Abstract
Multiple solitary plasmacytoma (MSP) is a rare plasma cell dyscrasia, characterised by multiple lesions of neoplastic monoclonal plasma cells. It differs from multiple myeloma by the lack of hypercalcaemia, renal insufficiency, anaemia and pathological monoclonal plasmocytosis on a random bone biopsy. We present the case of an MSP described for the first time in a patient on peritoneal dialysis. There are only few cases of MSP described in literature, and we performed a review of these cases trying to systematise the topic. The increasing clinical use of CT, MRI and positron emission tomography will enhance in the future the correct diagnosis of MSP.
Background
The International Myeloma Working Group in 20031 classified plasmacytomas as solitary plasmacytoma of bone (SBP) when a single bone lesion was present, solitary extramedullary plasmacytoma (SEP) when a solitary soft-tissue lesion was present and multiple solitary plasmacytoma (MSP) when multiple sites of disease were present in soft tissue, bone or both. SBP, SEP and MSP are rare clinical entities, characterised by a monoclonal plasma cell infiltrate in bone or soft tissue, cytologycally and immunophenotypically identical to multiple myeloma (MM). However they are distinct from the last by the lack of hypercalcaemia, renal failure, anaemia, pathological monoclonal plasmocytosis on a random (usually of the iliac crest) bone biopsy, bone lytic changes (except for the primary solitary lesion) and serum or urinary monoclonal protein.
The incidence of these entities is 0.35/100 000/year, representing 5–10% of all plasma cell neoplasms.2–6 The importance of diagnosis lies with the potential of these disorders to progress to MM. Indeed, more than half of subjects diagnosed with solitary plasmacytomas (52–80% of patients with SBP and 10–44% of patients with SEP) would develop MM later in life.7 8
We report the case of a patient with plasmacytoma involving several bones and extended to the surrounding soft tissues.
Case presentation
A 57-year-old Caucasian man was diagnosed with chronic renal failure in 2002 in the course of diagnostic workup for arterial hypertension. Owing to chronic kidney disease progression to end stage renal disease (ESRD), in July 2011 the patient started peritoneal dialysis. Two months later a soft tissue, relatively rapid growing, swelling was noticed in the median-left paramedian region of the back at the level of the first thoracic vertebra.
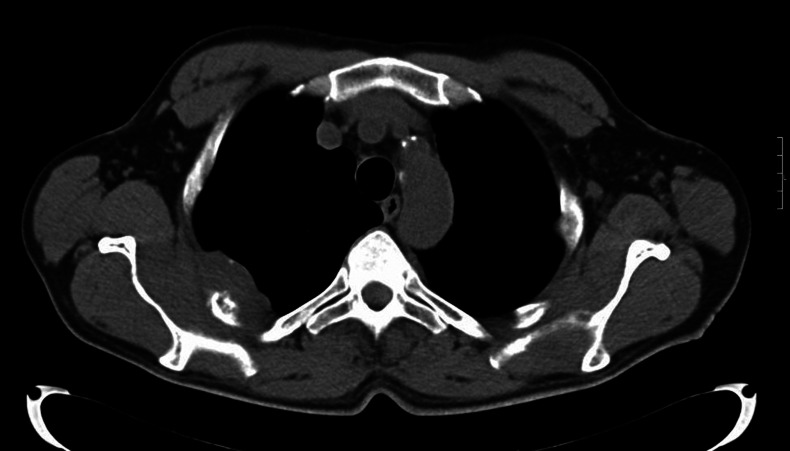
The high-resolution CT scan detected a solid plaque-like lesion (6×4 cm) infiltrating both the ribs and the pleura (figure 1). The lesion was in close relationship on the right with the posterior arches of the III, IV and X ribs and on the left with the lateral arch of the II rib, that showed lytic changes. No pathological findings were reported with regard to the lungs and mediastinal lymph nodes.
Figure 1.
Chest CT scan: solid tissue infiltrating both the ribs and the pleura.
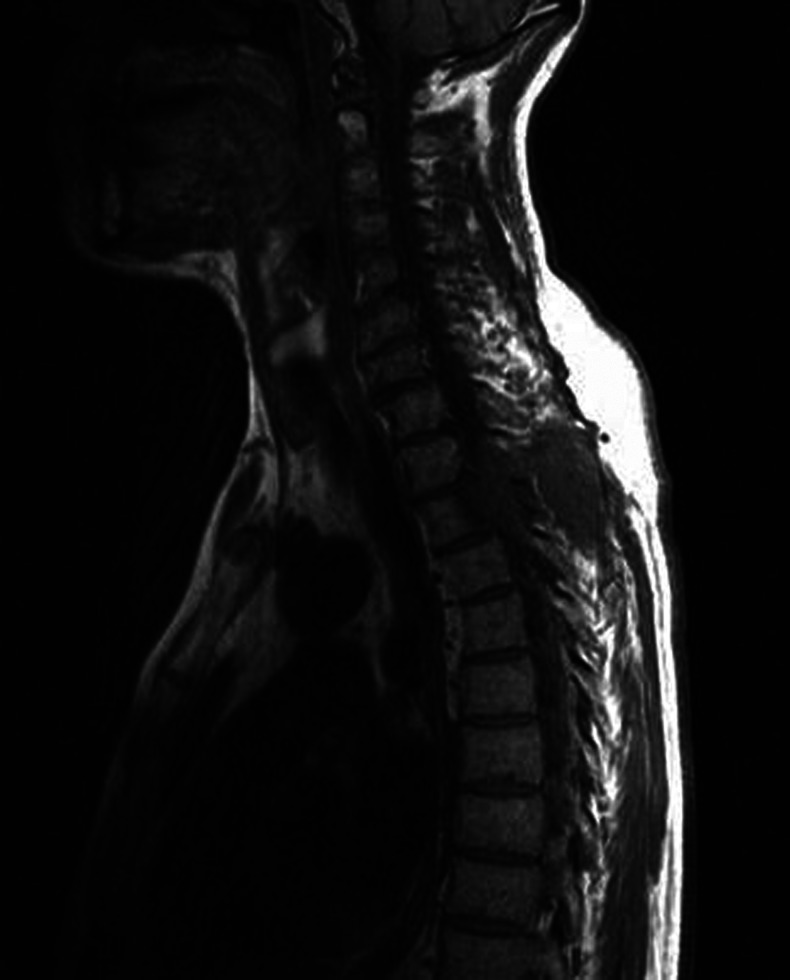
In November 2011 the patient was admitted to our hospital because of progressive ataxia. A spine MRI confirmed the previous findings but also showed a mass lesion substituting most of the vertebral body of D2–D3 (figure 2). The lesion spread into the spinal canal displacing the dural sac and extended to the surrounding soft tissues of the left paravertebral region. It showed enhancing on a subsequent contrast CT scan of the thoracic spine.
Figure 2.
Cervical and thoracic spine MRI scan: mass lesion substituting most of the vertebral body of D2–D3.
The surgical biopsy of the solid mass showed a monomorphic, CD38 positive, plasma cell population monotypic for λ-chains, consisting of medium-sized cells with a large ‘plasmacytoid’ cytoplasm. They were positive for CD38, CD45 (lightly), CD10 (lightly) and λ-light chains, negative for CD20, CD3, CD30, CD79a, CD56, anaplastic lymphoma kinase-1 (ALK1), cytokeratins AE1 and AE3. Diagnosis of plasmacytoma was made.
The bone marrow biopsy from iliac crest showed a normocellular bone marrow with only minimal interstitial plasmacytosis (<10% of all nucleated cells). On biochemical tests serum calcium was in the low-normal range and serum and urine immunofixation were negative for monoclonal (M) proteins.
Treatment
The patient was treated with dexamethasone intravenously, which was associated with a significant improvement of ataxia. The consultant neurosurgeon excluded any indication to surgery, and radiotherapy (total dose 45 Gy) on D3 was started. It was followed by 3 monthly cycles of chemotherapy with bortezomib and melphalan.
Outcome and follow-up
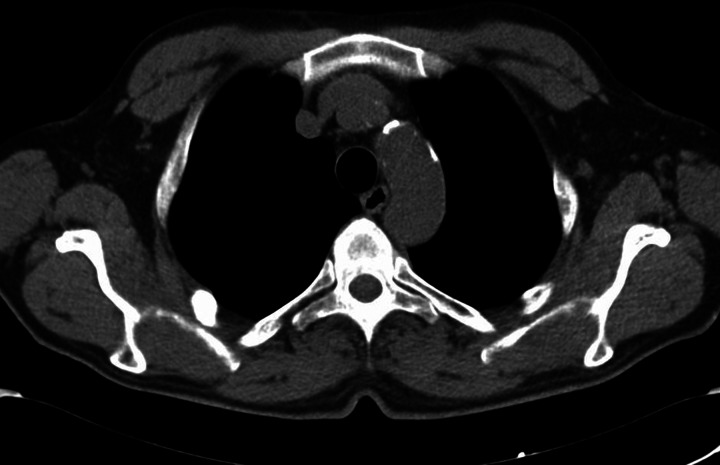
One year later, positron emission tomography (PET) (July 2012) and CT (October 2012—figure 3) documented total regression of mass lesion. No monoclonal proteins were found in serum and urine (December 2012). The patient was on the waiting list for renal transplantation and since diagnosis of MSP he has been ‘temporary’ removed. Now he is apparently free of the disease. As nowdays it is not clear what is the long-term prognosis of MSP and the possible interference of immunosuppressive therapy, the transplant centre is continuing to keep the patient out of the waiting list for transplantation. It will be interesting to follow the patient in the future to assess whether a complete healing has truly occurred.
Figure 3.
Chest CT scan after surgery, radiotherapy and chemotherapy: no evidence of mass lesions in vertebral body (D3), ribs and pleura.
Discussion
MSP is defined as a monoclonal plasma cell infiltrate in one or more lytic bone lesions (often spreading to the surrounding soft tissues) associated with no evidence of plasma cell proliferation on random (usually of iliac crest) bone marrow biopsy and without systemic abnormalities typical of MM (eg, hypercalcaemia, anaemia, renal failure, serum and/or urinary monoclonal proteins or monoclonal light chains). In our patient laboratory investigations and biopsy of the solid mass made it possible to exclude asymptomatic MM,9 non-secretory MM10 and non-Hodgkin lymphoma with plasma cell differentiation.11
Apart from renal insufficiency and anaemia, which clearly antedated the plasma cell disorder, the clinical and histological picture in our patient perfectly adapted to the diagnosis of MSP.
Unlike the majority of the cases described, where only one bone lesion was detected, in our case two vertebral bodies (D2–D3) and three ribs were involved. The neoplasia appeared as a multilobulated mass obliterating the foramen, displacing the dural sac and extending to the surrounding soft tissues (chest wall and pleura). The lesion also involved the rib arches at sites far from each other and not in continuity with the vertebral localisations, so enforcing the evidence of a ‘multifocal’ plasmacytoma.
Our patient was diagnosed with MSP while on chronic peritoneal dialysis. ESRD is characterised by several immunological changes and the uraemic state has been associated with an increased risk of malignancies,12 including MM,13–15 but a higher incidence of plasmacytomas has never been reported.
Up to now only few cases of MSP have been reported in literature.16–25 Kulbacki et al17 presented a case of MSP in a young patient with lytic lesions in C1 vertebrae, first left rib and hip. The biopsy of the lesion was positive for CD38, CD138, CD56 and κ-light chain but was negative for CD19, CD20, cyclin D1 and λ-light chain. Collier et al18 reported a case of an adult man with MSP who presented with raised intracranial pressure. Small lytic lesions were seen on radiographs of the skull, sacrum and left clavicle. Ooi et al20 describe six patients with MSP, four cases with multiple extramedullary plasmacytomas (MEMP) and two cases with both bone and extramedullary lesions. Shih et al23 collected 32 patients with SPB or SEP and they reported different kind of relapse after therapy: local single recurrence of SPB or SEP, multiple bone plasmacytomas, MEMP or MM. Katzmann et al reported 899 patients with plasma cell dyscrasia and monoclonal gammopathies; among these patients, there were 27 with SBP or SEP and 3 with MSP.
In our case, the initial constitutional symptoms (fatigue, weakness), only subsequently followed by more specific symptoms owing to tumour extension (soft tissue swelling, pain, ataxia) and the prevalent vertebral (rather than other skeletal sites) involvement were very similar to other cases of MSP previously described. Moreover, likewise many patients with MSP, our patient did not have serum paraprotein, urinary Bence-Jones protein or immunoglobulin subtype alterations detected at diagnosis.
An association between MM and a decrease in natural killer (NK) cell number and activity is well-known in literature. Also, the degree of NK impairment was correlated with the clinical severity of the disease and the response to therapy.26–29 Recently, Jurisic et al reported a patient affected from aggressive MEMP with a significant reduction in NK number and activity and a decrease in interleukin 2 production in cultured cells.27 In our case the analysis of lymphocyte phenotype showed depressed levels of NK too; however, no problems could be related to a decreased NK activity and in the next year the patient responded well to therapy.
The increasing use of CT, MRI and PET in the evaluation and staging of apparently solitary plasmacytomas will eventually reveal multiple soft tissue lesions or additional bone lesions20 30 31 and will enhance in the future the correct diagnosis of MSP.
Because of its rarity and heterogeneous presentation, no clear guidelines are available for MSP treatment. Most of the information available regarding the best treatments are found in single or multicentre retrospective series. Chemotherapy, radiotherapy and surgery have been tried with variable results. While for single-lesion cases of plasmacytoma (SBP and SEP) surgical excision followed by radiation therapy appears a reasonable approach, for multiple-lesions disease (MSP) surgery, radiotherapy and chemotherapy are indicated.
Learning points.
Multiple solitary plasmacytoma (MSP) is a rare plasma cell dyscrasia and it is reported in the classification of plasmacytomas (The International Myeloma Working Group, 2003). Up to now only few cases of MSP have been reported in literature but the increasing use of CT, MRI and positron emission tomography in the evaluation and staging of apparently solitary plasmacytomas will eventually reveal multiple soft tissue lesions or additional bone lesions and enhance in the future the diagnosis of MSP.
The differential diagnosis among multiple myeloma (MM), non-secretory MM, extramedullary plasmacytoma, solitary plasmacytoma and MSP is very important because these diseases may have different clinical course and prognosis.
Our case report appears also significative for the implications related to an eventual immunosuppressive treatment. The patient was on the waiting list for renal transplantation and since diagnosis of MSP he has been ‘temporary’ removed. He underwent radiotherapy and chemotherapy and now he is apparently free of the disease. As nowdays it is not clear what is the long-term prognosis of MSP and the possible interference of immunosuppressive therapy, the transplant centres is continuing to keep the patient out of the waiting list for transplantation. It will be interesting to follow the patient in the future to assess whether a complete healing has truly occurred.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.The International Myeloma Working Group Criteria for the classification monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003;2013:749–57 [PubMed] [Google Scholar]
- 2.Kilciksiz S, Karakoyun-Celik O, Agaoglu FY, et al. A review for solitary plasmacytoma of bone and extramedullary plasmacytoma. Scic World J 2012;2013:895765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.British Committee for Standards in Haematology Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Br J Haemato 2004;2013:717–26 [DOI] [PubMed] [Google Scholar]
- 4.Jawad MU, Scully SP. Skeletal plasmacytoma: progression of disease and impact of local treatment; an analysis of SEER database. J Hematol Oncol 2009;2013:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes M, Soutar R, Lucraft H, et al. Guidelines on the diagnosis and management of solitary plasmacytoma of bone, extramedullary plasmacytoma and multiple solitary plasmacytomas: 2009 Update, British Committee for Standards in Haematology, London, UK, 2009
- 6.Katzmann JA, Abraham RS, Dispenzieri A, et al. Diagnostic performance of quantitative kappa and lambda free light chain assays in clinical practice. Clin Chem 2005;2013:878–81 [DOI] [PubMed] [Google Scholar]
- 7.Dagan R, Morris CG, Kirwan J, et al. Solitary plasmacytoma. Am J Clin Oncol 2009;2013:612–17 [DOI] [PubMed] [Google Scholar]
- 8.Wiltshaw E. The natural history of extramedullary plasmacytoma and its relation to solitary myeloma of bone and myelomatosis. Mrdicine 1976;2013:217–38 [DOI] [PubMed] [Google Scholar]
- 9.Dimopoulos MA, Moulopoulos LA, Maniatis A, et al. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood 2000;2013:2037–44 [PubMed] [Google Scholar]
- 10.Chen LP, Sun TH, Hsieh PP, et al. Diagnostic pitfalls of nonsecretory myeloma manifesting as multiple foci of periosseous plasmacytomas. JCMA 2011;2013:464–8. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe N, Morijiri M, Shimizu M, et al. A case of retroperitoneal extramedullary plasmacytoma with multiple metastases. Clin Imaging 2000;2013:365–7 [DOI] [PubMed] [Google Scholar]
- 12.Maisonneuve P, Agodoa L, Gellert R, et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet 1999;2013:93–9 [DOI] [PubMed] [Google Scholar]
- 13.Shebl FM, Warren JL, Eggers PW, et al. Cancer risk among elderly persons with end-stage renal disease: a population-based case-control study. BMC Nephrol 2012;2013:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holley JL. Screening, diagnosis, and treatment of cancer in long-term dialysis patients. Clin J Am Soc Nephrol 2007;2013:604–10 [DOI] [PubMed] [Google Scholar]
- 15.Lee JE, Han SH, Cho BC, et al. Cancer in patients on chronic dialysis in Korea. J Korean Med Sci 2009;2013(Suppl):S95–S101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vassou A, Alymara V, Agelis E, et al. Multifocal extramedullary plasmacytoma with paraproteinemia, pleural involvement and retroperitoneal lymphadenopathy: an uncommon pattern of relapse of solitary osseous plasmacytoma. Ann Hematol 2006;2013:335–6 [DOI] [PubMed] [Google Scholar]
- 17.Kulbacki E, Wang E. Pathological bone fractures in a 20-year-old athletic male with multifocal solitary plasmacytoma of bone. Am J Hematol 2012;2013:626–7 [DOI] [PubMed] [Google Scholar]
- 18.Collier A, Ashworth B. Multiple plasmacytoma presenting as raised intracranial pressure. J Neurol Neurosurg Psychiatry 1987;2013:495–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carson GP, Ackerman LV, Maltby JD. Plasma cell myeloma. A clinical, pathologic and roentgenologic review of 90 cases. Am J Clin Pathol 1955;2013:849–88 [DOI] [PubMed] [Google Scholar]
- 20.Ooi GC, Chim JC, Au WY, et al. Radiologic manifestations of primary solitary extramedullary and multiple solitary plasmacytomas. AJR Am J Roentgenol 2006;2013:821–7 [DOI] [PubMed] [Google Scholar]
- 21.Kujat C, Reiche W, Koch B, et al. Rare intracranial plasmacytoma manifestations. Case reports and review of the literature in diffuse plasmocytoma, in primary solitary extramedullary plasmacytoma and in primary solitary osseous plasmacytoma. Radiologe 1996;2013:914–20 [DOI] [PubMed] [Google Scholar]
- 22.Antonijević N, Radosević-Radojković N, Colović M, et al. Simultaneous occurrence of localized plasmacytoma in hands and feet bones. Srp Arh Celok Lek 1994;2013:294–6 [PubMed] [Google Scholar]
- 23.Shih LY, Dunn P, Leung WM, et al. Localised plasmacytomas in Taiwan: comparison between extramedullary plasmacytoma and solitary plasmacytoma of bone. Br J Cancer 1995;2013:128–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bataille R, Sany J. Solitary myeloma: clinical and prognostic features of a review of 114 cases. Cancer 1981;2013:845–51 [DOI] [PubMed] [Google Scholar]
- 25.Knowling MA, Harwood AR, Bergsagel DE. Comparison of extramedullary plasmacytomas with solitary and multiple plasma cell tumors of bone. J Clin Oncol 1983;2013:255–62 [DOI] [PubMed] [Google Scholar]
- 26.Jurisic V, Srdic T, Markovic O, et al. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med Oncol 2007;2013:312–17 [DOI] [PubMed] [Google Scholar]
- 27.Jurisic V, Colovic N, Konjevic G, et al. An aggressive extramedullary cutaneous plasmacytoma associated with extreme alterations in the innate immune system. Onkologie 2010;2013:113–15 [DOI] [PubMed] [Google Scholar]
- 28.Godfrey J, Benson DM., Jr The role of natural killer cells in immunity against multiple myeloma. Leuk Lymphoma 2012;2013:1666–76 [DOI] [PubMed] [Google Scholar]
- 29.Costello RT, Boehrer A, Sanchez C, et al. Differential expression of natural killer cell activating receptors in blood vs bone marrow in patients with monoclonal gammopathy. Immunology 2013. doi:10.1111/imm.12082 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moulopoulos LA, Dimopoulos MA, Weber D, et al. Magnetic resonance imaging in the staging of solitary plasmacytoma of bone. J Clin Oncol 1993;2013:1311–15 [DOI] [PubMed] [Google Scholar]
- 31.Schirrmeister H, Buck AK, Bergmann L, et al. Positron emission tomography (PET) for staging of solitary plasmacytoma. Cancer Biother Radiopharm 2003;2013:841–5 [DOI] [PubMed] [Google Scholar]