Abstract
Enteral nutrition is the preferred route of feeding in critically ill patients. It has multiple advantages over parenteral nutrition and potentially improves patients’ outcome. Enteral nutrition is delivered via gastric or postpyloric (small intestine) feeding tubes. The latter option used to be a more challenging choice to achieve unless the feeding tube is placed endoscopically or by interventional radiology. Multiple technical advances have facilitated postpyloric feeding, including a new electromagnetically visualised jejunal feeding tube system (CORTRAK Enteral Access System). We are presenting a case of a 50-year-old woman who suffered a nasopharyngeal perforation caused by this novel technology. The complication was recognised promptly and managed successfully with conservative measures. This case illustrates the importance of recognising patients at high risk for feeding tube placement complications, meticulous placement technique and appropriate follow-up once the tube has been inserted.
Background
Enteral nutrition is the preferred route of feeding for critically ill patients who require nutritional support therapy.1 It maintains gut integrity, reduces infectious complications, complements efforts of stress ulcer prophylaxis and improves patients’ outcome.1
Enteral nutrition is acceptably delivered via gastric or postpyloric (small intestine) feeding tubes. The latter option offers potential advantages in patients at high risk for aspiration and in patients who were intolerant to gastric feeding.1
Traditionally, successful placement of postpyloric feeding tubes posed a challenge unless performed endoscopically which is costly, time consuming and technically challenging. A new electromagnetically visualised jejunal feeding tube system (CORTRAK Enteral Access System) compared favourably in a prospective randomised trial with the gold standard technique, endoscopic tube placement.2 The placement success rate of the new system was 91% with similar implantation time and safety profile, but at a much lower cost.2
We present a case of nasopharyngeal perforation caused by this new electromagnetically visualised feeding tube system. Malposition and gut perforation have been previously reported with enteral feeding tubes and continues to be a real concern even with this novel technology.3
Case presentation
A 50-year-old female patient presented with right-sided chest pain and cough of 2 weeks duration. She had a history of metastatic small cell lung cancer diagnosed 2 years ago, but has been off treatment for several months.
On examination, her vital signs were stable. Right-side chest exam revealed dullness to percussion and poor air entry on auscultation. Her laboratory tests showed haemoglobin of 10.9 g/dl and white cell count of 6.1×109/l. Her platelet count, kidney and liver function tests were within normal limits. A CT scan of the chest showed massive right-sided pleural effusion. Video-assisted thoracoscopy of the right pleural space confirmed the presence of malignant pleural effusion with pleural metastases. Pleurodesis was performed.
On postoperative day 3, while the patient was still on mechanical ventilation, the nurse who was in charge of the surgical intensive care unit attempted to place an electromagnetically visualised 12-French polyurethane feeding tube after the patient's nurse failed to place a regular 16-French orogastric tube. The nurse in charge was one of few designated ‘super-users’ of this novel technology. Minimal resistance was reported during the attempt, but the patient showed signs of distress, and a tender swelling on the right side of the face was noticed (figure 1). The procedure was aborted and the tube was left in place. An emergent CT of the neck showed the feeding tube perforating the right side of the nasopharynx near the fossa of Rosenmuller (figure 2). The tube travelled just anterior to the carotid artery and internal jugular vein (figure 3). The tip of the tube was visualised traversing the inferior part of the parotid gland and logging in the right subcutaneous facial tissues (figure 4). The surgical service was consulted, the feeding tube was removed and broad-spectrum antibiotics were initiated. The patient's complaints resolved without sequel. She was liberated successfully from mechanical ventilation and discharged home.
Figure 1.
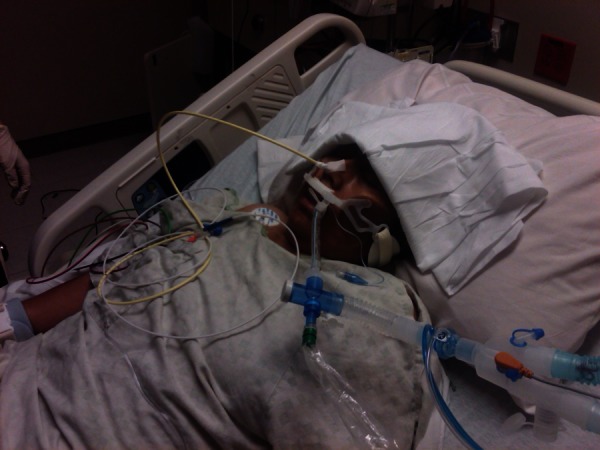
The electromagnetically visualised jejunal feeding tube placed through the right nostril.
Figure 2.
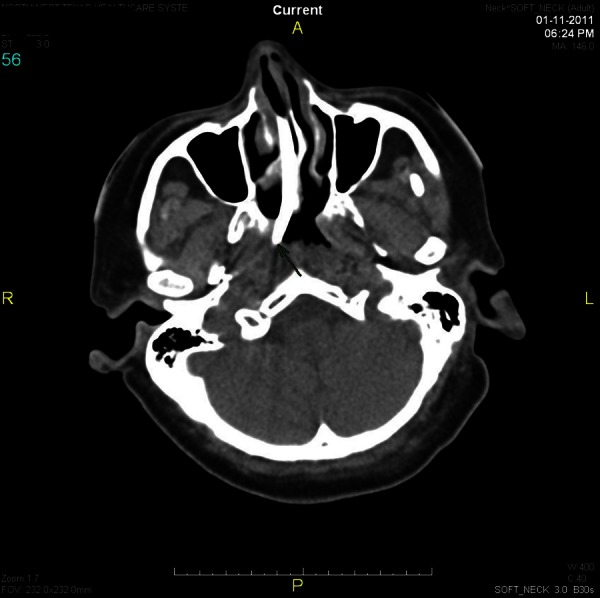
A CT cross-sectional view showing the feeding tube about to penetrate the oropharynx.
Figure 3.
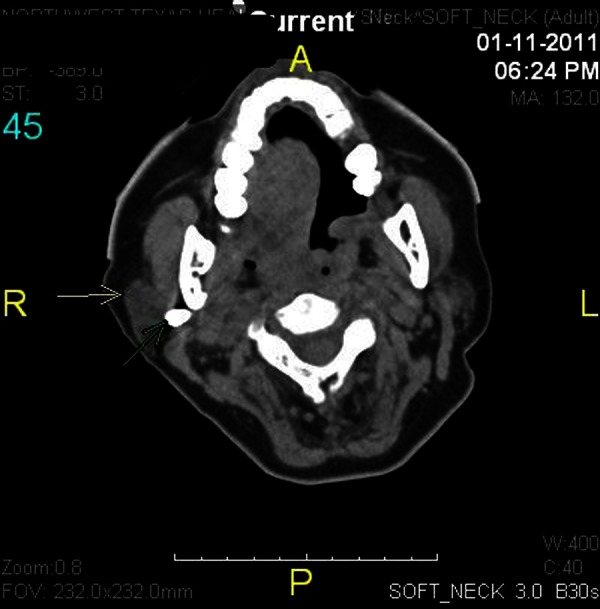
A CT cross-sectional view showing the feeding tube travelling just anterior to the carotid artery and internal jugular vein.
Figure 4.
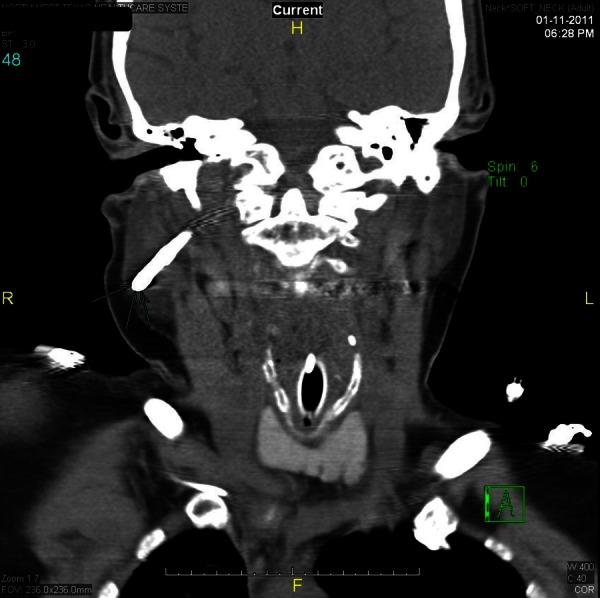
A CT coronal sectional view showing the feeding tube traversing the inferior part of the parotid gland and logging in the right subcutaneous facial tissues.
Discussion
The placement of enteral feeding tubes is associated with a favourable risk–benefit ratio. In comparison with large-bore nasogastric or orogastric feeding tubes, small-intestine feeding tubes are of small-diameter and are softer and more pliable. They are more comfortable to patients and potentially less likely to induce mucosal damage or block nasal sinuses ostia.3 Despite the above-mentioned advantages, ensuring a postpyloric placement used to be challenging. A new electromagnetically visualised jejunal feeding tube system (CORTRAK Enteral Access System) has a stylet with a transmitter that generates signals as it passes through the alimentary tract.4 A computer screen displays the graphic representation of the feeding tube's position as it passes through the gut and confirms the postpyloric placement.5 Our institution has been using this technology for the last 2 years and it has proven to be safe and easy to use, a fact that has been reported by others.6 Nevertheless, the potential for complications is inherently embedded in the use of any medical intervention. We presented a case of nasopharyngeal perforation caused by a new electromagnetically visualised jejunal feeding tube system. The complication was recognised promptly and managed appropriately without significant effect on the patient's outcome. The exact cause of the complication in this patient was not clear to us. We postulated that the excessive use of force, the small-diameter and the stiff tip of the tube in combination with the high-risk patient at hand caused the problem.
Proper placement of feeding tubes is facilitated by the patient's cooperation, swallowing repeatedly once the tube enters the oropharynx. Difficulties in tube placement are anticipated in uncooperative patients, demented patients as well as obtunded patients due to a disease process or medications, a situation commonly encountered in critically ill patients.3
Small-intestine feeding tubes have weighted tips and are equipped with a removable stylet for use during insertion. The aforementioned two features combined with the small-diameter of the tube makes it a stiff instrument that could perforate structures if excessive pressure is applied with the distal tip deflected against an organ.
Multiple cases of oesophageal and tracheobronchial tree perforations with subsequent pneumothorax and pulmonary haemorrhage have been reported in the literature.7–9 The exact perforation rates associated with the use of postpyloric feeding tubes have not been studied. Other complications associated with feeding tube placement have been reviewed extensively elsewhere.10
Based on the above, few steps can be followed to minimise perforation risk associated with small-intestine feeding tube placement:
Use small-intestine feeding tubes in patient populations most likely to benefit from this modality of feeding; patients at high risk for aspiration, patients who are not tolerating gastric feeding and if feeding is contemplated in patients with acute severe pancreatitis.1 There is no proven benefit for postpyloric feeding in critically ill patients, including those on mechanical ventilation, who are tolerating gastric feeding.11 12
Identify patients who are at high risk for this complication; patients with depressed sensorium or impaired gag reflex or laryngeal sensation. Maintain a high index of suspicion meanwhile inserting small-intestine feeding tubes in this group of patients. Withdraw the tube and re-insert it if resistance is met or the patient starts coughing or shows evidence of respiratory distress. Consider different approaches, endoscopic placement or placement under fluoroscopy, after repeated failed attempts.
Personnel experienced with these tubes should insert or supervise the procedure all the time. In our intensive care units, a number of experienced nurses, ‘super-users’, perform this task.
Once small-intestine feeding tubes are placed, perform appropriate procedures, for example, abdominal x-ray, to confirm its position. Epigastric bubbling after air insufflations or aspiration of fluid is not reliable enough to confirm the placement.3
Developing a protocol that follows appropriate indications and procedural steps will ensure adherence with the above suggested steps.
In conclusion, new technical developments have facilitated the successful postpyloric feeding tube placement at the bedside. Recognising patients at high-risk for complication during the tube insertion, meticulous placement technique and appropriate follow-up after the tube insertion will ensure optimal utilisation of this novel technology.
Learning points.
Patients with depressed sensorium or impaired gag reflex are at high risk for complications during oral or nasal feeding tube insertion.
Personnel experienced with these tubes should insert or supervise the procedure all the time.
Adhering to meticulous placement techniques followed by appropriate follow-up imaging will ensure optimal utilisation of these tubes.
Footnotes
Contributors: All authors participated in literature review and writing the manuscript. All authors approved this submission.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2009;2013:277–316 [DOI] [PubMed] [Google Scholar]
- 2.Holzinger U, Brunner R, Miehsler W, et al. Jejunal tube placement in critically ill patients: a prospective, randomized trial comparing the endoscopic technique with the electromagnetically visualized method. Crit Care Med 2011;2013:73–7 [DOI] [PubMed] [Google Scholar]
- 3.Miller KS, Tomlinson JR, Sahn SA. Pleuropulmonary complications of enteral tube feedings. Two reports, review of the literature, and recommendations. Chest 1985;2013:230–3 [DOI] [PubMed] [Google Scholar]
- 4.Gabriel SA, Ackermann RJ. Placement of nasoenteral feeding tubes using external magnetic guidance. JPEN J Parenter Enteral Nutr 2004;2013:119–22 [DOI] [PubMed] [Google Scholar]
- 5.Ozdemir B, Frost M, Hayes J, et al. Placement of nasoenteral feeding tubes using magnetic guidance: retesting a new technique. J Am Coll Nutr 2000;2013:446–51 [DOI] [PubMed] [Google Scholar]
- 6.Kaffarnik MF, Lock JF, Wassilew G, et al. The use of bedside electromagnetically guided nasointestinal tube for jejunal feeding of critical ill surgical patients. Technol Health Care 2013;2013:1–8 [DOI] [PubMed] [Google Scholar]
- 7.Dorsey JS. An unusual complication of naso-enteral feeding with small-diameter feeding tubes. Ann Surg 1984;2013:229–30 [PMC free article] [PubMed] [Google Scholar]
- 8.James RH. An unusual complication of passing a narrow bore nasogastric tube. Anaesthesia 1978;2013:716–18 [DOI] [PubMed] [Google Scholar]
- 9.Balogh GJ, Adler SJ, VanderWoude J, et al. Pneumothorax as a complication of feeding tube placement. AJR Am J Roentgenol 1983;2013:1275–7 [DOI] [PubMed] [Google Scholar]
- 10.Metheny NA, Meert KL, Clouse RE. Complications related to feeding tube placement. Curr Opin Gastroenterol 2007;2013:178–82 [DOI] [PubMed] [Google Scholar]
- 11.Ho KM, Dobb GJ, Webb SA. A comparison of early gastric and post-pyloric feeding in critically ill patients: a meta-analysis. Intensive Care Med 2006;2013:639–49 [DOI] [PubMed] [Google Scholar]
- 12.White H, Sosnowski K, Tran K, et al. A randomised controlled comparison of early post-pyloric versus early gastric feeding to meet nutritional targets in ventilated intensive care patients. Crit Care 2009;2013:R187. [DOI] [PMC free article] [PubMed] [Google Scholar]


