the art. World J. Gastroenterol 2011;2013:4747–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanis PJ, van der Gaag NA, Busch ORC, et al. Systematic review of pancreatic surgery for metastatic renal cell carcinoma. Br J Surg 2009;2013:579–92 [DOI] [PubMed] [Google Scholar]
- 8.Reddy S, Wolfgang CL. The role of surgery in the management of isolated metastases to the pancreas. Lancet Oncol 2009;2013:287–93 [DOI] [PubMed] [Google Scholar]
- 9.Reddy S, Edil BH, Cameron JL, et al. Pancreatic resection of isolated metastases from nonpancreatic primary cancers. Ann Surg Oncol 2008;2013:3199–206 [DOI] [PubMed] [Google Scholar]
- 10.Zerbi A, Ortolano E, Balzano G, et al. Pancreatic metastasis from renal cell carcinoma: which patients benefit from surgical resection? Ann Surg Oncol 2008;2013:1161–8 [DOI] [PubMed] [Google Scholar]
- 11.Konstantinidis IT, Dursun A, Zheng H, et al. Metastatic Tumors in the Pancreas in the Modern Era. J Am Coll Surg 2010;2013:749–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machado NO, Chopra P. Pancreatic metastasis from renal carcinoma managed by Whipple resection. A case report and literature review of metastatic pattern, surgical management and outcome. JOP 2009;2013:413–18 [PubMed] [Google Scholar]
- 13.Ravaud A, Wallerand H, Culine S, et al. Update on the medical treatment of metastatic renal cell carcinoma. Eur. Urol 2008;2013:315–25 [DOI] [PubMed] [Google Scholar]
- 14.McDermott DF. Immunotherapy of metastatic renal cell carcinoma. Cancer 2009;2013(10 Suppl):2298–305 [DOI] [PubMed] [Google Scholar]
- 15.Bellmunt J, Montagut C, Albiol S, et al. Present strategies in the treatment of metastatic renal cell carcinoma: an update on molecular targeting agents. BJU Int 2007;2013:274–80 [DOI] [PubMed] [Google Scholar]
- 16.Coppin C, Kollmannsberger C, Le L, et al. Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU Int 2011;2013:1556–63 [DOI] [PubMed] [Google Scholar]
- 17.Di Lorenzo G, Autorino R, Sternberg CN. Metastatic renal cell carcinoma: recent advances in the targeted therapy Era. Eur Urol 2009;2013:959–71 [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. J. Clin. Oncol 2006;2013:5601–8 [DOI] [PubMed] [Google Scholar]
- 19.Bellmunt J, Guix M. The medical management of metastatic renal cell carcinoma: integrating new guidelines and recommendations. BJU Int 2009;2013:572–7 [DOI] [PubMed] [Google Scholar]
- 20.Escudier B, Eisen T, Porta C, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;2013(Suppl 7):vii65–71 [DOI] [PubMed] [Google Scholar]
- 21.Molina AM, Motzer RJ. Clinical practice guidelines for the treatment of metastatic renal cell carcinoma: today and tomorrow. Oncologist 2011;2013(Suppl 2):45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]