Abstract
Heroin abuse and natural aging exert common influences on immunological cell functioning. This observation led to a recent and untested idea that aging may be accelerated in abusers of heroin. We examined this claim by testing whether heroin use is associated with premature aging at both cellular and brain system levels. A group of abstinent heroin users (n=33) and matched healthy controls (n=30) were recruited and measured on various biological indicators of aging. These measures included peripheral blood telomerase activity, which reflects cellular aging, and both structural and functional measures of brain magnetic resonance imaging. We found that heroin users were characterized by significantly low telomerase activity (0.21 vs 1.78; 88% reduction; t(61)=6.96, P<0.001; 95% confidence interval=1.12–2.02), which interacted with heroin use to affect the structural integrity of gray and white matter of the prefrontal cortex (PFC; AlphaSim corrected P<0.05), a key brain region implicated in aging. Using the PFC location identified from the structural analyses as a ‘seed' region, it was further revealed that telomerase activity interacted with heroin use to impact age-sensitive brain functional networks (AlphaSim corrected P<0.05), which correlated with behavioral performance on executive functioning, memory and attentional control (Pearson correlation, all P<0.05). To our knowledge, this study is the first to attempt a direct integration of peripheral molecular, brain system and behavioral measures in the context of substance abuse. The present finding that heroin abuse is associated with accelerated aging at both cellular and brain system levels is novel and forms a unique contribution to our knowledge in how the biological processes of drug abusers may be disrupted.
Keywords: addiction, aging, heroin, MRI, prefrontal cortex, resting state, telomerase
Introduction
Heroin abuse is crippling to both society and individual abusers. One of the many adverse effects of heroin on individuals' biopsychosocial functioning is in immunological cell functioning.1, 2 It is therefore no surprise that immunological conditions such as liver and heart diseases ranked second and third (after unintentional accidents) as the causes of heroin-related mortality,3 the prevalence of which is substantially higher than the general population.3, 4, 5, 6 Incidentally, altered immune processes are hallmarks to natural aging, as indicated by various biochemical changes as a result of aging.7 Owing to such parallels between the impact of heroin abuse and aging have on immunological processes, an intriguing hypothesis that heroin abuse accelerates aging has been suggested.8 Despite so, the present study is the first to report an attempt to explore the association between heroin abuse and biological aging, measured at both molecular and brain system levels.
Biomarkers of cellular aging include telomeres and telomerase activity,9, 10 which have referred to as ‘psychobiomarkers' of aging due to their association with stress.11 Telomeres are chromatin structures at the ends of chromosomes that shorten as cells divide because the method of replication is incomplete (‘the end replication problem'). To compensate for this telomere shortening, the enzyme telomerase is recruited to elongate telomeres in order to prevent cell senescence and apoptosis.12 Telomerase activity is therefore an important index of cellular age, confirmed by previous studies that demonstrated an association between telomerase activity and aging, aging-related diseases and mortality.9, 13 It was also reported that various mental states, including stress,14, 15 depressed mood,16 schizophrenia17 and meditation,18 could have detrimental or beneficial effects on telomerase activity. Whether substance abuse, specifically heroin abuse, impacts telomerase activity remains an unexplored question.
Another outstanding question that remains to be explored is whether heroin abuse accelerates aging at the brain system level. It is well documented that the prefrontal cortex (PFC) and medial temporal lobe (MTL) are the two brain areas most implicated in the aging process.19, 20, 21 Using magnetic resonance imaging (MRI), it has been shown that both the structural and functional integrity of the PFC are profoundly affected by the aging process.22, 23, 24 Moreover, the impact on the PFC correlates with a decline in higher cognitive abilities.25 On the other hand, hippocampal degeneration due to old age and age-related diseases (for example, dementia) is associated with marked impairment of memory functions.26 In a parallel line of studies, both PFC and MTL structure27, 28, 29, 30 and function31, 32, 33, 34 were found to show deficits in relation to heroin abuse. Abnormality in the brains of heroin abusers likely mediates deficits in various cognitive domains such as decision making,35 impulse control36, 37 and memory.38 In sum, separate studies converge to suggest that both aging and heroin abuse affect the structural and functional integrity of the PFC and the MTL, and such effects on the brain reflect cognitive impairment. However, it remains untested whether and to what extent aging and heroin abuse interact to impact similar brain systems.
To this end, the present study sought to investigate the impact that heroin abuse may have on telomerase activity and MRI brain measures, which included both structural (volumetric) and functional (resting-state connectivity) indices. Specifically, we examined group differences (heroin users vs healthy controls) in telomerase activity and tested for group-by-telomerase activity interactions in both structural and functional measures of the brain. It was hypothesized that: (1) heroin users would show a deficiency in telomerase activity as measured from peripheral blood; (2) there are interactions between telomerase activity and heroin abuse at the brain system level; and (3) such interactions would occur in brain areas implicated in aging, namely the PFC and the MTL. In addition, we sought to identify the behavioral relevance of significant functional brain regions by correlating these regions' signals with behavioral performance on a set of cognitive tasks that are known to relate to heroin abuse and/or aging.
Materials and methods
Participants
Participants included in this study were 33 abstinent heroin users and 30 healthy controls without a history of substance abuse. All were males and right-handed. Heroin users were recruited from two male drug rehabilitation centers. In order to avoid medication effects, only heroin users who were not receiving replacement treatment, such as methadone maintenance therapy, were included in this study. All drug users in this study were diagnosed with heroin abuse or dependence based on structured clinical interviews.39 As poly-drug use is the rule rather than the exception, it is difficult to recruit drug abusers whose drug of abuse was exclusively heroin. Therefore, the heroin users were defined as those who reported using heroin for over 50% of their overall drug use. The healthy controls were recruited through advertisement in the local communities. Exclusion criteria for both the heroin users and the controls were history of neurological disorder, psychiatric disorder other than substance-related disorder, contraindication for MRI scanning and history of substance use for the control group.
All participants completed the Raven's Progressive Matrices40 to ensure matching between the heroin users and the healthy controls on intelligence. The affective status of the participants was also measured, using the Hospital Anxiety and Depression Scale (HADS).41, 42 See Table 1 for comparison in demographic variables of heroin users and the controls, and also the heroin users' drug abuse records.
Table 1. Demographic and clinical details of heroin users and healthy controls.
| Heroin users (n=33) | Controls (n=30) | Statistics | ||||
|---|---|---|---|---|---|---|
| |
Mean |
s.d. |
Mean |
s.d. |
T |
P-value |
| Age (years) | 35.1 | 4.0 | 33.1 | 9.9 | −1.05 | NS |
| Body mass index | 22.1 | 2.0 | 23.3 | 3.1 | 1.75 | NS |
| Years of educationa | 8.5 | 1.8 | 11.5 | 2.9 | 5.02 | <0.001 |
| Estimated intelligence (RPM)b | 43.5 | 7.6 | 46.2 | 8.8 | 1.32 | NS |
| HADS anxiety | 14.4 | 3.5 | 5.5 | 3.3 | −10.4 | <0.001 |
| HADS depression | 14.1 | 2.5 | 4.0 | 3.0 | −14.5 | <0.001 |
| Smokers | 33/33 | 8/30 | ||||
| Cigarette per dayc | 18.5 | 4.8 | 1.6 | 3.5 | ||
| Drinkers | 24/33 | 3/30 | ||||
| Drink per dayc | 2.7 | 1.8 | 2.3 | 1.2 | ||
| Duration of previous heroin use (months) | 67.0 | 65.2 | ||||
| Duration of abstinence (months) | 12.1 | 9.8 | ||||
| Route of administration | 26 Injection | |||||
| 4 Smoke | ||||||
| 3 Oral | ||||||
Abbreviations: HADS=Hospital Anxiety and Depression Scale, NS=nonsignificant, P=associated P-value, RPM=Raven progressive matrices, t=t-statistics.
All participants were males and right-handed.
Starting from primary school and excluding latest incomplete year.
Based on raw score with maximum of 60.
Calculated as the mean from those who smoke/drink.
This study was approved by the Institutional Review Board of the University of Hong Kong and the Hospital Authority (Hong Kong West Cluster), and conducted according to the Declaration of Helsinki.
Telomerase activity assay
Peripheral blood was collected in EDTA containing tubes. Telomerase activity was measured according to the telomeric repeat amplification protocol, in line with some previous studies.43, 44 Collected blood samples were centrifuged at 3000 g at 4 °C for 10 min. Ten microliter of mononuclear layer of cells was extracted and resuspended in 200 μl of lysis buffer, which was followed by incubation on ice for 30 min. The lysate was then centrifuged at 16 000 g for 20 min and the supernatant was used for the quantification of telomerase activity using Telo TAGGG Telomerase PCR ELISA kit (Roche Molecular Biochemicals, Basal, Switzerland) according to the manufacturer's protocol. The centrifuged lysates were added into the reaction buffer to allow the addition of telomeric repeats (TTAGGG) to the biotin-labeled primers by the telomerase in the samples. Then the elongated products were amplified by PCR. After PCR, an aliquot of the PCR product was denatured and hybridized to a digoxigenin-labeled, telomeric repeat-specific detection probe. The resulting product was immobilized to a streptavidin-coated microplate. The detection probe and the hybridization conditions have been optimized for obtaining the highest specificity and sensitivity. The immobilized PCR product was then detected with an antibody against digoxigenin (anti-digoxigenin-POD) that is conjugated to peroxidase. Finally, the probe was visualized by virtue of peroxidase metabolizing tetramethylbenzidine to form a colored reaction product. Telomerase activity values were normalized to the positive control included in the ELISA kit.
Structural MRI preprocessing and analysis
MRI was performed using a 3T GE Signa Propeller HD MR scanner (GE Healthcare, Milwaukee, WI, USA) equipped with a standard whole-head coil. High-resolution whole-brain volume T1-weight structural images were acquired with the three-dimensional fast spoiled gradient-echo sequence, with the following parameters: repetition time=9.5 ms; echo time=3.9 ms; inversion time=450 ms; flip angle=20o; partial field of view factor=0.9; receiver bandwidth=±31.25 kHz; acquisition matrix=350 × 224; number of excitations=1; field of view=240 × 240 mm2; slice thickness=0.9 mm; 216 slices in sagittal plane; voxel resolution=1.07 × 0.90 × 0.69 mm3.
Acquired structural brain images were preprocessed and analyzed using the toolbox VBM8 (http://dbm.neuro.uni-jena.de/vbm/) implemented in SPM8 (FIL, London, UK). Specifically, each image was reoriented to match that of the template by manually locating the anterior commissure as the point of origin. The East Asian template was used for affine registration. Thorough cleanup was used to optimize the removal of non-brain tissues. The images were segmented into three tissue types: gray matter (GM), white matter (WM) and cerebrospinal fluid. High-dimensional DARTEL45 was used for spatial normalization, as it is an optimal approach for whole-brain alignment and particularly sensitive for examining small brain structures, including deep brain nuclei.46 Images were modulated with Jacobian determinants to allow for the testing of effects at the local level, having adjusted for individual differences in global brain size. Normalized, unsegmented images were visually inspected for gross artifacts resulting from normalization. In addition, to identify potential outliers, covariance for both normalized GM and WM segments were checked for sample homogeneity. Finally, both the GM and WM segments were smoothed with an 8-mm full-width half-maximum Gaussian kernel.
Analysis of GM and WM segments were carried out independently. To characterize group differences in the association between brain structure and telomerase activity, whole-brain interaction analyses were carried out. The factor of group was tested for its interaction with telomerase activity. The resulting contrasts showed brain regions where the association between GM/WM volume and telomerase activity was significantly different between the heroin and the control groups. For each significant brain cluster, the average GM/WM volume values were derived and included in correlation analyses to test for the relationship with telomerase activity, separately for each group. This aimed to establish the direction of each significant interaction effect.
The statistical analyses were carried out with years of education and HADS scores as covariates. The resulting analyses, therefore, revealed effects between groups that were matched in sex, age, body mass index and estimated intelligence quotient, and adjusted for education and affective status. Absolute threshold masking of <0.1 was used. The statistical analyses were thresholded at P<0.001, corrected for cluster size extent using AlphaSim. AlphaSim correction involves running a Monte Carlo simulation that determines the minimum size of clusters, given a particular threshold, required to maintain corrected experiment-wise error rate at the canonical level (P=0.05). Based on imaging parameters of this study, the results from 10 000 iterations revealed that the minimum number of voxels required in a cluster in order to prevent inflation of type 1 errors were 156 and 147, respectively, for GM and WM.
Functional MRI preprocessing and analysis
Resting state functional MRI data were acquired while subjects' eyes were closed but they remained awake for 6 min inside the same MRI machine as the structural MRI. Functional MRI images were acquired with a T2*-weighted echo-planar imaging sequence. The parameters used are as follow: repetition time=2000 ms; echo time=30 ms; flip angle=90o; acquisition matrix=64 × 64; number of excitation=1; field of view=22 cm; phase field of view=1; slice thickness=3 mm; 36 slices in axial plane; slice gap=0.6 mm; voxel size=3.44 × 3.44 × 3.60 mm3. Acquired functional brain images were preprocessed and analyzed using the toolboxes DPARSF,47 REST48 and SPM8.
Resting state functional images were preprocessed using SPM8 and DPARSF. For each participant, the first 10 volumes were discarded to avoid interference stemming from instable signals and inability of participants to adapt during the initial stage of scanning. The preserved images were first corrected for the acquisition time difference among multiple slices within an image and spatially realigned to the first volume in the whole session for participant's head motion. Functional images were co-registered to each individual's high-resolution T1 image and normalized to the Montreal Neurological Institute template in resolution of 3 × 3 × 3 mm3 by unified segmentation. Normalized images were spatially smoothed with a 6-mm full-width half-maximum Gaussian kernel. Then, using the REST toolkit, images were band-pass filtered (at 0.01–0.08 Hz) and had their linear trend removed and nuisance variables regressed. Nuisance variables included six head motion parameters and mean signals of the whole brain (that is, global trend), WM and cerebrospinal fluid. For each subject, the neural activity of the seed region was computed as the mean time series of all voxels within the region-of-interest mask. Functional connectivity between the mean time series in the seed region and all the voxels in the brain was then calculated by Pearson correlations. The correlation R values were transformed into Fisher's Z scores in each voxel of the brain.
The seed-based correlation method was employed to examine the functional connectivity during rest. The seed region was chosen based on the significant group-by-telomerase activity interaction on the GM volume in the previous structural MRI analysis (that is, the right dorsolateral prefrontal cortex (DLPFC)). The Z-images representing the standardized functional connectivity were entered into the same second-level whole-brain interaction analyses to characterize group differences in the association between functional connectivity with DLPFC and telomerase activity. The factor of group was tested for its interaction with telomerase activity. For each significant brain cluster, the average Z values were derived and included in further correlation analyses to establish the directions of these interactions. Similar to the structural MRI analyses, years of education and HADS scores were included as covariates in the functional MRI analyses. The statistical analyses were thresholded at P<0.005 and cluster extent of 26 voxels (AlphaSim corrected P<0.05).
Behavioral measures and analysis
To establish the behavioral relevance for each of the above significant functional brain cluster, the average Z values for the heroin users were correlated (Pearson correlation) with their performance on six behavioral tasks that are well established to measure the following cognitive domains: executive functioning, attentional control, sustained attention, working memory, learning and memory, and visuospatial processing.
Executive functioning was measured using a computerized, 128-card version of the Wisconsin Card Sorting Test (WCST). This task required participants to derive the implicit rules, based on feedback from previous trials, in order to correctly sort cards according to the color, shape or number of stimuli on each card. Dependent measures were the number of correct trials, percentage of perseverative response, percentage of perseverative error and percentage of nonperseverative error.49
Attentional control was measured using a computerized task based on the classic Eriksen and Eriksen flankers task.50 In this task, participants were instructed to press the corresponding keys as quickly as possible in response to the central arrow while ignoring the two distracting flanker arrows at either side of the central arrow. There were 60 trials for each of the congruent (distracters compatible with central target) and incongruent (distracters incompatible with central target) condition. Dependent measures were rate of accuracy and reaction time for each condition.
Sustained attention was measured using the computerized Conners' Continuous Performance Test II, which consisted of 120 trials in which participants were required to remain continuous vigilance in order to correctly press a button in response to targets (alphabetical letters), with the exception of non-targets, which were relatively rare. Dependent measures were number of omission errors, number of commission errors, reaction time, standard error of reaction time, d′, and β.51
Working memory was measured using a computerized, 2-back version of the n-back test.52 In this test, participants were required to manipulate and update information in working memory in order to correctly indicate whether the current stimulus (an Arabic number) was identical to the stimulus in the second preceding trial. There were 100 trials in total. Dependent measures were overall accuracy and reaction time.
Learning and memory was measured using a computerized, visuospatial version of the paired-associate learning test (PAL).53 In this test, participants were first instructed to learn the location of different visual figures shown sequentially on the computer screen and then asked to recall each of the figure's correct location. If the location of each figure was not recalled correctly, the participant was instructed to try again until the participant achieved perfect recall or until the maximum of 10 trials was reached. There were five sets of blocks: one with three figures, two with five figures and two with eight figures. Dependent measures for the PAL were total number of trials attempted (a higher number reflects poorer performance) and overall accuracy.
Visuospatial processing was measured using Benton's Judgment of Line Orientation test, H-form.54 This test assesses visuospatial ability by asking participants to decide which of the response choice lines corresponded to the two stimulus lines according to their orientations. There was 30 items on this test. The dependent measure was the total number of correct responses.
Results
Telomerase activity
An independent-groups t-test revealed that the heroin users had significantly lower telomerase activity (mean=0.21, s.d.=0.15) relative to the healthy controls (mean=1.78, s.d.=1.29; 88% reduction of telomerase activity in heroin users; t(61)=6.96, P<0.001; confidence interval=1.12–2.02).
Brain structural analyses: interaction between group and telomerase activity
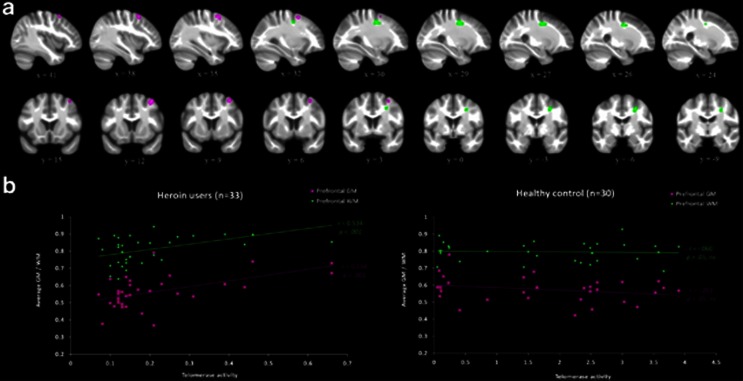
Interaction analyses revealed clusters where the association between GM/WM volume and telomerase activity significantly differed between the heroin users and the matched healthy controls (Table 2). First, in terms of the association between GM and telomerase activity, a cluster within the right middle frontal gyrus, part of the DLPFC, was more positively associated with telomerase activity for the heroin users, relative to the healthy controls (Figure 1a). Correlation analyses revealed that within the heroin group, lower telomerase activity was associated with lower amounts of DLPFC GM (r=0.53, P=0.001). For the healthy controls, telomerase activity was not associated with the amount of GM in this region (r=−0.26, P>0.05; Figure 1b). Corroboratively, a cluster of WM in a nearby brain region of right DLPFC also revealed a significant interaction between group and telomerase activity (Figure 1a). Again, for the heroin group, there was a positive correlation between the amount of WM and telomerase activity (r=0.53, P=0.001). On the other hand, for healthy controls, there was no correlation between WM in the same cluster and telomerase activity (r=−0.06, P>0.05; Figure 1b).
Table 2. Whole-brain structural analyses revealing interactions between group (heroin users and controls) and telomerase activity.
| Anatomical region | Laterality | x | y | z | Number of voxels | Peak t-value |
|---|---|---|---|---|---|---|
| Association between telomerase activity and GM | ||||||
| Controls>heroin users | ||||||
| No suprathreshold cluster | – | – | – | – | – | |
| Heroin users>controls | ||||||
| Middle frontal gyrus (DLPFC) | R | 36 | 12 | 51 | 184 | 3.93 |
| Association between telomerase activity and WM | ||||||
| Controls>heroin users | ||||||
| No suprathreshold cluster | – | – | – | – | – | |
| Heroin users>controls | ||||||
| DLPFC | R | 29 | −6 | 43 | 201 | 3.62 |
Abbreviations: DLPFC, dorsolateral prefrontal cortex; GM, grey matter; L, left; R, right; WM, white matter.
Years of education and Hospital Anxiety and Depression Scale scores were included as covariates of no interest. The statistical threshold was set at P<0.001, corrected for cluster-size extent with a minimum of 156 (GM) or 147 (WM) voxels within a cluster in order to be classified as significant (AlphaSim corrected P<0.05). Coordinates are in Montreal Neurological Institute space.
Figure 1.
Volumetric analyses revealing brain regions with significant interaction between group and telomerase activity, and the corresponding scatter plots that depict correlations within each group. (a) The dorsolateral prefrontal cortex (DLPFC) GM cluster (depicted in violet) and WM cluster (depicted in green) that showed significant interactions between group and telomerase activity are shown in sagittal (top panel) and coronal (bottom panel) slices. The left side of the brain is shown on the left. The template brain image is the bias-corrected average image from all participants. Coordinates are in MNI space. (b) Scatter plots showing significant positive correlation between telomerase activity and both clusters in the DLPFC for heroin users (left panel) and nonsignificant (ns) correlation between telomerase activity and both clusters in the DLPFC for the healthy controls (right panel). GM, gray matter; WM, white matter; r=Pearson's correlation coefficient, P=associated P-value.
Brain connectivity analyses: interaction between group and telomerase activity
Having identified the right DLPFC as the location where its structural integrity was compromised by heroin abuse and low telomerase activity, we next sought to characterize the functional connectivity of this region during resting state. An interaction analysis was used to test for brain regions where the association between telomerase activity and functional connectivity with the right DLPFC significantly differed between the heroin users and the healthy controls (Table 3).
Table 3. Whole-brain connectivity analyses revealing interactions between group (heroin users and controls) and telomerase activity.
| Anatomical region | Laterality | x | y | z | Number of voxels | Peak t-value |
|---|---|---|---|---|---|---|
| Controls>heroin users | ||||||
| Medial orbitofrontal cortex | L | −12 | 36 | −21 | 34 | 4.10 |
| Entorhinal cortex | L | −18 | 9 | −27 | 27 | 3.85 |
| Superior occipitoparietal cortex | R | 30 | -81 | 48 | 38 | 3.48 |
| 21 | −84 | 48 | 3.11 | |||
| Anterior cingulate cortex | B | 3 | 27 | 18 | 42 | 3.28 |
| 0 | 27 | 30 | 2.99 | |||
| Heroin users>controls | ||||||
| Superior temporal gyrus | R | 57 | −27 | 9 | 135 | 4.47 |
| 63 | −9 | 3 | 3.12 | |||
Abbreviations: B, bilateral; L, left; R, right.
The seed region was chosen as the right dorsolateral prefrontal cortex gray matter cluster identified from the structural brain analyses. Years of education and Hospital Anxiety and Depression Scale scores were included as covariates of no interest. The statistical threshold was set at P<0.005, with a minimum of 26 voxels within a cluster in order to be classified as significant (AlphaSim corrected P<0.05). Coordinates are in Montreal Neurological Institute space.
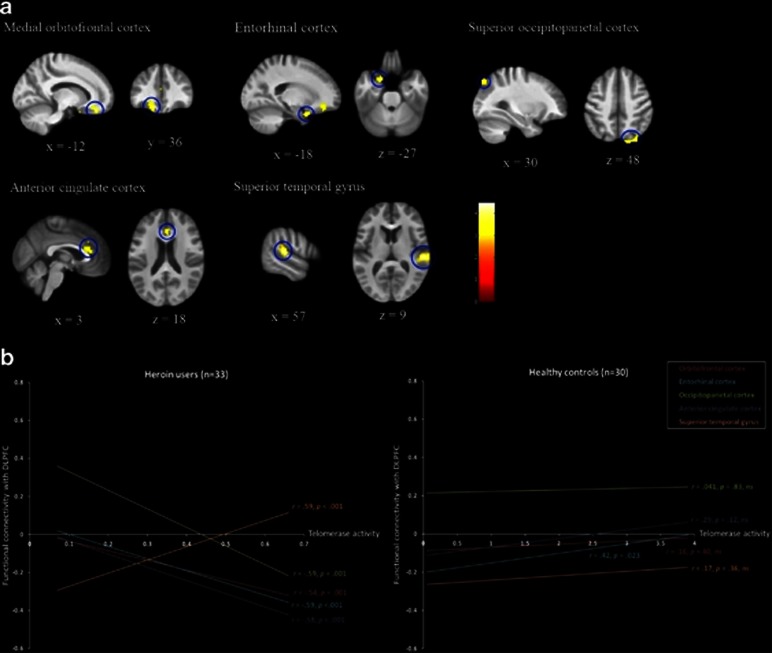
In the contrast that revealed brain regions where the slope between telomerase activity and functional connectivity with DLPFC was significantly more positive for the control group compared with the heroin group, four brain regions were identified (Figure 2). First, a region in the left medial orbitofrontal cortex (OFC) was found to be negatively correlated with telomerase activity within the heroin group (r=−0.54, P=0.001), but not correlated within the control group (r=0.16, P>0.05). Specifically, for heroin users there was a negative coupling between DLPFC and OFC and this coupling decreased in proportion to decrease in telomerase activity. Second, a region corresponding to the right entorhinal cortex (EC), part of the MTL, was found to correlate negatively with telomerase activity within the heroin group (r=−0.59, P<0.001) but positively within the control group (r=0.42, P<0.05). Similar to the OFC, for heroin users, there was a negative coupling between the DLPFC and the EC, but this coupling diminished as telomerase activity decreased. Third, the right superior occipitoparietal cortex (OP) was negatively correlated with telomerase activity within the heroin group (r=−0.59, P=0.001) but not within the control group (r=0.041, P>0.05). For heroin users, the DLPFC-OP coupling shifted from negative to positive coupling as telomerase activity decreased, whereas the coupling was positive and stable for the healthy controls. Fourth, the bilateral anterior cingulate cortex (ACC) was negatively correlated with telomerase activity within the heroin group (r=−0.58, P<0.001) but not within the control group (r=0.29, P>0.05). For heroin users, the DLPFC and ACC were negatively coupled, but this coupling diminished as telomerase activity decreased.
Figure 2.
Connectivity analyses (with the right dorsolateral prefrontal cortex (DLPFC) as the seed region) revealing brain regions with significant interaction between group and telomerase activity, and the corresponding scatter plots that depict correlations within each group. (a) The medial OFC, EC, OP, ACC and STG showed significant interactions between group and telomerase activity. The left side of the brain is shown on the left. The template brain image is the bias-corrected average image from all participants. Coordinates are in MNI space. (b) Scatter plots showing significant negative correlations between telomerase activity and OFC/EC/OP/ACC, positive correlation between telomerase activity and STG for the heroin users (left panel), positive correlation between telomerase activity and EC, and nonsignificant (ns) correlation between telomerase activity and OFC/OP/ACC/STG for the healthy controls (right panel). Individual points are not presented for reason of clarity. GM, gray matter; WM,white matter; r=Pearson's correlation coefficient, P=associated P-value.
In the reverse contrast that revealed brain regions where the association between telomerase activity and functional connectivity with DLPFC was more positive for the heroin users compared with the controls, a cluster in the right superior temporal gyrus (STG) was identified (Figure 2). The functional connectivity of this cluster with DLPFC was positively correlated with telomerase activity within the heroin group (r=0.59, P<0.001) but not correlated within the control group (r=0.17, P>0.05). For heroin users, there was a shift from positive to negative coupling between the DLPFC and the ACC as telomerase activity decreased.
Supplementary analysis: alcohol and cigarette consumption
In order to explore whether the observed interaction effects between group and telomerase activity were driven by the heroin users being heavier consumers of cigarettes and alcohol, we correlated the number of cigarette and the amount of alcohol consumed each day with the volume amount or connectivity strength of clusters identified from the above interaction analyses. Results revealed that none of the identified cluster correlated with cigarette or alcohol consumption (all P>0.05). This suggests that the group difference in the association between telomerase activity and brain structure or functional connectivity could not have been driven by the group difference in cigarette and alcohol use.
Correlation between brain signals and behavioral performance
Supplementary Table 1 presents summary statistics for each of the six behavioral tasks, which measured the following cognitive domains: executive functioning (WCST), attentional control (flankers task), sustained attention (Continuous Performance Test), working memory (n-back task), learning and memory (PAL) and visuospatial processing (Judgment of Line Orientation test). We correlated the heroin users' behavioral performance with functional brain index in order to establish the behavioral relevance for each significant brain region (Table 4). Results revealed that the DLPFC-OFC connectivity correlated with the WCST (number of correct trials: r=0.41, P<0.05). The DLPFC-EC connectivity correlated with the WCST (number of correct trials: r=0.44, P=0.01; nonperseverative errors: r=−0.43, P<0.05), n-back test (accuracy: r=0.44, P<0.05) and PAL test (trials attempted: r=−0.54, P=0.001; accuracy: r=0.46, P<0.01). The DLPFC-OP connectivity correlated with the flankers task (incongruent condition accuracy: r=−0.51, P<0.01), which also correlated with the DLPFC-ACC connectivity (congruent condition accuracy: r=0.40, P<0.05; congruent condition reaction time: r=−0.49, P=0.01; incongruent condition reaction time: r=−0.41, P<0.05). Last, the DLPFC-STG connectivity did not correlate with any behavioral tasks.
Table 4. Pearson correlation coefficients between heroin users' behavioral performance and functional connectivity of the DLPFC.
| Functional connectivity between DLPFC and | |||||
|---|---|---|---|---|---|
| |
OFC |
EC |
ACC |
OP |
STG |
| WCST | |||||
| Number of correct trials | 0.41** | 0.44** | 0.25 | 0.10 | −0.10 |
| Perseverative response (%) | −0.31 | −0.20 | −0.27 | 0.079 | 0.038 |
| Perseverative error (%) | −0.32 | −0.25 | −0.28 | 0.047 | 0.06 |
| Nonperseverative error (%) | −0.069 | −0.43* | −0.096 | −0.064 | −0.098 |
| Flankers | |||||
| Accuracy congruent (%) | −0.15 | 0.25 | 0.40* | −0.001 | −0.018 |
| Accuracy incongruent (%) | 0.077 | 0.074 | −0.046 | −0.51** | 0.038 |
| RT congruent (ms) | −0.072 | −0.22 | −0.49** | −0.069 | −0.17 |
| RT incongruent (ms) | −0.21 | −0.34 | −0.41* | −0.077 | −0.082 |
| CPT | |||||
| Omission errors | −0.048 | −0.036 | 0.22 | 0.16 | −0.21 |
| Commission errors | 0.16 | 0.17 | 0.19 | −0.031 | −0.28 |
| RT (ms) | 0.003 | 0.006 | 0.10 | −0.039 | −0.049 |
| RT standard error | 0.17 | 0.11 | 0.28 | 0.10 | −0.18 |
| d′ | −0.25 | −0.016 | −0.10 | −0.061 | 0.28 |
| β | −0.12 | −0.17 | −0.05 | 0.23 | −0.049 |
| n-Back | |||||
| Accuracy (%) | 0.11 | 0.44* | −0.16 | −0.23 | −0.022 |
| RT (ms) | 0.18 | 0.19 | −0.27 | −0.28 | 0.13 |
| PAL | |||||
| Trials attempted | −0.11 | −0.54** | −0.28 | −0.011 | 0.33 |
| Accuracy (%) | 0.083 | 0.46** | 0.12 | −0.16 | −0.11 |
| JOLO | |||||
| Correct responses | 0.092 | 0.20 | −0.12 | −0.055 | −0.022 |
Abbreviations: ACC, anterior cingulate cortex; CPT, Continuous Performance Test; DLPFC, dorsolateral prefrontal cortex; EC, entorhinal cortex; JOLO, Judgment of Line Orientation; OFC, medial orbitofrontal cortex; OP, superior occipitoparietal cortex; PAL, paired-associate learning test; RT, reaction time; STG, superior temporal gyrus; WCST, Wisconsin Card Sorting Test. *P<0.05, **P<0.01.
Discussion
This study aimed to investigate the relationship between heroin use and aging, specifically we examined whether heroin abuse is associated with a deficiency in telomerase activity, and whether such deficiency would interact with heroin use to impact brain systems and functions implicated in aging. Four main results emerged: (1) long-term heroin users had significantly lower telomerase activity, which is an index of cellular aging; (2) low telomerase activity in the heroin users was associated with compromised structural integrity of the right DLPFC; (3) low telomerase activity in the heroin users was associated with an altered pattern of functional connectivity between the right DLPFC and brain regions implicated in heroin abuse and aging; and (4) functional brain regions found to interact with heroin abuse and aging correlated with behavioral performance that are consistent with each of the brain regions' cognitive domain, namely executive functioning, memory and attentional control.
Heroin and telomerase: acceleration in cellular aging
The observation that long-term heroin abuse is associated with significantly lower telomerase activity suggests that cellular aging may be accelerated in heroin users, consistent with a previously established hypothesis.8 It is also consistent with studies that demonstrated a close-knit relationship between telomerase activity and mental states known to impact physical health. Specifically, acute and chronic stress could elevate and alleviate telomerase activity, respectively.14, 15 Studies on psychiatric conditions reported elevated telomerase activity in unmedicated patients with depression16 and alleviated telomerase activity in an animal model of schizophrenia.17 It was also reported that mentally enhancing activities, such as meditation, could elevate telomerase activity.18 Although these studies seemingly disagreed about whether a pathological state increases or decreases activity levels of telomerase, they need not be incompatible with each other. It is possible that decreased telomerase activity reflects both pathological (reduced cell protection) and beneficial (reduced need to protect) processes. Owing to heroin's deleterious impact on immunologically related biomarkers,2 it is reasonable to assume that the current observation of lowered telomerase activity in heroin users fits into the pathological model in which heroin abuse exacerbates cellular aging.
DLPFC structural alteration: aging at brain system level
If lower telomerase activity in heroin users indeed reflects accelerated aging, then heroin abuse and telomerase activity should interact to impact brain systems related to both heroin abuse and aging. Our observation that the heroin users' low telomerase activity was associated with greater DLPFC atrophy was therefore consistent with this prediction. The PFC is a key brain area implicated in the neuropathology of drug addiction. In particular, DLPFC is the likely region that mediates the link between substance abuse and impaired higher-cognitive processes.55 Previous neuroimaging studies reported both structural and functional deficits of DLPFC in heroin abusers, especially in the context of cognitively demanding tasks.37, 56 In the context of aging, the DLPFC is one of the most consistently reported brain areas to show an age-sensitive decline,20 which is related to the deterioration of various cognitive functions.25 Furthermore, our finding that the right, but not left, DLPFC is implicated in heroin-associated aging is compatible with the ‘right hemi-aging' hypothesis, which suggests that the right lateral PFC is most vulnerable to age-related decline.57 Therefore, the finding that heroin abuse and telomerase activity interacted on the right DLPFC provides us with an additional hint that heroin abuse may accelerate biological aging, and such aging may extend from the cellular to the brain system level.
Functional connectivity: the executive functioning, memory and attentional control networks
Resting state functional connectivity is a measure of the intrinsic, spontaneous functional organization of brain systems58 and is reflective of neuronal metabolic processes.59 Functional connectivity of five brain regions with the right DLPFC was found to be abnormally associated with heroin abuse and telomerase activity. In line with the observed behavioral correlates of these regions, they could be broadly divided into three networks in conjunction with the DLPFC: the executive functioning network (OFC), the memory network (EC) and the attentional control network (ACC and OP).
Interaction between the DLPFC and the OFC has been implicated in executive functions and decision-making processes.60 One key theory of decision making posits that the DLPFC mediates ‘cold' cognition, which acts as an executive control over the emotive ‘hot' cognition of the OFC.61 It has been proposed that the breakdown in the DLPFC-OFC balance underlies the irresistible urge that leads to compulsive drug taking.55 In the context of heroin abuse, we have previously presented evidence that support this neurocognitive disease model. Specifically, it was found that heroin abusers were characterized by deficits in impulse control,62, 63 which forms a vital component of executive functioning.64 A recent study provides additional support for the DLPFC-OFC imbalance proposal by showing reduced resting-state functional connectivity between these brain regions in heroin abusers.65 The present finding indicates that for the heroin users, there was a negative coupling between the DLPFC and the OFC (which correlated with the performance on executive functioning), but such coupling diminished as telomerase activity decreased. This suggests that a greater acceleration of the cellular aging process in the heroin users was associated with more severe atrophy in the DLPFC-OFC functional connectivity. This interpretation is consistent with both the prefrontal theories of drug addiction55 and the aging literature, which has demonstrated that aging can have a detrimental impact on OFC functions, including reward-based decision-making66 and learning reversal.67
The EC, part of the MTL, was also negatively coupled with the DLPFC in the heroin users, and this negative coupling also diminished as telomerase activity decreased. The DLPFC-EC coupling correlated with performance on executive functioning, suggesting that the connection between these regions form part of the executive functioning network. The DLPFC-EC coupling also correlated with two behavioral tasks related to memory: the n-back task (working memory) and the PAL task (learning and memory). This finding supports our view that the abnormal DLPFC-EC functional connectivity in heroin users also mediates a memory network. There seems to be an intricate relationship between memory and addiction processes at both cellular and brain system levels.38, 68, 69 In relation to prefrontal functioning, it has been suggested that the failure in prefrontal control mechanisms prevents successful suppression of drug-related memory, which triggers addiction processes such as relapse.38 The present observation that EC, but not the hippocampus proper, is related to heroin abuse and aging is an interesting finding. Despite prominent atrophy to the hippocampus as a function of increasing age, the EC is relatively resistant to damage from healthy aging.70 Importantly, the EC seems particularly implicated in age-related diseases, such as dementia. It has been shown that the extent of EC atrophy is predictive of future progression from the healthy to disease state.71, 72, 73 The present EC finding therefore suggests that low telomerase activity in heroin users is also involved in triggering mechanisms that prelude age-related diseases, rather than plain acceleration of healthy aging.
Heroin abuse and low telomerase activity was also related to the functional connectivity between the DLPFC and three other brain regions, namely, the superior OP, the ACC and the STG. Two of these brain regions, the OP and the ACC, are part of the several regions implicated in attention and cognitive control processes.74 The observation that both the DLPFC-OP and the DLPFC-ACC couplings correlated with the performance on the flanker task supported our view that these regions mediate an attentional control network. The attentional control system in heroin abusers (and addiction in general) is known to characterize a bias such that an abnormally large attentional focus is put on drug-related stimuli.75 A recent functional connectivity study on cannabis abuse reported converging evidence of an abnormal connectivity pattern between the PFC and the OP in relation to attentional processes.76 Likewise, deficit in attentional control and related neural circuitry is characteristic of aging.77, 78 It is therefore not a surprise that the attentional system was affected by both heroin abuse and low telomerase activity. Last, the DLPFC-STG functional connectivity was not found to relate to any of the behavioral measures included in this study. An explanation for this observation is that the DLPFC-STG coupling denotes a function that was not part of our primary interest (for instance, auditory processing, which is a known key function of the STG).
Limitations and future directions
The present study consists of several limitations that must be taken into consideration. First, the correlative nature of this study prevents an inference on cause and effect. Although the present findings suggest that heroin abuse may accelerate biological aging, prospective studies are needed to establish the causative mechanisms that mediate heroin abuse and biological aging. Second, telomerase activity was measured via peripheral blood rather than directly from the brain where system level measures were derived and examined with telomerase. Despite the existence of telomerase in neural progenitor cells of the brain,79 to measure it in vivo is not yet feasible. For this reason, we used peripheral telomerase activity as a solution for measuring cellular aging. Moreover, future studies should adopt a multi-aging molecular biomarker approach to further elucidate the relationship between heroin abuse and aging. Third, only male participants were recruited as female abusers of heroin are less prevalent to their male counterparts. Finally, it is important to test whether the present findings are specific to heroin use or a general pattern observable in people who abuse other substances. Previously documented effects of heroin on age-related immunological biomarkers led us to investigate heroin addiction in the present study. However, a recent study provides important evidence that cocaine abuse could also accelerate aging at the brain system level.80
Conclusion
Based on a novel integration of peripheral molecular and brain system measures, the present study presents evidence that the long-term heroin abuse is associated with an acceleration of both cellular and brain system aging. Specifically, heroin users were characterized by significantly lower telomerase activity, which interacted with heroin use to impact age-related brain systems and functions. These findings constitutes a significant contribution to our understanding of how heroin abuse influences the brain and body, and lays an important foundation for studies that seek to further characterize the mechanisms that mediate substance abuse and biological aging. Understanding such mechanisms raises the possibility of reversing the detrimental effects of drug addiction, and ultimately enriches past abusers with a greater potential to endeavor in life.
Acknowledgments
This work was supported by the May Endowed Professorship of the University of Hong Kong, the Research Grant Council General Research Fund (Ref: HKU747612H), research fund from KKHo International Charitable Foundation and the Fundamental Research Funds for the Central Universities Grant 21609101. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ. Opioids, opioid receptors, and the immune response. Drug Alcohol Depend. 2001;62:111–123. doi: 10.1016/s0376-8716(00)00181-2. [DOI] [PubMed] [Google Scholar]
- Reece AS. Evidence of accelerated ageing in clinical drug addiction from immune, hepatic and metabolic biomarkers. Immun Ageing. 2007;4:6. doi: 10.1186/1742-4933-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth B, Hoffman V, Fan J, Hser YI. Years of potential life lost among heroin addicts 33 years after treatment. Prev Med. 2007;44:369–374. doi: 10.1016/j.ypmed.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugal MT, Domingo-Salvany A, Puig R, Barrio G, Garcia de Olalla P, de la Fuente L. Evaluating the impact of methadone maintenance programmes on mortality due to overdose and aids in a cohort of heroin users in Spain. Addiction. 2005;100:981–989. doi: 10.1111/j.1360-0443.2005.01089.x. [DOI] [PubMed] [Google Scholar]
- Oppenheimer E, Tobutt C, Taylor C, Andrew T. Death and survival in a cohort of heroin addicts from London clinics: a 22-year follow-up study. Addiction. 1994;89:1299–1308. doi: 10.1111/j.1360-0443.1994.tb03309.x. [DOI] [PubMed] [Google Scholar]
- Gronbladh L, Ohlund LS, Gunne LM. Mortality in heroin addiction: impact of methadone treatment. Acta Psychiatr Scand. 1990;82:223–227. doi: 10.1111/j.1600-0447.1990.tb03057.x. [DOI] [PubMed] [Google Scholar]
- Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211:144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece AS. Chronic immune stimulation as a contributing cause of chronic disease in opiate addiction including multi-system ageing. Med Hypotheses. 2010;75:613–619. doi: 10.1016/j.mehy.2010.07.047. [DOI] [PubMed] [Google Scholar]
- Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Epel ES. Telomeres in a life-span perspective: a new ‘Psychobiomarker'. Curr Dir Psychol Sci. 2009;18:6–10. [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Dhabhar FS, Wolkowitz OM, Puterman E, Karan L, et al. Dynamics of telomerase activity in response to acute psychological stress. Brain Behav Immun. 2010;24:531–539. doi: 10.1016/j.bbi.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Reus VI, Rosser R, et al. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Mol Psychiatry. 2012;17:164–172. doi: 10.1038/mp.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SA, Melnik A, Kempermann G. Physical exercise increases adult neurogenesis and telomerase activity, and improves behavioral deficits in a mouse model of schizophrenia. Brain Behav Immun. 2011;25:971–980. doi: 10.1016/j.bbi.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Jacobs TL, Epel ES, Lin J, Blackburn EH, Wolkowitz OM, Bridwell DA, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36:664–681. doi: 10.1016/j.psyneuen.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R.Neuroimaging of healthy cognitive agingIn: Craik FIM, Salthouse TA (eds)The Handbook of Aging and Cognition3rd edn.Psychology Press: New York; 20081–54. [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13:572–586. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Eyler LT, Sherzai A, Kaup AR, Jeste DV. A review of functional brain imaging correlates of successful cognitive aging. Biol Psychiatry. 2011;70:115–122. doi: 10.1016/j.biopsych.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Long-term potentiation and the ageing brain. Philos Trans R Soc Lond B Biol Sci. 2003;358:765–772. doi: 10.1098/rstb.2002.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Hao Y, Kaneko Y, Ouyang X, Zhang Y, Xu L, et al. Frontal and cingulate gray matter volume reduction in heroin dependence: optimized voxel-based morphometry. Psychiatry Clin Neurosci. 2009;63:563–568. doi: 10.1111/j.1440-1819.2009.01989.x. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Pollack MH, Silveri MM, Ahn KH, Diaz CI, Hwang J, et al. Prefrontal and temporal gray matter density decreases in opiate dependence. Psychopharmacology (Berl) 2006;184:139–144. doi: 10.1007/s00213-005-0198-x. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zhu Z, Shi J, Zou Z, Yuan F, Liu Y, et al. Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain Cogn. 2009;71:223–228. doi: 10.1016/j.bandc.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Yuan K, Qin W, Dong M, Liu J, Sun J, Liu P, et al. Gray matter deficits and resting-state abnormalities in abstinent heroin-dependent individuals. Neurosci Lett. 2010;482:101–105. doi: 10.1016/j.neulet.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Britten S, et al. Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. Am J Psychiatry. 2001;158:1680–1686. doi: 10.1176/appi.ajp.158.10.1680. [DOI] [PubMed] [Google Scholar]
- Li Q, Wang Y, Zhang Y, Li W, Yang W, Zhu J, et al. Craving correlates with mesolimbic responses to heroin-related cues in short-term abstinence from heroin: an event-related fMRI study. Brain Res. 2012;1469:63–72. doi: 10.1016/j.brainres.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Lee T, Zhang JX, Wu Q, Wu R, Weng X, et al. Thirsty heroin addicts show different fMRI activations when exposed to water-related and drug-related cues. Drug Alcohol Depend. 2006;83:157–162. doi: 10.1016/j.drugalcdep.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Zijlstra F, Veltman DJ, Booij J, van den Brink W, Franken IH. Neurobiological substrates of cue-elicited craving and anhedonia in recently abstinent opioid-dependent males. Drug Alcohol Depend. 2009;99:183–192. doi: 10.1016/j.drugalcdep.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Schultz W. Potential vulnerabilities of neuronal reward, risk, and decision mechanisms to addictive drugs. Neuron. 2011;69:603–617. doi: 10.1016/j.neuron.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends Cogn Sci. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Lee TM, Zhou WH, Luo XJ, Yuen KS, Ruan XZ, Weng XC. Neural activity associated with cognitive regulation in heroin users: a fMRI study. Neurosci Lett. 2005;382:211–216. doi: 10.1016/j.neulet.2005.03.053. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Society Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR4th edn.American Psychiatric Association Press: Washington, DC; 2000 [Google Scholar]
- Raven JC. Standard Progressive Matrices Plus Version and Mill Hill Vocabulary Scale. Pearson Assessment: London; 2008. [Google Scholar]
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- Vasa M, Breitschopf K, Zeiher AM, Dimmeler S. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ Res. 2000;87:540–542. doi: 10.1161/01.res.87.7.540. [DOI] [PubMed] [Google Scholar]
- Yang H, Ou CC, Feldman RI, Nicosia SV, Kruk PA, Cheng JQ. Aurora-A kinase regulates telomerase activity through c-Myc in human ovarian and breast epithelial cells. Cancer Res. 2004;64:463–467. doi: 10.1158/0008-5472.can-03-2907. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. Neuroimage. 2009;44:319–327. doi: 10.1016/j.neuroimage.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Yan CC, Zang YF. DPARSF: A MATLAB Toolbox for ‘Pipeline' data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton KH. Wisconsin Card Sorting Test: Computer Version 4 Research Edition. PAR Psychological Assessment Resources, Inc: North Florida; 2003. [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- Conners CK. Conners' Continuous Performance Test II V.5. Mutli-Health Systems Inc.: Toronto; 2004. [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Map. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1995;33:1–24. doi: 10.1016/0028-3932(94)00098-a. [DOI] [PubMed] [Google Scholar]
- Benton AL, Sivan AB, Hamsher KS, Varney NR, Spreen O. Contributions to Neuropsychological Assessment. Oxford University Press: New York; 1994. [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Lewis SJ, Clark L, Stocks-Gee G, London M, et al. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology (Berl) 2005;180:612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, D'Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Lee TM, Pau CW. Impulse control differences between abstinent heroin users and matched controls. Brain Inj. 2002;16:885–889. doi: 10.1080/02699050210128915. [DOI] [PubMed] [Google Scholar]
- Pau CW, Lee TM, Chan SF. The impact of heroin on frontal executive functions. Arch Clin Neuropsychol. 2002;17:663–670. [PubMed] [Google Scholar]
- Kim S, Lee D. Prefrontal cortex and impulsive decision making. Biol Psychiatry. 2011;69:1140–1146. doi: 10.1016/j.biopsych.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, et al. Addiction related alteration in resting-state brain connectivity. Neuroimage. 2010;49:738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denburg NL, Cole CA, Hernandez M, Yamada TH, Tranel D, Bechara A, et al. The orbitofrontal cortex, real-world decision making, and normal aging. Ann N Y Acad Sci. 2007;1121:480–498. doi: 10.1196/annals.1401.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar M, Resnick SM. Aging and prefrontal functions: dissociating orbitofrontal and dorsolateral abilities. Neurobiol Aging. 2004;25:553–558. doi: 10.1016/j.neurobiolaging.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem. 2002;78:637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62:433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiol Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hanninen T, Laakso MP, et al. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging. 2004;25:303–310. doi: 10.1016/S0197-4580(03)00084-8. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Harding IH, Solowij N, Harrison BJ, Takagi M, Lorenzetti V, Lubman DI, et al. Functional connectivity in brain networks underlying cognitive control in chronic cannabis users. Neuropsychopharmacology. 2012;37:1923–1933. doi: 10.1038/npp.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Prefrontal deficits in attention and inhibitory control with aging. Cereb Cortex. 1997;7:63–69. doi: 10.1093/cercor/7.1.63. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND.Aging and functional brain networks Mol Psychiatry 201217471549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso GL, Lim DA, Alvarez-Buylla A, Chao MV. Telomerase activity in the subventricular zone of adult mice. Mol Cell Neurosci. 2003;23:693–702. doi: 10.1016/s1044-7431(03)00103-9. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Robbins TW, Bullmore ET. Cocaine dependence: a fast-track for brain ageing. Mol Psychiatry. 2013;18:134–135. doi: 10.1038/mp.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.