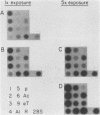
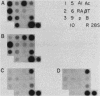
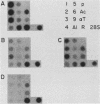
Abstract
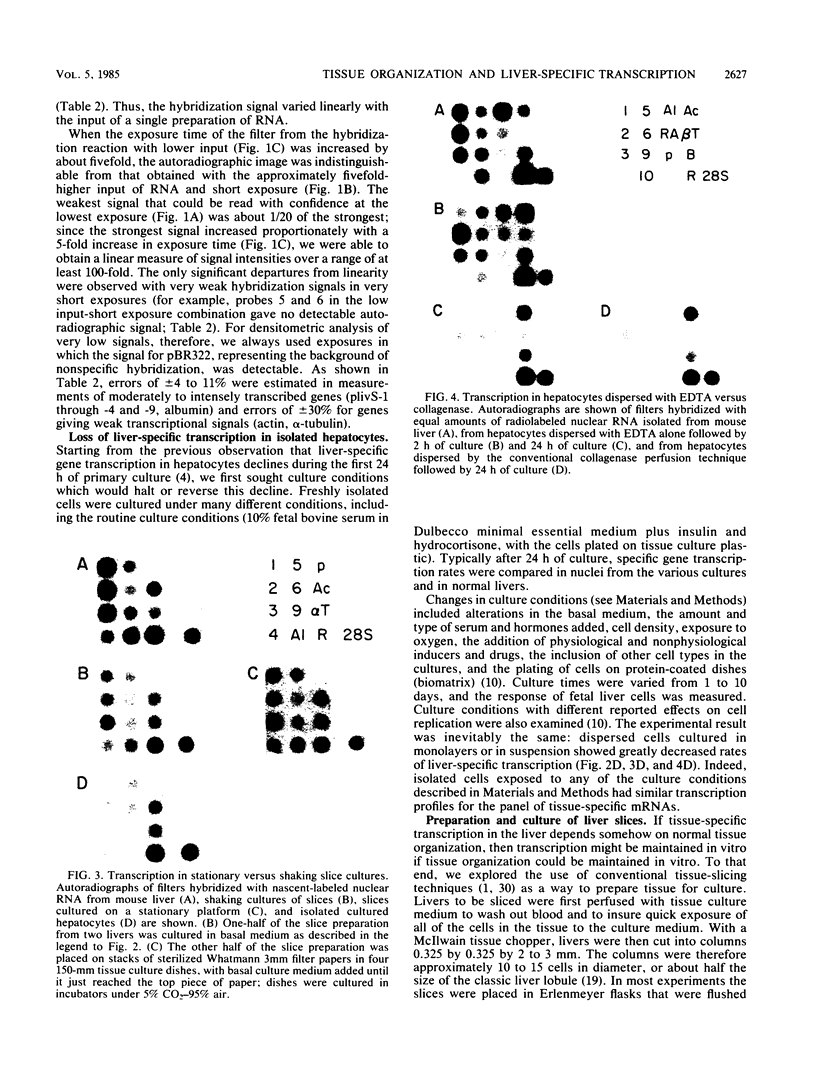
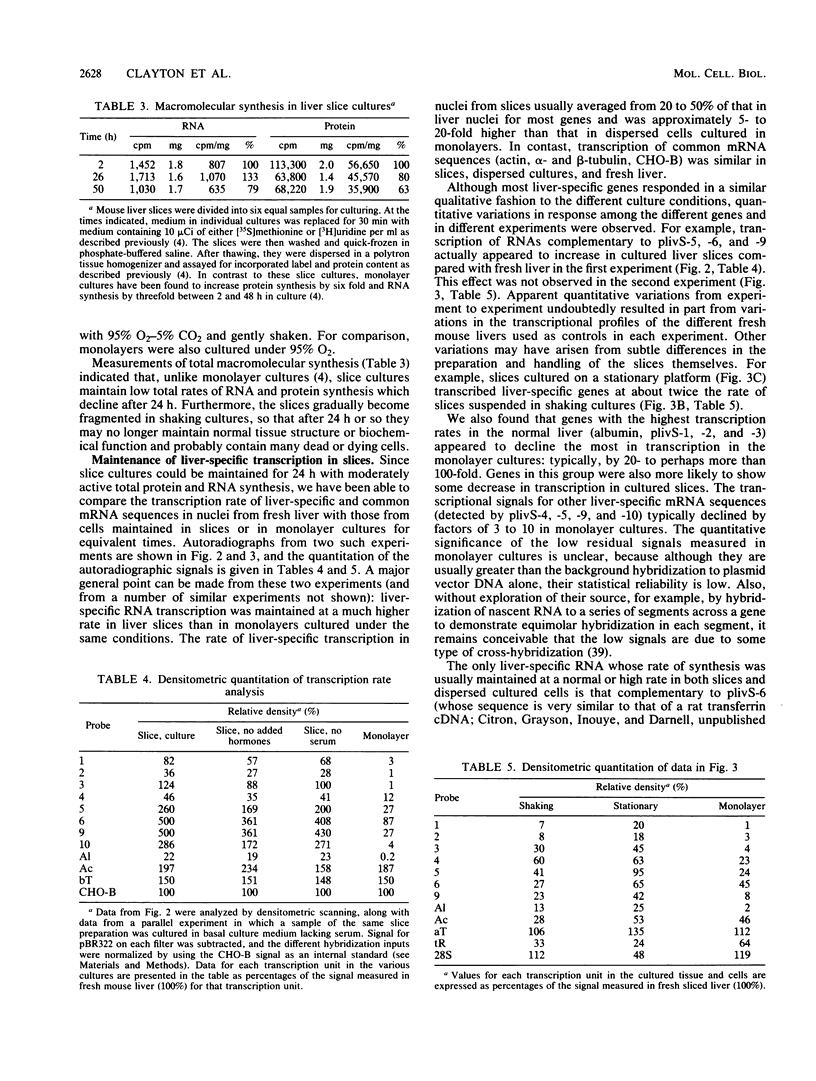
When the liver is disaggregated and hepatocytes are cultured as a cellular monolayer for 24 h, a sharp decline (80 to 99% decrease) in the transcription of most liver-specific mRNAs, but not common mRNAs, occurs (Clayton and Darnell, Mol. Cell. Biol. 2:1552-1561, 1983). A wide variety of culture conditions involving various hormones and substrates and cocultivation with other cells failed to sustain high rates of liver-specific mRNA synthesis in cultured hepatocytes, although they continued to synthesize common mRNAs at normal or elevated rates. In contrast, when slices of intact mouse liver tissue were placed in culture, the transcription of liver-specific genes was maintained at high levels (20 to 100% of normal liver). Furthermore, we found that cells in the liver could be disengaged and immediately reengaged in a tissue-like structure by perfusing the liver with EDTA followed by serum-containing culture medium. Slices of reengaged liver continued to transcribe tissue-specific mRNA sequences at significantly higher rates after 24 h in culture than did individual cells isolated by EDTA perfusion followed by culturing as a monolayer. Therefore we conclude that a mature tissue structure plays an important role in the maintenance of maximum tissue-specific transcription in liver cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bak I. J., Misgeld U., Weiler M., Morgan E. The preservation of nerve cells in rat neostriatal slices maintained in vitro: a morphological study. Brain Res. 1980 Sep 22;197(2):341–353. doi: 10.1016/0006-8993(80)91120-8. [DOI] [PubMed] [Google Scholar]
- Barth R. K., Gross K. W., Gremke L. C., Hastie N. D. Developmentally regulated mRNAs in mouse liver. Proc Natl Acad Sci U S A. 1982 Jan;79(2):500–504. doi: 10.1073/pnas.79.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. F., Darnell J. E., Jr Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol Cell Biol. 1983 Sep;3(9):1552–1561. doi: 10.1128/mcb.3.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. F., Weiss M., Darnell J. E., Jr Liver-specific RNA metabolism in hepatoma cells: variations in transcription rates and mRNA levels. Mol Cell Biol. 1985 Oct;5(10):2633–2641. doi: 10.1128/mcb.5.10.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
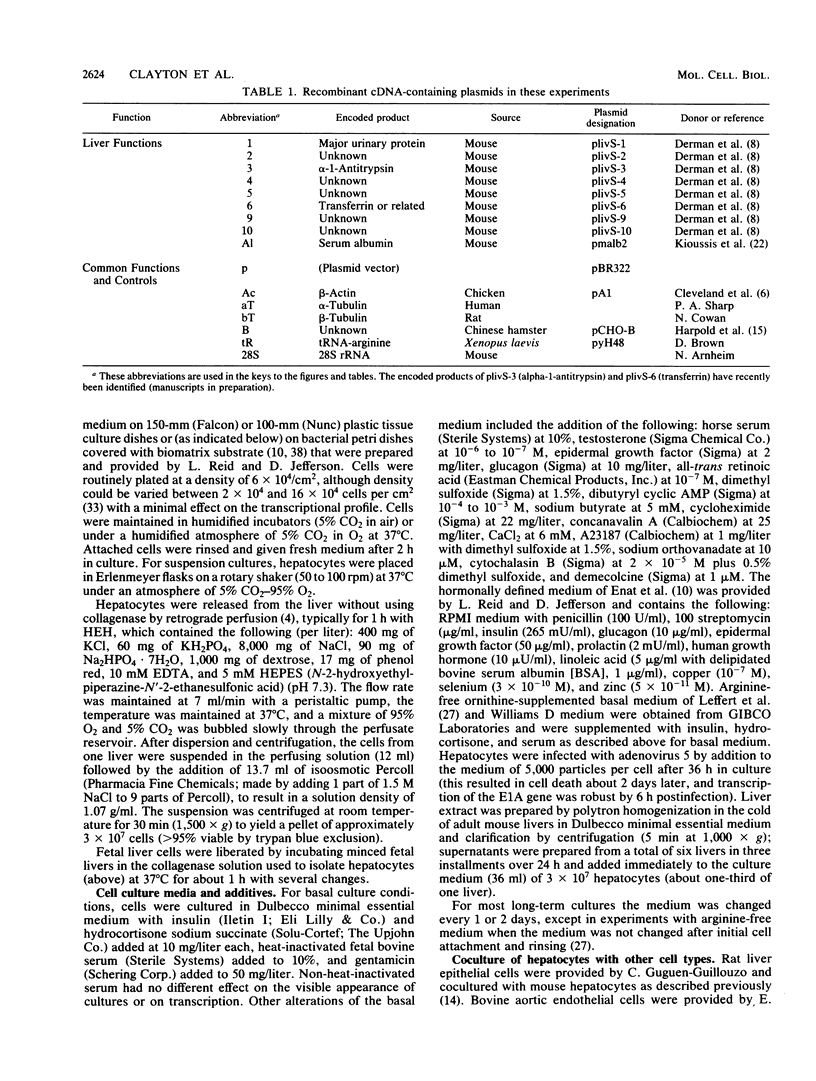
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Douarin N. M. An experimental analysis of liver development. Med Biol. 1975 Dec;53(6):427–455. [PubMed] [Google Scholar]
- Enat R., Jefferson D. M., Ruiz-Opazo N., Gatmaitan Z., Leinwand L. A., Reid L. M. Hepatocyte proliferation in vitro: its dependence on the use of serum-free hormonally defined medium and substrata of extracellular matrix. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1411–1415. doi: 10.1073/pnas.81.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J. M., Chung E. Y., Darnell J. E., Jr Gene expression during liver regeneration. J Mol Biol. 1984 Oct 15;179(1):37–53. doi: 10.1016/0022-2836(84)90305-x. [DOI] [PubMed] [Google Scholar]
- Granner D. K., Hargrove J. L. Regulation of the synthesis of tyrosine aminotransferase: the relationship to mRNATAT. Mol Cell Biochem. 1983;53-54(1-2):113–128. doi: 10.1007/BF00225249. [DOI] [PubMed] [Google Scholar]
- Greengard O., Federman M., Knox W. E. Cytomorphometry of developing rat liver and its application to enzymic differentiation. J Cell Biol. 1972 Feb;52(2):261–272. doi: 10.1083/jcb.52.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guguen-Guillouzo C., Clément B., Baffet G., Beaumont C., Morel-Chany E., Glaise D., Guillouzo A. Maintenance and reversibility of active albumin secretion by adult rat hepatocytes co-cultured with another liver epithelial cell type. Exp Cell Res. 1983 Jan;143(1):47–54. doi: 10.1016/0014-4827(83)90107-6. [DOI] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Houssaint E. Differentiation of the mouse hepatic primordium. I. An analysis of tissue interactions in hepatocyte differentiation. Cell Differ. 1980 Oct;9(5):269–279. doi: 10.1016/0045-6039(80)90026-3. [DOI] [PubMed] [Google Scholar]
- Jeejeebhoy K., Phillips M. J. Isolated mammalian hepatocytes in culture. Gastroenterology. 1976 Dec;71(6):1086–1096. [PubMed] [Google Scholar]
- Jefferson D. M., Clayton D. F., Darnell J. E., Jr, Reid L. M. Posttranscriptional modulation of gene expression in cultured rat hepatocytes. Mol Cell Biol. 1984 Sep;4(9):1929–1934. doi: 10.1128/mcb.4.9.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungermann K., Katz N. Functional hepatocellular heterogeneity. Hepatology. 1982 May-Jun;2(3):385–395. doi: 10.1002/hep.1840020316. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioussis D., Eiferman F., van de Rijn P., Gorin M. B., Ingram R. S., Tilghman S. M. The evolution of alpha-fetoprotein and albumin. II. The structures of the alpha-fetoprotein and albumin genes in the mouse. J Biol Chem. 1981 Feb 25;256(4):1960–1967. [PubMed] [Google Scholar]
- Knopf J. L., Gallagher J. F., Held W. A. Differential, multihormonal regulation of the mouse major urinary protein gene family in the liver. Mol Cell Biol. 1983 Dec;3(12):2232–2240. doi: 10.1128/mcb.3.12.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh H., Yasuda K., Okada T. S. Tissue-specific expression of a cloned chick delta-crystallin gene in mouse cells. Nature. 1983 Feb 3;301(5899):440–442. doi: 10.1038/301440a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leffert H. L., Moran T., Boorstein R., Koch K. S. Procarcinogen activation and hormonal control of cell proliferation in differentiated primary adult rat liver cell cultures. Nature. 1977 May 5;267(5606):58–61. doi: 10.1038/267058a0. [DOI] [PubMed] [Google Scholar]
- McGuire E. J., Burdick C. L. Intercellular adhesive selectivity. I. An improved assay for the measurement of embryonic chick intercellular adhesion (liver and other tissues). J Cell Biol. 1976 Jan;68(1):80–89. doi: 10.1083/jcb.68.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R., Friend D. S., Perrelet A., Orci L. In vivo assembly of tight junctions in fetal rat liver. J Cell Biol. 1975 Nov;67(2PT1):310–319. doi: 10.1083/jcb.67.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muglia L., Locker J. Developmental regulation of albumin and alpha-fetoprotein gene expression in the rat. Nucleic Acids Res. 1984 Sep 11;12(17):6751–6762. doi: 10.1093/nar/12.17.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Yoshimoto K., Nakayama Y., Tomita Y., Ichihara A. Reciprocal modulation of growth and differentiated functions of mature rat hepatocytes in primary culture by cell--cell contact and cell membranes. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7229–7233. doi: 10.1073/pnas.80.23.7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M. O., Sperling L., Herbomel P., Yaniv M., Weiss M. C. Tissue-specific expression is conferred by a sequence from the 5' end of the rat albumin gene. EMBO J. 1984 Nov;3(11):2505–2510. doi: 10.1002/j.1460-2075.1984.tb02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D. J., Friedman J. M., Oulette A. J., Krauter K. S., Darnell J. E., Jr Transcriptional and post-transcriptional control of specific messenger RNAs in adult and embryonic liver. J Mol Biol. 1984 Oct 15;179(1):21–35. doi: 10.1016/0022-2836(84)90304-8. [DOI] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Salditt-Georgieff M., Sheffery M., Krauter K., Darnell J. E., Jr, Rifkind R., Marks P. A. Induced transcription of the mouse beta-globin transcription unit in erythroleukemia cells. Time-course of induction and of changes in chromatin structure. J Mol Biol. 1984 Feb 5;172(4):437–450. doi: 10.1016/s0022-2836(84)80016-9. [DOI] [PubMed] [Google Scholar]
- Sasse D. Chemomorphologie der Glykogensynthese und des Glykogengehalts während der Histogenese der Leber. Histochemie. 1969;20(2):159–170. doi: 10.1007/BF00268710. [DOI] [PubMed] [Google Scholar]
- Stafford J., Queen C. Cell-type specific expression of a transfected immunoglobulin gene. Nature. 1983 Nov 3;306(5938):77–79. doi: 10.1038/306077a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD R. L. AN ELECTRON MICROSCOPE STUDY OF DEVELOPING BILE CANALICULI IN THE RAT. Anat Rec. 1965 Apr;151:507–529. doi: 10.1002/ar.1091510403. [DOI] [PubMed] [Google Scholar]
- Walker M. D., Edlund T., Boulet A. M., Rutter W. J. Cell-specific expression controlled by the 5'-flanking region of insulin and chymotrypsin genes. Nature. 1983 Dec 8;306(5943):557–561. doi: 10.1038/306557a0. [DOI] [PubMed] [Google Scholar]