Abstract
Intracranial haemorrhage is a known complication after fibrinolytic therapy and occurs usually in the first 24 h. We report a 35-year-old woman who presented with severe central chest pain and she was diagnosed as anterior ST elevation myocardial infarction. She was given fibrinolytic therapy with Tenecteplase. She responded well to the treatment with a decrease in the intensity of chest pain and resolution of the ST segment elevation. She was taken for coronary angiogram the next day, which revealed an occlusion of the left anterior descending (LAD) artery, and stenting of LAD was carried out. Four days later, she developed severe headache, confusion, slurring of speech and right haemiparesis. CT brain revealed intracerebral haemorrhage and she was referred to an neurosurgeon who advised for conservative management. Her condition gradually improved with physiotherapy and was discharged home with no marked functional impairment.
Background
We present this case since, first of all, acute coronary syndrome is relatively uncommon in very young women, and second the complication of intracerebral haemorrhage after fibrinolytic therapy in ST elevation myocardial infarction (MI) is rare after 4 days of treatment.
Case presentation
A 35-year-old Sri Lankan lady presented to the emergency department with severe central chest pain, radiating to both arms of 4 h duration associated with nausea and vomiting. There was no history of similar episodes and of exertional angina or dyspnoea. She is a non-smoker, non-alcoholic and not on any regular medications including oral contraceptive pills. She has a family history of premature coronary artery disease. Her father developed MI at the age of 40 and died at the age of 45.
On examination, blood pressure was 130/95 mm hg, pulse 84/min, regular.
Rest of the clinical examination was unremarkable.
Twelve lead ECG showed sinus rhythm with ST elevation in anterior leads fromV1 to V6 (Ofigure 1).
Figure 1.
ECG showing ST elevation in V1–V6 on presenting to the emergency department with chest pain.
She was diagnosed as acute coronary syndrome (anterior ST elevation MI) and was given fibrinolytic therapy with tenecteplase. The chest pain subsided and there was a good resolution of the ST elevation (Ofigure 2). After 30 h, she was taken for coronary angiogram which showed 95% stenosis of mid-LAD artery. The other arteries were normal (Ofigure 3). Angioplasty of the LAD artery was performed with a drug-eluting stent (Ofigure 4).
Figure 2.
ECG showing the resolution of the ST segment elevation 90 min after receiving fibrinolytic therapy.
Figure 3.
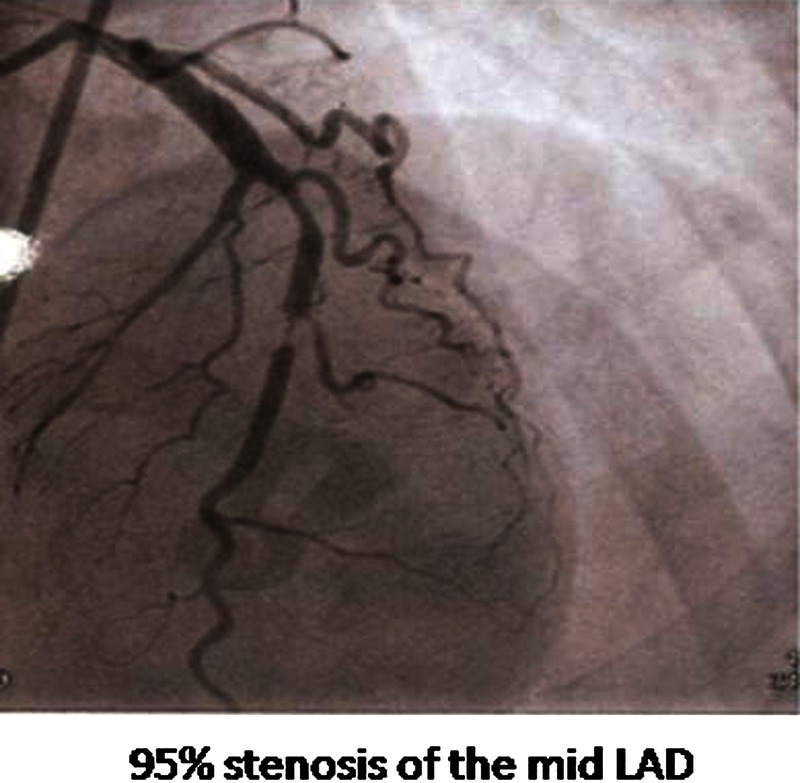
Coronary angiogram showing stenosis of the mid left anterior descending artery.
Figure 4.
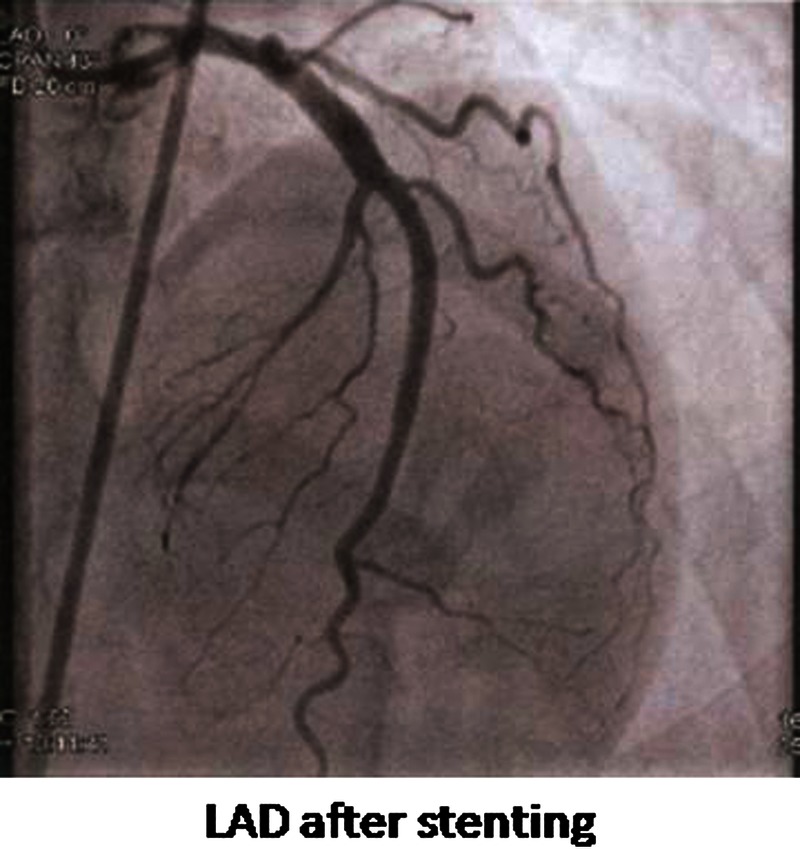
Coronary angiogram after stenting of the left anterior descending artery.
She remained stable for the next 4 days and was planned for discharge when she developed headache, slurring of speech and confusion. A CT of the brain performed showed left frontoparietal haemorrhage 5×4.2×3.3 cm in diameter along with moderate perifocal oedema with compression of ipsilateral ventricle causing midline shift to the right side (Ofigure 5). Her Glasgow Coma Scale was 14/15, pupils 3 mm, equal and reactive to light, right facial nerve palsy and left-sided haemiparesis (power 4/5 in the left upper and lower limbs). She was referred to the neurosurgeon who advised conservative management and she was started on Mannitol injection to reduce cerebral oedema. Aspirin was discontinued, but she was continued on Clopidogrel. However, her condition did not improve and the weakness increased. A CT of the brain was repeated 72 h later which showed a slight increase in the haematoma and the neurosurgeons decided to continue with the conservative management. A CT cerebral angiogram showed no evidence of aneurysmal dilation or arteriovenous malformation.
Figure 5.

A CT of the brain on the day she developed neurological symptoms and signs showing left frontoparietal haemorrhage 5×4.2×3.3 cm in diameter along with moderate perifocal oedema with compression of ipsilateral ventricle causing midline shift to the right side.
Investigations
Echocardiography showed normal-sized cardiac chambers, moderate left ventricular systolic dysfunction with ejection fraction of 33%, mild mitral regurgitation and right ventricular systolic pressure 34 mm hg. There was a thin rim of pericardial effusion.
Lipid profile showed total cholesterol 281 mg/dl, low-density lipoprotein 205 mg/dl, high-density lipoprotein 49 mg/dl, triglycerides 189 mg/dl, glycated haemoglobin 6.8%. Peak creatine kinase, and troponin were 1476 U/l, 182 U/l and 3.61 ng/ml, respectively.
Differential diagnosis
The haemiparesis in a patient after a MI could also be due to an ischaemic stroke secondary to showering of a left ventricular thrombus. A CT scan of the brain helps to differentiate this from a haemorrhagical cause.
Outcome and follow-up
During the course of the hospital stay, she developed pneumonia and lung collapse and required intubation and ventilator support. With the help of intravenous antibiotics and chest physiotherapy, her condition improved and she was extubated a week later. She received regular physiotherapy for her limbs and was able to walk without support at discharge.
At 3 months postdischarge, she was doing well. She has returned to her previous normal activities without any significant functional impairment.
Discussion
Cardiovascular diseases are one of the most common causes of morbidity and mortality in women in the developed countries.1 2 Although coronary artery disease primarily occurs in postmenopausal women and those with multiple risk factors, younger women can also be affected.1 2 In the Framingham Heart Study, the incidence of MI over a 10-year period was 5.2/1000 in women 35–44 years old. The incidence of MI was 8–9 times greater in women aged 55–64 years.3
Young patients with MI usually have multiple risk factors for coronary heart disease. Younger patients more often have a family history of premature coronary heart disease.4 The association between family history and premature coronary heart disease can be due to both genetic and environmental factors.5 Hypertriglyceridaemia is the most common lipid abnormality in young patients with MI. Young patients have lower mean serum HDL concentrations (35 vs 43 mg/dl) and higher serum triglycerides (239 vs 186 mg/dl) when compared to older patients. It may be associated with glucose intolerance and a predominance of small atherogenic LDL particles, both of which predispose to atherosclerosis.6
Two other important coronary risk factors, diabetes mellitus and hypertension, appear to be less common in young patients with coronary heart disease than in older patients.7 However, young patients frequently have subtle problems with glucose metabolism, and impaired glucose tolerance in the absence of overt diabetes is considered a risk factor for coronary disease.8
The intracranial haemorrhage which developed in our patient was 4 days after the fibrinolytic therapy. The fibrinolytic agent she received was tenecteplase which was given according to her body weight. The thrombolytic agents used in ST elevation MI are alteplase, tenecteplase, reteplase and streptokinase.9 Alteplase had better outcomes than streptokinase in the GUSTO-1 trial.10 A review of 38 trials involving therapy with reteplase and tenecteplase found that the efficacy and safety were similar to alteplase and these agents were more convenient to use because of bolus administration.11 Tenecteplase (TNK-tPA) is the easiest fibrinolytic to use (it can be given as a single bolus), and in ASSENT-2 trial it was associated with a significantly lower rate of non-cerebral bleeding complications and the need for transfusions compared to reteplase. However, there was no much difference in the rate of intracerebral haemorrhage when compared to alteplase or reteplase.12 13 Tenecteplase is the fibrinolytic agent of choice in many hospitals.14
Intracranial haemorrhage represents the most serious complication of fibrinolytic therapy and may be fatal in half to two-third of patients.15 The incidence of intracranial haemorrhage is highest in the first 24 h and patients should be very closely observed for any signs and symptoms of intracranial haemorrhage.16
The risk of intracranial haemorrhage with the newer drugs, particularly tenecteplase, is less than 1%.17 The predictors of intracranial haemorrhage after thrombolytic are old age, lower bodyweight (weight less than 65 kg for women or 80 kg for men), female gender, prior cerebrovascular disease and uncontrolled hypertension. Moderate or severe bleeding is 1.9-fold higher in women compared to men. Bleeding risk remained higher even after the adjustment of baseline differences.18 But, the 1-year mortality is lower in women compared to men with intracranial bleeding and the reason for this is unclear.19
In patients who have received fibrinolytic therapy, catheterisation with intent to revascularise should be done ideally within 24 h. In a meta-analysis that included seven randomised controlled trials of early transfer for catheterisation, a strategy of routine early catheterisation (within 24 h) after fibrinolysis was associated with a statistically significant reduction in the incidence of death or MI at 30 days and at 1 year, without an increase in the risk of major bleeding.20
However, catheterisation should not be performed within the first 2–3 h after the administration of fibrinolytic therapy as there is an increased risk of bleeding in patients who are taken for angioplasty.21
Intracranial haemorrhage should be suspected in any patient who develops sudden neurological deterioration, a decline in the level of consciousness, new headache, nausea and vomiting or a sudden rise in BP after fibrinolytic therapy. Although the risk is highest in the first 24 h, any of the symptoms that occur even beyond 24 h should alert the physician to consider intracranial haemorrhage to be the cause and brain imaging with a CT brain should be done to rule this out.
Learning points.
Although myocardial infarction commonly affects men and postmenopausal women, a high degree of suspicion is needed in anyone presenting with chest pain and treatment be provided without delay.
The most serious complication of fibrinolytic therapy is intracranial haemorrhage and it usually occurs within 24 h. However, any neurological symptoms or signs that develop even after 24 h should alert the physician a high possibility of intracranial haemorrhage, and brain imaging should be carried out to rule this out.
Women have a higher incidence of intracranial haemorrhage after thrombolytic therapy, but have lower mortality than men.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Eaker ED, Chesebro JH, Sacks FM, et al. Cardiovascular disease in women. Circulation 1993;2013:1999. [DOI] [PubMed] [Google Scholar]
- 2.Merz CN Bairey, Shaw LJ, Reis SE, et al. Insights from the NHLBI-sponsored Women's Ischemia Syndrome Evaluation (WISE) Study, part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol 2006;2013:S21–9 [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Abbott RD. Incidence and prognosis of unrecognized myocardial infarction. An update on the Framingham study. N Engl J Med 1984;2013:1144. [DOI] [PubMed] [Google Scholar]
- 4.Cipriani V, Mannucci PM, Ardissino D, et al. Familial aggregation of early-onset myocardial infarction. Eur Jl Inter Med 2010;2013:511–5 [DOI] [PubMed] [Google Scholar]
- 5.Michos ED, Vasamreddy CR, Becker DM, et al. Women with a low Framingham risk score and a family history of premature coronary heart disease have a high prevalence of subclinical coronary atherosclerosis. Am Heart J 2005;2013:1276. [DOI] [PubMed] [Google Scholar]
- 6.Topol EJ, McCarthy J, Gabriel S, et al. Single nucleotide polymorphisms in multiple novel thrombospondin genes may be associated with familial premature myocardial infarction. Circulation 2001;2013:2641. [DOI] [PubMed] [Google Scholar]
- 7.Malmberg K, Båvenholm P, Hamsten A. Clinical and biochemical factors associated with prognosis after myocardial infarction at a young age. J Am Coll Cardiol 1994;2013:592. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman FH, Cameron A, Fisher LD, et al. Myocardial infarction in young adults: angiographic characterization, risk factors and prognosis (Coronary Artery Surgery Study Registry). J Am Coll Cardiol 1995;2013:654. [DOI] [PubMed] [Google Scholar]
- 9.Hilleman DE, Tsikouris JP, Seals AA, et al. Fibrinolytic agents for the management of ST-segment elevation myocardial infarction. Pharmacotherapy 2007;2013:1558–70 [DOI] [PubMed] [Google Scholar]
- 10.Hudson MP, Granger CB, Topol EJ. Experience from the global utilization of streptokinase and tissue plasminogen activator (alteplase) for occluded coronary arteries(GUSTO I) and global use of strategies to open occluded coronary arteries (GUSTO III)trials. ACC Curr J Rev 2002;2013:12. [DOI] [PubMed] [Google Scholar]
- 11.Llevadot J, Giugliano RP, Antman EM. Bolus fibrinolytic therapy in acute myocardial infarction. JAMA 2001;2013:442. [DOI] [PubMed] [Google Scholar]
- 12.Al-Zakwani I, Ali A, Zubaid M, et al. Impact of type of thrombolytic agent on in-hospital outcomes in ST-segment elevation myocardial infarction patients in the Middle East. J Thrombolysis 2012;2013:280–6 [DOI] [PubMed] [Google Scholar]
- 13.Panduranga P, Al-Zakwani I, Sulaiman K, et al. Clinical profile and mortality of ST-segment elevation myocardial infarction patients receiving thrombolytic therapy in the Middle East. Heart Views 2012;2013:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunadian V, Gibson CM. Thrombolytics and myocardial infarction. Cardiovasc Ther 2012;2013:e81–8 [DOI] [PubMed] [Google Scholar]
- 15.Gurwitz JH, Gore JM, Goldberg RJ, et al. Risk for intracranial hemorrhage after tissue plasminogen activator treatment for acute myocardial infarction. Participants in the National Registry of Myocardial Infarction 2. Ann Intern Med 1998;2013:597. [DOI] [PubMed] [Google Scholar]
- 16.The Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)-3 Investigators Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT-3 randomised trial in acute myocardial infarction Original Research Article. Lancet 2001;2013:605–11 [DOI] [PubMed] [Google Scholar]
- 17.Assessment of the safety and efficacy of a New Thrombolytic Investigators Single bolus tenecteplase compared with front loaded alteplase in acute myocardial infarction: the ASSENT- 2 double blind randomized trial. Lancet 1999;2013:716–22 [DOI] [PubMed] [Google Scholar]
- 18.Berkowitz SD, Granger CB, Pieper KS, et al. Incidence and predictors of bleeding after contemporary thrombolytic therapy for myocardial infarction. The Global Utilization of Streptokinase and Tissue plasminogen activator for Occluded coronary arteries (GUSTO) I Investigators. Circulation 1997;2013:2508. [DOI] [PubMed] [Google Scholar]
- 19.Mehta RH, Stebbins AS. Comparison of incidence of bleeding and mortality of men versus women with ST elevation myocardial infarction treated with fibrinolysis. Am J Cardiol 2012;2013:320–6 [DOI] [PubMed] [Google Scholar]
- 20.Borgia F, Goodman SG, Halvorsen S, et al. Early routine percutaneous coronary intervention after fibrinolysis vs. standard therapy in ST segment elevation myocardial infarction: a meta-analysis. Eur Heart J 2010;2013:2156–69 [DOI] [PubMed] [Google Scholar]
- 21.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guidelines for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol 2013;2013:e78–e140 [DOI] [PubMed] [Google Scholar]




