Abstract
Although rarely reported in the literature, adenomatous polyp and adenocarcinoma can occur as a late complication in an interposed colonic segment. We describe a case of a late stage adenocarcinoma in a colonic interposition performed for benign oesophageal stricture.
Background
Colon interposition, although often well tolerated, can be associated with restenosis, polyps or rarely adenocarcinoma.1 Very few cases of adenocarcinoma of the interposed colonic segment have been reported in the literature. Early detection and treatment can improve morbidity and mortality, especially if patients are young with good life expectancy. Here, we discuss the presentation, diagnostic modalities and treatment options for carcinoma of the interposed colonic segment.
Case presentation
A 60-year-old Hispanic man presented to the emergency department with increasing episodes of dysphagia for 3 weeks. Although he had intermittent episodes of dysphagia in the past 2 years, this gradually worsened over the past 3 weeks, more so to solid foods than liquids. This was associated with minimal epigastric pain, weakness, progressive weight loss of about 12 kg over 2 months, intermittent black stool, light headedness and poor appetite. He denied nausea, vomiting and haematemesis. He had 30 pack-year smoking history and moderate alcohol consumption in the past. His medical history was significant for severe oesophageal stricture secondary to caustic lye ingestion for which he underwent right colonic interposition (approximately 4 cm) in the stricture segment 30 years ago. The specific details of possible colonoscopy before he underwent the interposition could not be obtained as this was done in a different medical centre. After the procedure was performed 30 years previously, he was lost to follow-up for 15 years. His last endoscopic dilation for anastomotic stenosis was 15 years ago. He denied the history of inflammatory bowel disease and colorectal cancer in the past. He denied a family history of colon cancer. Physical examination was remarkable for pale conjunctiva without icterus. Digital rectal examination revealed black tarry, haemoccult-positive stool.
Investigations
Laboratory investigation revealed significant anaemia with haemoglobin of 5.9 g/dl, Haematocrit 20.5, mean corpuscular volume 67.4 fl, iron 14 μg/dl, transferrin 329 mg/dl and ferritin 3 ng/ml. Vitamin B12, folic acid levels and liver function tests were all within normal limits. Although his history suggested that dysphagia was progressive over the past 2 years, the exact degree could not be ascertained as he had not had any endoscopic evaluation for the past 15 years.
Differential diagnosis
Oesophageal carcinoma, gastric carcinoma, benign oesophageal lesions (polyp, oesophagitis) and strictures were considered as the possible differentials. Although rare, the possibility of colonic polyp and carcinoma of the interposed segment were also taken into consideration.
Treatment
To rule out these, an oesophagogastroduodenoscopy was performed which revealed a 4×3 cm ulcerated mass in his colonic oesophagus (figure 1). Histopathological examination of the biopsy specimen was consistent with well-differentiated invasive adenocarcinoma of the colon (figure 2A,B). A CT scan of the abdomen showed multiple liver masses concerning for metastases which was further delineated with an MRI scan. Barium studies were also obtained which revealed a thickened fold and mass in the antipyloric region. Carcinoembryonic antigen level and chest x-ray were within normal limits. He received blood transfusion and iron therapy. Since his metastatic lesion was not surgically resectable, he was put on chemotherapy (5-fluorouracil). However, he had progression of his disease and he subsequently developed gastric outlet obstruction. Palliative percutaneous endoscopic gastrostomy (PEG) tube was placed for decompression and a jejunal tube was placed for the feeding. Ultimately, the patient chose palliative care.
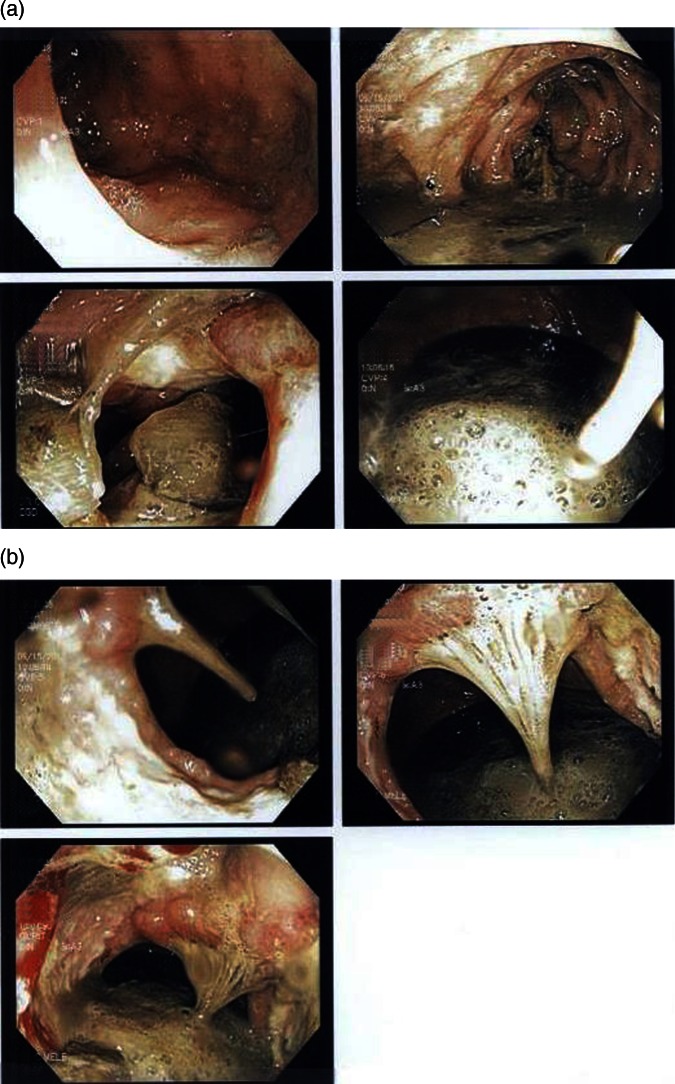
Figure 1.
(A and B) Images obtained via upper gastrointestinal endoscopy showing abnormal appearing malignant mass (about 4 cm) at 60 cm from the incisors.
Figure 2.

(A and B) Histological sections obtained from colonic interposition segment of oesophagus showing well-differentiated invasive adenocarcinoma of colonic origin.
Discussion
Colonic interposition for oesophageal reconstruction has been performed for almost 100 years. It is generally performed for not only malignant disease of the oesophagus but also for benign oesophageal disease, such as lye-induced strictures or oesophageal injury from trauma if gastric pull-up procedures are not technically feasible. It has been shown to lower postoperative morbidity and mortality as well as improve long-term quality of life.2 3 Perioperative mortality for colonic interposition for benign strictures is 5–8% with a high morbidity associated with it.4 Since the occurrence of pre-existing polyp and carcinoma is a possibility, patients being considered for this procedure need to undergo colonoscopy before surgery. Also, diverticulosis with inflammatory fibrosis, extensive polyposis or malignancy need to be ruled out which are contraindications for the interposition.5 6 The detection of colorectal neoplasm in screening population depends on various factors including the size of the adenoma. Heresbach et al reported an overall miss rate of 23.4% in the colonoscopic detection of neoplasia including both adenomas and colorectal carcinomas. Therefore, upper endoscopic screening within 1 year of colonic interposition and periodic surveillance has been recommended as the lesions may be detected early and removed safely.7 8 Anastomotic dehiscence followed by fistula, fibrosis and stricture (22%) are relatively common complications after the oesophageal resection and interposition of large bowel6 with the most common postoperative complaint being dysphagia.3 9 As in our case, patients with corrosive-induced oesophageal stricture have a 1000-fold increased risk of developing carcinoma of oesophagus.10
Adenocarcinoma in the colonic graft is a rare late complication with only a few cases reported in the literature. For this reason, the late development of dysphagia in a patient with a colonic interposition segment should always be taken seriously and thoroughly investigated. Early detection is likely to give the best chance of cure with a complete surgical resection.11 Risk factors for malignant change in the interposition includes a history of colonic polyps, colitis and a positive family history of colonic carcinoma.12 Altorjay et al13 noticed that muscle contraction of the interposed colon was segmental without spreading waves on manometry. This takes food contents in grafted colon longer transition time than normal oesophagus. Also, the interposed segment is vulnerable to the reflux contents from stomach due to the lack of sphincter. This leads to a prolonged duration of exposure to noxious agents and carcinogens14 which help in promoting dysplastic changes in the interposition segment and augment precancerous conditions.15 Lindahl et al16 reported that the interposed colonic mucosa tends to undergo frequent premalignant transformation such as fundic-type gastric metaplasia of the interposed colon and colonic dysplasia and increased risk of Barrett's oesophagus. Early and regular screening of the interposed colonic graft to detect and treat malignancy may be necessary as malignancy arising from interposed colon can be asymptomatic, but associated with high mortality, and reoperation is often difficult.17 18 It is particularly important where colonic interposition has been performed for benign disease when the subject may be young and the life expectancy long as in our case.12 18 If a polyp is found in either the remaining colon or the interposition segment, surveillance of the both the remaining colon and interposition segment should occur at the same surveillance interval.9
No guidelines are currently available for the management of cancer occurring in the transposed bowel. If the tumour is operable, surgery is a possibility and endoscopic resection is recommended for early cancers limited to the mucosa.6 7 Other procedures commonly performed include gastric pull-up, jejunal graft, Roux-en-Y oesophagojejunostomy or a permanent salivary fistula with feeding jejunostomy if inoperable.11 19–21 Resection becomes technically more difficult when the tumour has already infiltrated the submucosa and impossible when it has penetrated the muscle layer. Endoscopic ultrasonography in such situations would be helpful in assessing the depth of tumour infiltration.22 23 However, if the patient is in poor health and still recovering from the previous surgery or the presence of metastasis, palliative procedures to relieve dysphagia can be considered in an effort to improve nutritional status.6
Our case emphasises the need for continued screening and surveillance for colorectal cancer where ever colonic tissue is found. Although there are no specific guidelines as to when this should be done, we propose that it should be done at least every 5 years. The onset of cancer on the interposed segment in our case might have been preventable if he was not lost to follow-up. It is particularly important when patients develop symptoms such as dysphagia as this may herald serious pathology. This is especially true for patients who have normal life expectancy and undergo interposition surgery for benign pathologies like caustic ingestion or Barrett’s oesophagus.
Learning points.
Although rarely reported in the literature, adenomatous polyp and adenocarcinoma can occur as a late complication in an interposed colonic segment.
Regular screening with endoscopy of the interposed segment is important for the early detection of polyp and adenocarcinoma.
Early detection and treatment can improve morbidity and mortality especially if patients are young with good life expectancy.
Footnotes
Contributors: MRA and LJ wrote the initial manuscript and obtained the histopathological slide. NRM took the patient consent and revised the initial manuscript. MRA and JFA revised the final manuscript and prepared the caption.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Shersher DD, Hong E, Warren W, et al. Adenocarcinoma in a 40-year-old colonic interposition treated with Ivor Lewis esophagectomy and esophagogastric anastomosis. Ann Thorac Surg 2011;2013:e113–14 [DOI] [PubMed] [Google Scholar]
- 2.Radovanović N, Simić A, Kotarac M, et al. Colon interposition for pharyngoesophageal postcorrosive strictures. Hepatogastroenterology 2009;2013:139–43 [PubMed] [Google Scholar]
- 3.Briel JW, Tamhankar AP, Hagen JA, et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg 2004;2013:536–41; discussion 541–542 [DOI] [PubMed] [Google Scholar]
- 4.Keenan DJ, Hamilton JR, Gibbons J, et al. Surgery for benign esophageal stricture. J Thorac Cardiovasc Surg 1984;2013:182–8 [PubMed] [Google Scholar]
- 5.Watson TJ, Peters JH, DeMeester TR. Esophageal replacement for end-stage benign esophageal disease. Surg Clin North Am 1997;2013:1099–113 [DOI] [PubMed] [Google Scholar]
- 6.Fritscher-Ravens A, Sriram PV, Thonke F, et al. Synchronous adenocarcinoma in the transposed colonic conduit after esophagectomy for squamous cell cancer: endoscopic palliative resection while awaiting surgery. Gastrointest Endosc 1999;2013:852–4 [DOI] [PubMed] [Google Scholar]
- 7.Bando H, Ikematsu H, Fu K-I, et al. A laterally-spreading tumor in a colonic interposition treated by endoscopic submucosal dissection. World J Gastroenterol 2010;2013:392–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heresbach D, Barrioz T, Lapalus MG, et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy 2008;2013:284–90 [DOI] [PubMed] [Google Scholar]
- 9.Altomare JF, Komar MJ. A tubular adenoma arising in a colonic interposition. J Clin Gastroenterol 2006;2013:765–6 [DOI] [PubMed] [Google Scholar]
- 10.Kochhar R, Sethy PK, Kochhar S, et al. Corrosive induced carcinoma of esophagus: report of three patients and review of literature. J Gastroenterol Hepatol 2006;2013:777–80 [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Koay CB, Thompson H, et al. Adenocarcinoma arising in an oesophageal colonic interposition graft. J Laryngol Otol 1994;2013:80–3 [DOI] [PubMed] [Google Scholar]
- 12.Houghton AD, Jourdan M, McColl I. Dukes A carcinoma after colonic interposition for oesophageal stricture. Gut 1989;2013:880–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altorjay A, Kiss J, Vörös A, et al. Malignant tumor developed in colon-esophagus. Hepatogastroenterology 1995;2013:797–9 [PubMed] [Google Scholar]
- 14.Bucknell A, Clark C. An experimental method for recording the behaviour of human isolated colonic segments. Gut 1967;2013:569–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuwabara Y, Kimura M, Mitsui A, et al. Adenocarcinoma arising in a colonic interposition following a total gastrectomy: report of a case. Surg Today, 2009;2013:800–2 [DOI] [PubMed] [Google Scholar]
- 16.Lindahl H, Rintala R, Sariola H, et al. Long-term endoscopic and flow cytometric follow-up of colon interposition. J Pediatr Surg 1992;2013:859–61 [DOI] [PubMed] [Google Scholar]
- 17.Licata AA, Fecanin P, Glowitz R. Metastatic adenocarcinoma from oesophageal colonic interposition. Lancet 1978;2013:285. [DOI] [PubMed] [Google Scholar]
- 18.Hwang HJ, Song KH, Youn YH, et al. A case of more abundant and dysplastic adenomas in the interposed colon than in the native colon. Yonsei Med J 2007;2013:1075–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roos D, Busch ORC, Van Lanschot JJB. Primary colon carcinoma in a colon interposition graft after oesophageal resection. Ned Tijdschr Geneeskd 2007;2013:2111–14 [PubMed] [Google Scholar]
- 20.Goldsmith HS, Beattie EJ., Jr Malignant villous tumor in a colon bypass. Ann Surg 1968;2013:98–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martín MA, Ferrás A. Colon cancer: a rare complication in a colonic esophageal segment after coloesophagoplasty. Cir Esp 2005;2013:46–7 [DOI] [PubMed] [Google Scholar]
- 22.Soehendra N, Binmoeller KF, Bohnacker S, et al. Endoscopic snare mucosectomy in the esophagus without any additional equipment: a simple technique for resection of flat early cancer. Endoscopy 1997;2013:380–3 [DOI] [PubMed] [Google Scholar]
- 23.Akahoshi K, Chijiiwa Y, Hamada S, et al. Endoscopic ultrasonography: a promising method for assessing the prospects of endoscopic mucosal resection in early gastric cancer. Endoscopy 1997;2013:614–19 [DOI] [PubMed] [Google Scholar]