Abstract
Polymorphous low-grade adenocarcinoma (PLGA) originating mostly in the minor salivary glands of the posterior hard and soft palate is characterised by its indolent growth and a slower rate of metastasis. Seldom does the PLGA present an aggressive behaviour and demonstrate distant metastasis, as in the present case where a 73-year-old female patient with a swelling in the maxillary alveolus was diagnosed as PLGA exhibiting high-grade transformation, subsequently metastasizing to the abdomen and lungs. The importance of immunomarkers, c-kit and ki-67 in deciphering the clinical behaviour of this PLGA is highlighted. Distant metastasis to the abdomen has not yet been reported; hence, this case of PLGA emphasises the importance of immunohistochemistry in assessing its aggressiveness and understanding a novel aspect of its pathogenesis.
Background
Polymorphous low-grade adenocarcinoma (PLGA) is a malignant epithelial neoplasm characterised by cytological uniformity, morphological diversity, infiltrative growth pattern and a low metastatic potential. PLGA is considered as the second most common intraoral malignant salivary gland tumour accounting for 26% of intraoral minor salivary gland carcinomas.1 It is more common in elderly women with the palate being the most common site of involvement.2 Histologically, it shows striking similarities to adenoid cystic carcinoma (AdCC) with both exhibiting cribriform morphology and infiltrative growth. However, the differentiation of these two entities is important as the treatment and prognosis vary for both. A case of PLGA of the maxillary alveolus, which displayed aggressive behaviour and metastases to lungs and abdomen is presented, which, to the best of our knowledge, is the first such reported case. The immunohistochemical markers used to diagnose and distinguish it from AdCC as well as to assess its clinical behaviour are highlighted.
Case presentation
A 79-year-old female patient was presented to the university dental college hospital complaining of a painless swelling in the right upper posterior jaw for the last 1 month. Clinical examination revealed a swelling measuring 3.5×2 cm in the missing premolar area, with the overlying mucosa being erythematous and causing buccolingual expansion of cortical plates (figure 1). The lesion was excised under local anaesthesia and the tissue submitted for histopathological examination.
Figure 1.

Clinical appearance of the palatal tumour presenting as a swelling extending in the missing premolar area.
Microscopic examination of the lesion revealed a partially circumscribed neoplasm in which the tumour cells were arranged in a variety of patterns ranging from solid sheets, small tubules, cribriform and single file pattern in a hyalinised stroma (figure 2A–C). Individual tumour cells were small and uniform, displaying bland, minimally hyperchromatic nuclei with prominent nucleoli. All the above features were suggestive of PLGA; however, certain areas exhibited dedifferentiation showcasing high-grade tumour cells with abundant mitosis (figure 3). Immunohistochemical staining was consistent with a PLGA as tumour cells were positive for pan cytokeratin (CK), ki-67, S-100 (figure 4A–C) and negative for carcinoembryonic antigen (CEA), Smooth muscle antigen (SMA), c-kit (figure 4D) (table 1). Focal positivity for ki-67 was observed with the labelling index (LI) being 20%. Thus, taking into account the presence of dedifferentiated areas, a markedly high proliferative index with ki-67 and the absence of c-kit and CEA reactivity, this case was signed out as PLFA, dedifferentiated variant.
Figure 2.
Polymorphous low-grade adenocarcinoma (PLGA) tumour cells arranged in (A) solid pattern, (B) tubular pattern and (C) single file pattern.
Figure 3.
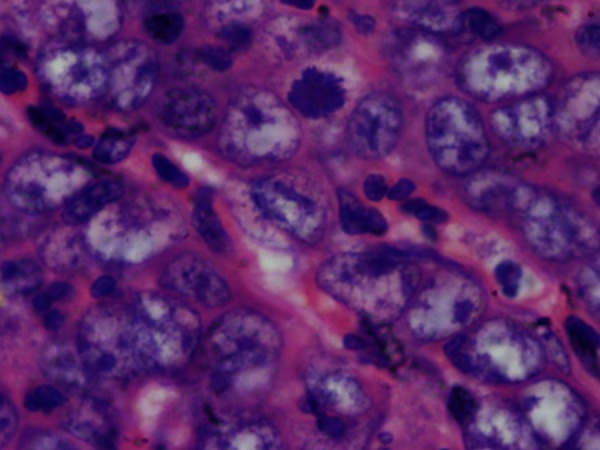
Cytoarchitectural features of dedifferentiated tumour cells showing abundant mitotic figures.
Figure 4.
Polymorphous low-grade adenocarcinoma (PLGA) tumour cells with a strong diffuse positivity for (A) pan CK and (B) S100. (C) Dedifferentiated areas of PLGA with strong focal positivity for ki-67. (D) Tumour cells, negative for c-kit.
Table 1.
Antibody panel used for diagnostic work-up of polymorphous low-grade adenocarcinoma
| Antibody | Source | Dilution | Expression |
|---|---|---|---|
| Pan CK | Dako | 1 : 50 | Positive, diffuse |
| CEA | Dako | 1 : 400 | Negative |
| Ki-67 | Sigma | 1 : 20 | Positive; LI—20% |
| S100 | Dako | 1 : 800 | Positive, diffuse |
| SMA | Sigma | 1 : 50 | Negative |
| c-kit | Dako | 1 : 200 | Negative |
CEA, carcinoembryonic antigen; c-kit—CD117; LI, labelling index; Pan CK, pan cytokeratin, S100; SMA, smooth muscle actin.
Histopathology of the resected maxilla revealed no residual tumour although metastatic deposits of poorly differentiated adenocarcinoma were evident in 1 of the 14 nodes in the neck. During surgery, a small lump of 1×1 cm, which was of hard consistency was noted in the right anterior abdominal wall and excised. Disseminated tumour cells in the anterior abdominal wall were noted. The patient was discharged with stable vital parameters and positron emission tomography scan was planned to rule out a primary tumour spread from the lungs. Although the patient did not return for a review, information obtained later revealed that there was a tumour focus present in the lungs, which showed metastatic deposit of poorly differentiated adenocarcinoma from the maxillary lesion.
Discussion
PLGA was aptly named as a separate entity from adenocarcinoma not otherwise specified in an effort to be clinically and morphologically descriptive by Evans and Batsakis.3 It is considered polymorphous as it demonstrates a myriad of tumour cell patterns microscopically and a low grade as it exhibits an indolent behaviour with metastasis seen in a minority of cases. Metastasis when reported is mostly confined to the regional nodes while spread to distant sites is a rarity.4 Even rarer is the transformation of this low-grade entity into a high grade one and subsequent metastasis.4
Histologically, the tumour is composed of neoplastic cells thought to be derived from the luminal and abluminal myoepithelial cells arranged in various patterns and although distinctive, it has to be differentiated from its closest differential diagnosis—AdCC. Immunohistochemistry (IHC) has been utilised in a number of case series to distinguish between the two but there is considerable overlap (table 1).1 5 In this case, the positivity for CK, S100 and negativity for c-kit confirmed the diagnosis of PLGA from AdCC.6
Past studies conducted regarding the expression of ki-67 have consistently reported a very low LI in PLGA which can assist in differentiating it from AdCC.1 7 Results obtained from these studies also showed that the LI of ki-67 in AdCC usually has rates of greater than 10% versus less than 6.4% in PLGA whereas a high LI of ki-67 (>20%) was observed in our case. This unusual finding of a high ki-67 LI in this case would therefore explain the aggressiveness of this particular case of PLGA which has manifested clinically with metastases to distant sites.
The concept of high-grade transformation is applied to the progression of cells to a less differentiated stage where the original line of differentiation is no longer evident.8 There have been five cases of PLGA with transformation to a high-grade state reported in the literature (table 2).4 9 10 Histologically, there are almost no points of difference between the two; with a few cases exhibiting a separate area of dedifferentiation to help in the diagnosis. All the cases reported, presented as a swelling in the palate, characterised by a higher proportion of solid and cystic growth pattern, nuclear atypia with prominent nucleoli and necrosis. IHC can help in dissociating PLGA from its aggressive manifestation. The high LI of ki-67 in the present case showcased the aggressiveness of this PLGA. According to Costa et al,11 the most useful tool in identifying the dedifferentiated/high-grade transformed component is ki-67 expression analysis.
Table 2.
Published cases of polymorphous low-grade adenocarcinoma showing high-grade change and distant metastasis
| S. No. | Reference | Cases | Site | Dedifferentiation/high-grade | Distant metastasis |
|---|---|---|---|---|---|
| 1 | Simpson et al | 2 | Palate | Present | Absent |
| 2 | Pelkey et al | 2 | Palate | Present | Absent |
| 3 | Lloreta et al | 1 | Nasal cavity | Present | Absent |
| 4 | Our case | 1 | Maxillary alveolus | Present | Present, lung and anterior abdomen |
In conclusion, we report, to the best of our knowledge, the first reported case of PLGA, occurring in the maxillary alveolus region that dedifferentiated and showcased distant metastases to the abdomen and lung. This case suggests that PLGA, although an indolent tumour can show a shift from its usual pathogenesis and can behave in a belligerent fashion. The use of an IHC marker panel of CK, SMA, S100, CEA, c-kit and ki-67 can help differentiate it from AdCC and serve as an initial clue to its behaviour thereby helping the surgeon make an appropriate treatment plan with a rigorous follow up schedule.
Learning points.
Although polymorphous low-grade adenocarcinoma is considered a low-grade malignancy, it can show distant metastasis.
C-kit immunostaining is most useful in differentiating polymorphous low-grade adenocarcinoma (PLGA) from its closest differential diagnosis adenoid cystic carcinoma.
Ki-67 labelling index is useful in assessing the aggressiveness of PLGA.
First reported case of PLGA showing metastasis to the abdomen.
Footnotes
Contributors: All authors have made an individual contribution to the writing of the article; AT was involved in conception and design; RR and AT were involved in drafting the article or revising it critically for important intellectual content and RR and AT was involved in final approval of the version published.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Waldron CA, El-Mofty S, Gnepp DR. Tumors of the intraoral minor salivary glands: a demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol 1988;2013:323–33 [DOI] [PubMed] [Google Scholar]
- 2.Castle JT, Thompson LDR, Frommelt RA, et al. Polymorphous low grade adenocarcinoma (PLGA): a clinicopathologic study of 164 cases. Cancer 1999;2013:207–19 [PubMed] [Google Scholar]
- 3.Evans HL, Batsakis JG. Polymorphous low grade adenocarcinoma of minor salivary glands: a study of 14 cases of a distinct neoplasm. Cancer 1984;2013:935–42 [DOI] [PubMed] [Google Scholar]
- 4.Simpson RHW, Pereira EM, Ribeiro AC, et al. Polymorphous low-grade adenocarcinoma of the salivary glands with transformation to high-grade adenocarcinoma. Histopathology 2002;2013:250–9 [DOI] [PubMed] [Google Scholar]
- 5.Beltran D, Faquin WC, Gallagher G, et al. Selective immunohistochemical comparison of polymorphous low grade adenocarcinoma and adenoid cystic carcinoma. J Oral Maxillofac Surg 2006;2013:415–23 [DOI] [PubMed] [Google Scholar]
- 6.Penner CR, Folpe AL, Budnick SD. C-kit expression distinguishes salivary gland adenoid cystic carcinoma from polymorphous low-grade adenocarcinoma. Mod Pathol 2002;2013:687–91 [DOI] [PubMed] [Google Scholar]
- 7.Perez-Ordonez B, Linkow I, Huvos AG. Polymorphous low-grade adenocarcinoma of minor salivary glands. A study of 17 cases with emphasis on cell differentiation. Histopathology 1998;2013:521–9 [DOI] [PubMed] [Google Scholar]
- 8.Katoh M, Shaw C, Xu Q, et al. An orderly retreat: dedifferentiation is a regulated process. Proc Natl Acad Sci USA 2004;2013:7005–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelkey MJ, Mills SE. Histologic transformation of polymorphous low-grade adenocarcinoma of salivary gland. Am J Clin Pathol 1999;2013:785–91 [DOI] [PubMed] [Google Scholar]
- 10.Lloreta J, Serrano S, Corominas JM, et al. Polymorphous low-grade adenocarcinoma arising in the nasal cavities with an associated undifferentiated carcinoma. Ultrastruct Pathol 1995;2013:365–70 [DOI] [PubMed] [Google Scholar]
- 11.Costa AF, Altemani A, Hermsen M. Current concepts on dedifferentiation/high-grade transformation in salivary gland tumors. Patholog Res Int 2011;2013:325965. [DOI] [PMC free article] [PubMed] [Google Scholar]




