Abstract
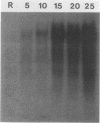
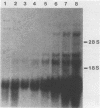
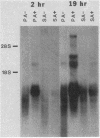
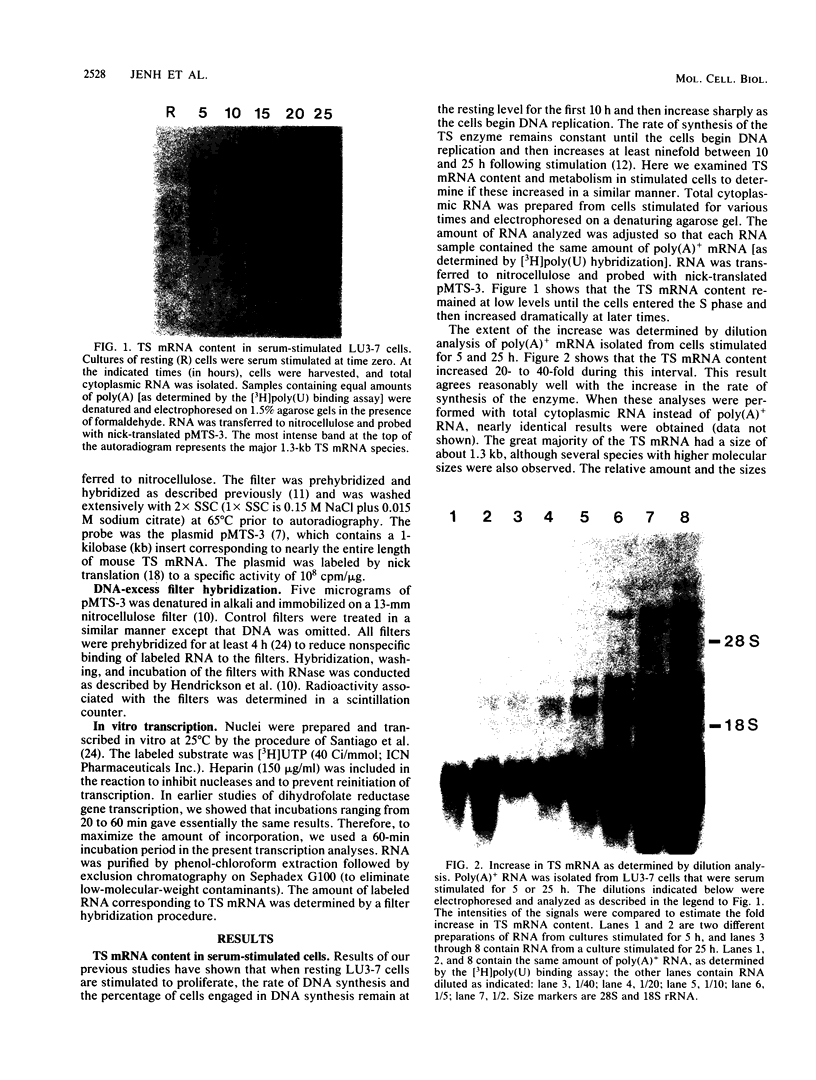
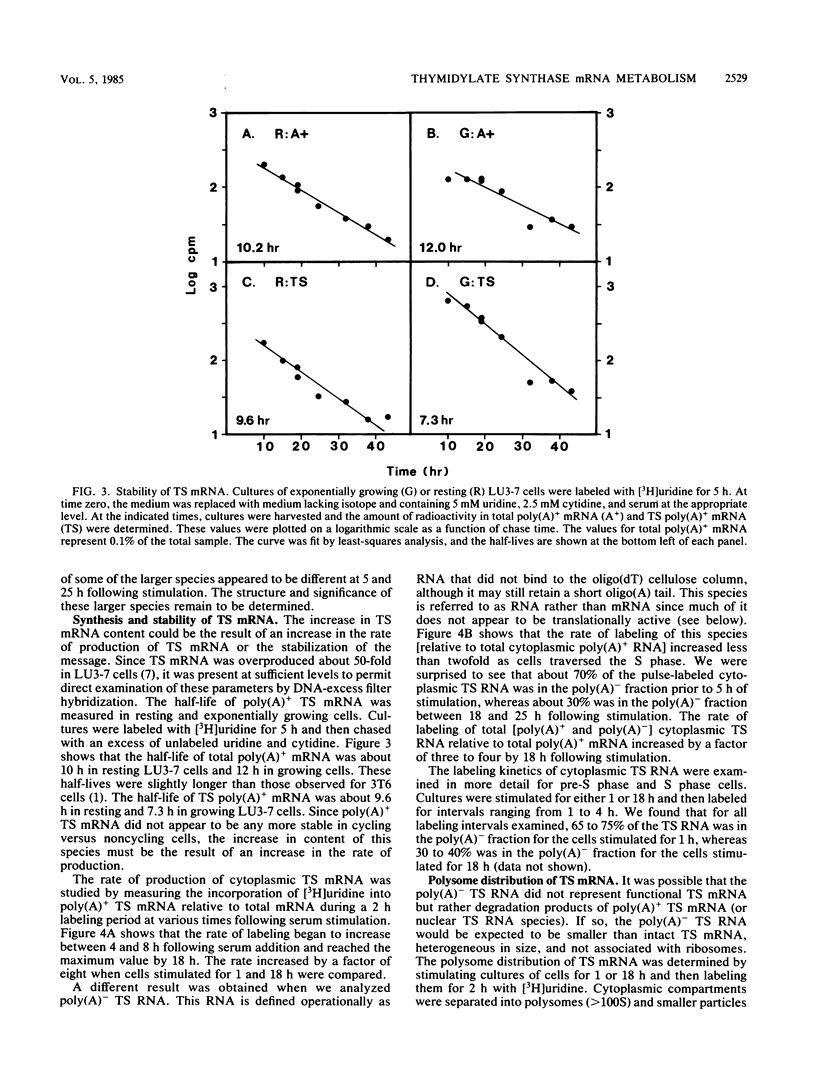
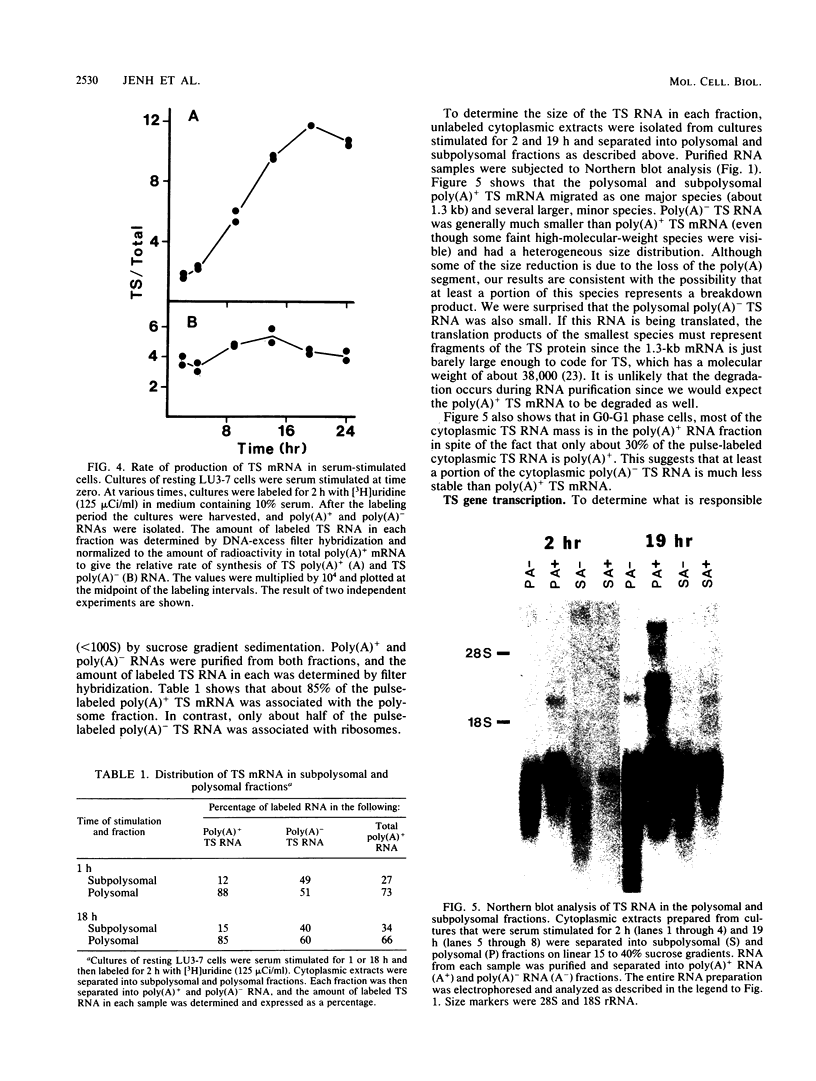
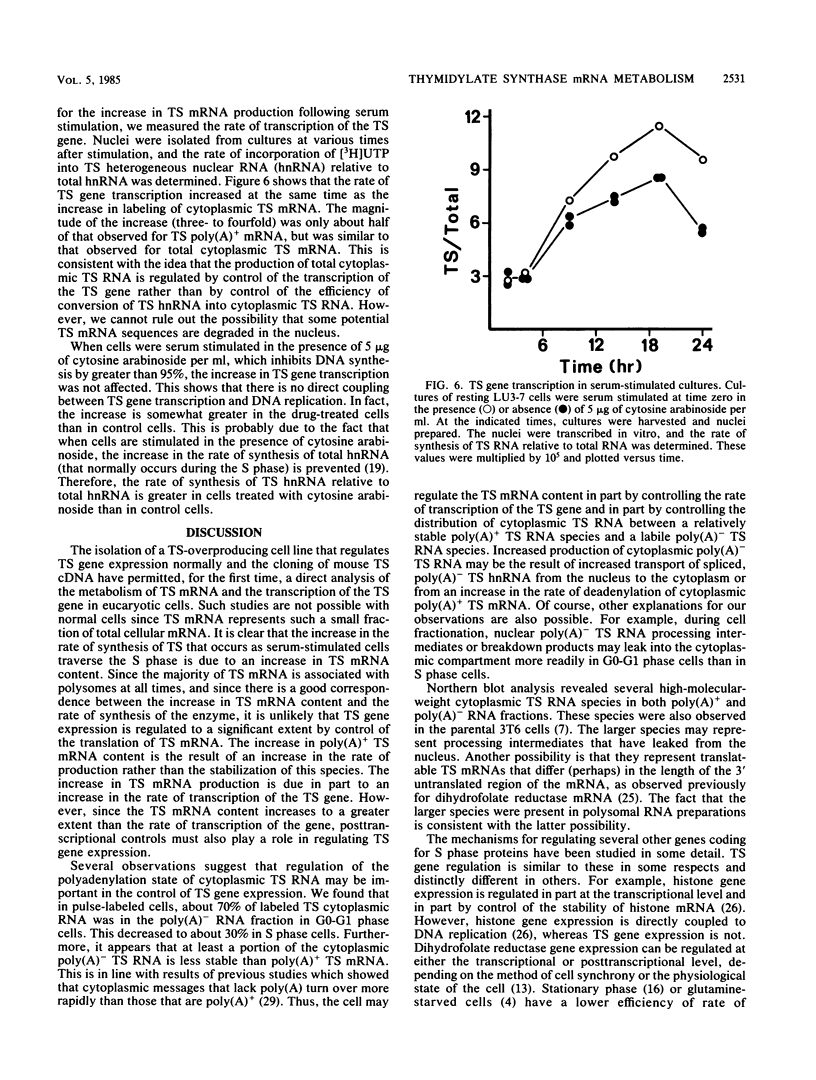
We studied the content and metabolism of thymidylate synthase mRNA in cultured mouse fibroblasts that were undergoing a serum-induced transition from the resting to growing state. The studies were performed with a 5-fluorodeoxyuridine-resistant 3T6 cell line (LU3-7) that over produces the enzyme and its mRNA about 50-fold and that regulates the expression of the thymidylate synthase gene in the same manner as the parental cell line. We have previously shown that the rate of synthesis of thymidylate synthase increases at least ninefold when the serum-stimulated cells traverse the S phase. Here we show, by Northern blot analysis, that thymidylate synthase mRNA increased 20- to 40-fold as cells progressed from resting to late S phase. About 85% of poly(A)+ thymidylate synthase mRNA was associated with polysomes at all times. The increase in thymidylate synthase poly(A)+ mRNA content was the result of an eightfold increase in the rate of production of this species, as shown by pulse-labeling studies. Pulse-chase analysis revealed that the half-life of thymidylate synthase poly(A)+ mRNA was similar in resting (9 h) and growing (7 h) cells. The rate of transcription of the thymidylate synthase gene, as determined in isolated nuclei, increased only by a factor of three to four during the S phase. Since the content of the message increased to a much greater extent than the rate of transcription of the gene, posttranscriptional controls must also play a role in regulating the content of thymidylate synthase mRNA under these conditions. Our results suggest that the cell may regulate the distribution of thymidylate synthase mRNA between a relatively stable poly(A)+ RNA species and a labile poly(A)- RNA species.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O., Rosbash M. Polynucleotide sequences in eukaryotic DNA and RNA that form ribonuclease-resistant complexes with polyuridylic acid. J Mol Biol. 1974 May 5;85(1):75–86. doi: 10.1016/0022-2836(74)90130-2. [DOI] [PubMed] [Google Scholar]
- Collins M. L., Wu J. S., Santiago C. L., Hendrickson S. L., Johnson L. F. Delayed processing of dihydrofolate reductase heterogeneous nuclear RNA in amino acid-starved mouse fibroblasts. Mol Cell Biol. 1983 Oct;3(10):1792–1802. doi: 10.1128/mcb.3.10.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad A. H., Ruddle F. H. Regulation of thymidylate synthetase activity in cultured mammalian cells. J Cell Sci. 1972 Mar;10(2):471–486. doi: 10.1242/jcs.10.2.471. [DOI] [PubMed] [Google Scholar]
- Danenberg P. V. Thymidylate synthetase - a target enzyme in cancer chemotherapy. Biochim Biophys Acta. 1977 Dec 23;473(2):73–92. doi: 10.1016/0304-419x(77)90001-4. [DOI] [PubMed] [Google Scholar]
- Geyer P. K., Johnson L. F. Molecular cloning of DNA sequences complementary to mouse thymidylate synthase messenger RNA. J Biol Chem. 1984 Jun 10;259(11):7206–7211. [PubMed] [Google Scholar]
- Geyer P. K., Meyuhas O., Perry R. P., Johnson L. F. Regulation of ribosomal protein mRNA content and translation in growth-stimulated mouse fibroblasts. Mol Cell Biol. 1982 Jun;2(6):685–693. doi: 10.1128/mcb.2.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Casimir C. Post-transcriptional regulation of the chicken thymidine kinase gene. Nucleic Acids Res. 1984 Feb 10;12(3):1427–1446. doi: 10.1093/nar/12.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson S. L., Wu J. S., Johnson L. F. Cell cycle regulation of dihydrofolate reductase mRNA metabolism in mouse fibroblasts. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5140–5144. doi: 10.1073/pnas.77.9.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenh C. H., Geyer P. K., Baskin F., Johnson L. F. Thymidylate synthase gene amplification in fluorodeoxyuridine-resistant mouse cell lines. Mol Pharmacol. 1985 Jul;28(1):80–85. [PubMed] [Google Scholar]
- Jenh C. H., Rao L. G., Johnson L. F. Regulation of thymidylate synthase enzyme synthesis in 5-fluorodeoxyuridine-resistant mouse fibroblasts during the transition from the resting to growing state. J Cell Physiol. 1985 Jan;122(1):149–154. doi: 10.1002/jcp.1041220122. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Leys E. J., Crouse G. F., Kellems R. E. Dihydrofolate reductase gene expression in cultured mouse cells is regulated by transcript stabilization in the nucleus. J Cell Biol. 1984 Jul;99(1 Pt 1):180–187. doi: 10.1083/jcb.99.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALEY F., MALEY G. F. Nucleotide interconversions. II. Elevation of deoxycytidylate deaminase and thymidylate synthetase in regenerating rat liver. J Biol Chem. 1960 Oct;235:2968–2970. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J. C., Green H. Regulation of RNA synthesis in fibroblasts during transition from resting to growing state. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2819–2822. doi: 10.1073/pnas.70.10.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill G. F., Hauschka S. D., McKnight S. L. tk Enzyme expression in differentiating muscle cells is regulated through an internal segment of the cellular tk gene. Mol Cell Biol. 1984 Sep;4(9):1777–1784. doi: 10.1128/mcb.4.9.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navalgund L. G., Rossana C., Muench A. J., Johnson L. F. Cell cycle regulation of thymidylate synthetase gene expression in cultured mouse fibroblasts. J Biol Chem. 1980 Aug 10;255(15):7386–7390. [PubMed] [Google Scholar]
- Rode W., Scanlon K. J., Moroson B. A., Bertino J. R. Regulation of thymidylate synthetase in mouse leukemia cells (L1210). J Biol Chem. 1980 Feb 25;255(4):1305–1311. [PubMed] [Google Scholar]
- Rossana C., Gollakota Rao L., Johnson L. F. Thymidylate synthetase overproduction in 5-fluorodeoxyuridine-resistant mouse fibroblasts. Mol Cell Biol. 1982 Sep;2(9):1118–1125. doi: 10.1128/mcb.2.9.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago C., Collins M., Johnson L. F. In vitro and in vivo analysis of the control of dihydrofolate reductase gene transcription in serum-stimulated mouse fibroblasts. J Cell Physiol. 1984 Jan;118(1):79–86. doi: 10.1002/jcp.1041180114. [DOI] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Storms R. K., Ord R. W., Greenwood M. T., Mirdamadi B., Chu F. K., Belfort M. Cell cycle-dependent expression of thymidylate synthase in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Dec;4(12):2858–2864. doi: 10.1128/mcb.4.12.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevi M., Nevins J. R., Darnell J. E., Jr Newly formed mRNA lacking polyadenylic acid enters the cytoplasm and the polyribosomes but has a shorter half-life in the absence of polyadenylic acid. Mol Cell Biol. 1982 May;2(5):517–525. doi: 10.1128/mcb.2.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]