Abstract
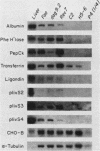
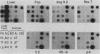
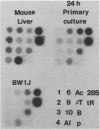
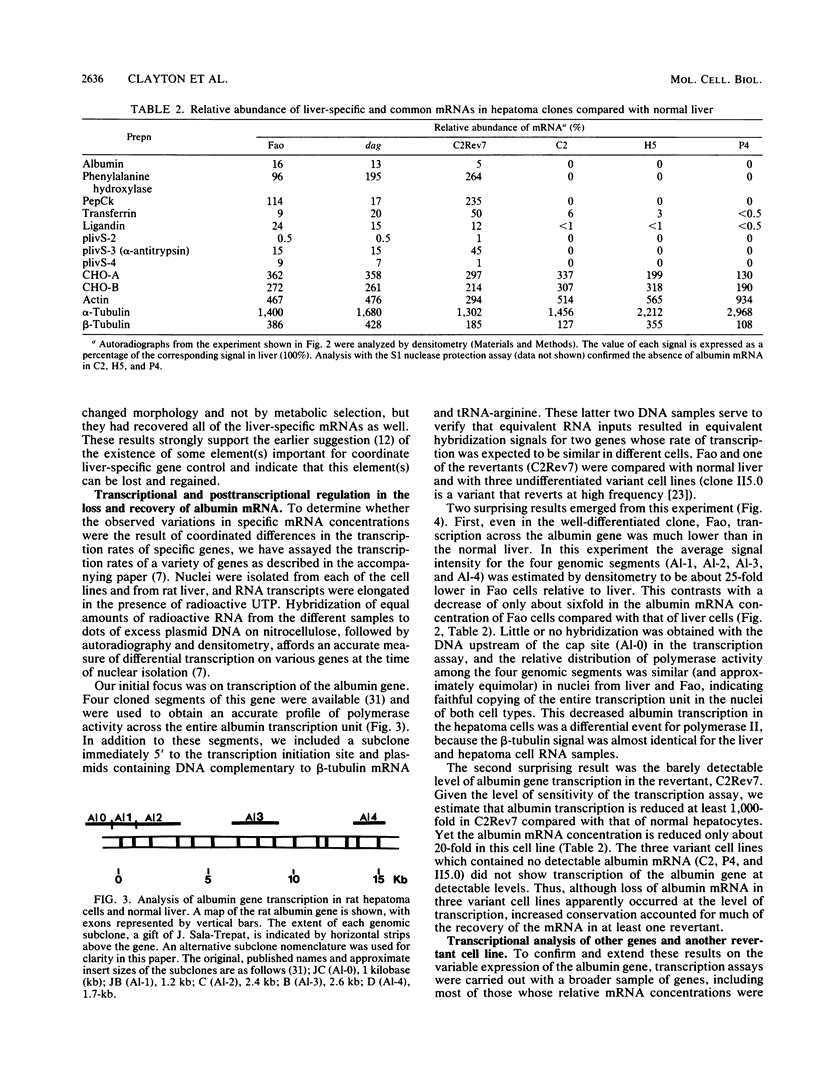
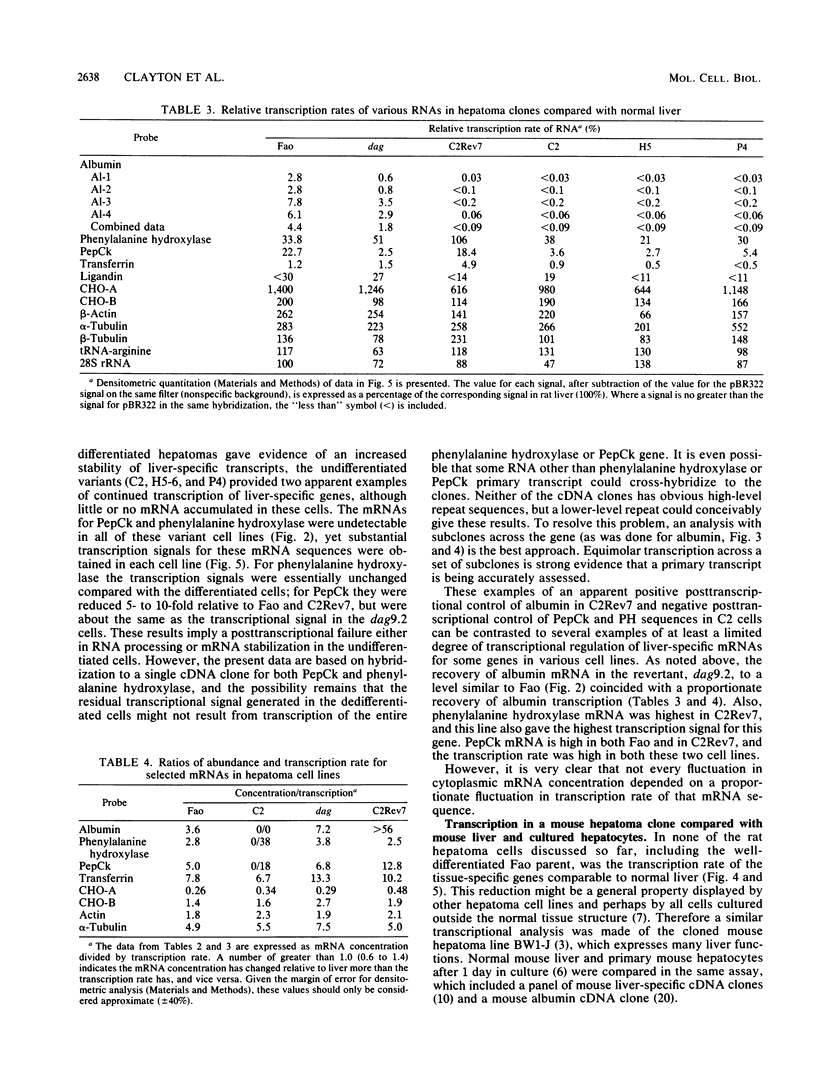
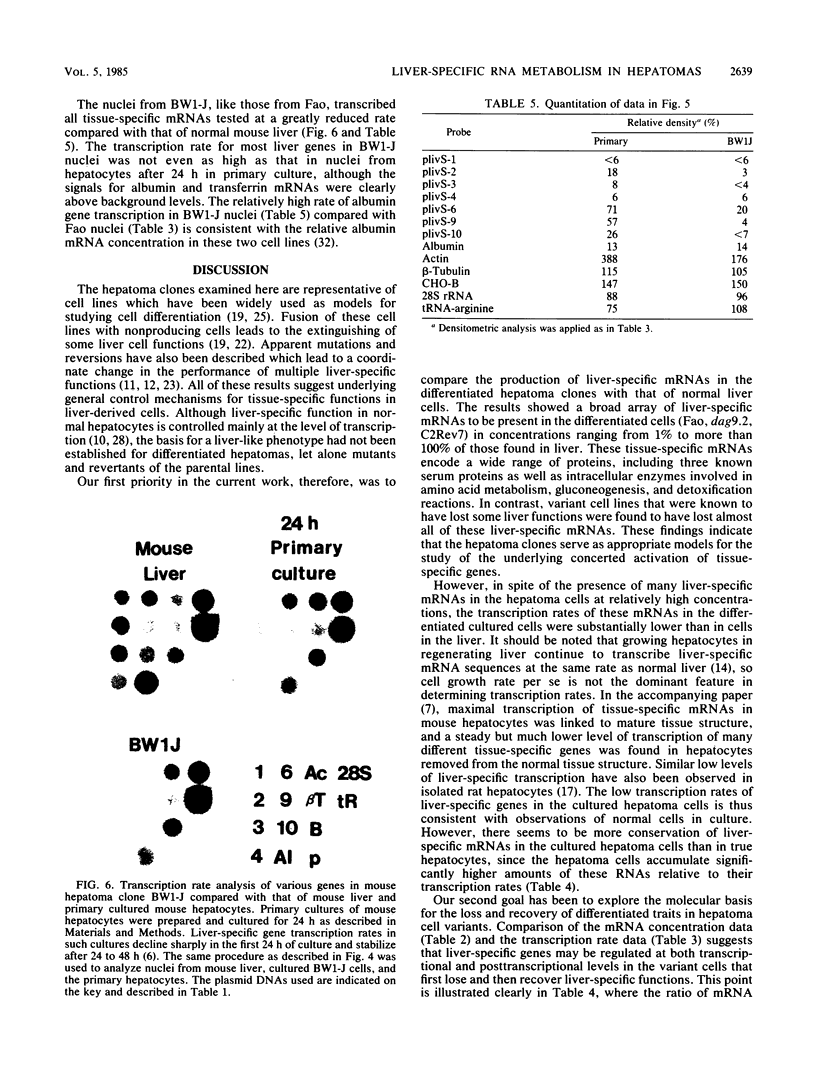
The transcription rate and abundance of several liver-specific mRNAs as well as mRNAs common to many cell types were compared in a series of rodent hepatoma cell lines, normal liver cells, and primary hepatocyte cultures. The rat hepatoma cell line, Fao, which displays a liver-specific phenotype, contained eight of eight liver-specific mRNAs examined. However, the transcription rates of most liver-specific mRNAs were found to be low (1 to 30%) compared with normal liver in this and other differentiated cell lines. This low rate is similar to the transcription rates of liver-specific mRNA sequences measured in primary cultures of hepatocytes. Several variant cell lines that had lost differentiated traits contained few or none of the liver-specific mRNAs; clonal descendents which had regained differentiated function regained the tissue-specific mRNAs as a group, but at various concentrations. Because all of the changes observed in mRNA levels were not accompanied by parallel changes in transcription of the same sequences, differential posttranscriptional stabilization of the liver-specific mRNAs must also occur in the different cell lines. These results qualify the utility of cultured cell lines in the study of tissue-specific transcriptional control, but raise the possibility that posttranscriptional mechanisms act in cooperation with transcriptional controls to bring the level of tissue-specific mRNAs closer to those found in liver cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertolotti R. A selective system for hepatoma cells producing gluconeogenic enzymes. Somatic Cell Genet. 1977 Jul;3(4):365–380. doi: 10.1007/BF01542966. [DOI] [PubMed] [Google Scholar]
- Bertolotti R. Expression of differentiated functions in hepatoma cell hybrids: selection in glucose-free media of segregated hybrid cells which reexpress gluconeogenic enzymes. Somatic Cell Genet. 1977 Nov;3(6):579–602. doi: 10.1007/BF01539067. [DOI] [PubMed] [Google Scholar]
- Cassio D., Weiss M. C. Expression of fetal and neonatal hepatic functions by mouse hepatoma-rat hepatoma hybrids. Somatic Cell Genet. 1979 Nov;5(6):719–738. doi: 10.1007/BF01542637. [DOI] [PubMed] [Google Scholar]
- Cassio D., Weiss M. C., Ott M. O., Sala-Trepat J. M., Friès J., Erdos T. Expression of the albumin gene in rat hepatoma cells and their dedifferentiated variants. Cell. 1981 Dec;27(2 Pt 1):351–358. doi: 10.1016/0092-8674(81)90418-9. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clayton D. F., Darnell J. E., Jr Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol Cell Biol. 1983 Sep;3(9):1552–1561. doi: 10.1128/mcb.3.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. F., Harrelson A. L., Darnell J. E., Jr Dependence of liver-specific transcription on tissue organization. Mol Cell Biol. 1985 Oct;5(10):2623–2632. doi: 10.1128/mcb.5.10.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Coon H. G., Weiss M. C. A quantitative comparison of formation of spontaneous and virus-produced viable hybrids. Proc Natl Acad Sci U S A. 1969 Mar;62(3):852–859. doi: 10.1073/pnas.62.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Deschatrette J. Dedifferentiated variants of a rat hepatoma: partial reversion induced by cell aggregation. Cell. 1980 Nov;22(2 Pt 2):501–511. doi: 10.1016/0092-8674(80)90360-8. [DOI] [PubMed] [Google Scholar]
- Deschatrette J., Moore E. E., Dubois M., Weiss M. C. Dedifferentiated variants of a rat hepatoma:reversion analysis. Cell. 1980 Apr;19(4):1043–1051. doi: 10.1016/0092-8674(80)90095-1. [DOI] [PubMed] [Google Scholar]
- Deschatrette J., Weiss M. C. Characterization of differentiated and dedifferentiated clones from a rat hepatoma. Biochimie. 1974;56(11-12):1603–1611. doi: 10.1016/s0300-9084(75)80286-0. [DOI] [PubMed] [Google Scholar]
- Friedman J. M., Chung E. Y., Darnell J. E., Jr Gene expression during liver regeneration. J Mol Biol. 1984 Oct 15;179(1):37–53. doi: 10.1016/0022-2836(84)90305-x. [DOI] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Jefferson D. M., Clayton D. F., Darnell J. E., Jr, Reid L. M. Posttranscriptional modulation of gene expression in cultured rat hepatocytes. Mol Cell Biol. 1984 Sep;4(9):1929–1934. doi: 10.1128/mcb.4.9.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinyak J. E., Taylor J. M. Rat glutathione S-transferase. Cloning of double-stranded cDNA and induction of its mRNA. J Biol Chem. 1982 Jan 10;257(1):523–530. [PubMed] [Google Scholar]
- Killary A. M., Fournier R. E. A genetic analysis of extinction: trans-dominant loci regulate expression of liver-specific traits in hepatoma hybrid cells. Cell. 1984 Sep;38(2):523–534. doi: 10.1016/0092-8674(84)90507-5. [DOI] [PubMed] [Google Scholar]
- Kioussis D., Eiferman F., van de Rijn P., Gorin M. B., Ingram R. S., Tilghman S. M. The evolution of alpha-fetoprotein and albumin. II. The structures of the alpha-fetoprotein and albumin genes in the mouse. J Biol Chem. 1981 Feb 25;256(4):1960–1967. [PubMed] [Google Scholar]
- Moore E. E., Weiss M. C. Selective isolation of stable and unstable dedifferentiated variants from a rat hepatoma cell line. J Cell Physiol. 1982 Apr;111(1):1–8. doi: 10.1002/jcp.1041110102. [DOI] [PubMed] [Google Scholar]
- Mével-Ninio M., Weiss M. C. Immunofluorescence analysis of the time-course of extinction, reexpression, and activation of albumin production in rat hepatoma-mouse fibroblast heterokaryons and hybrids. J Cell Biol. 1981 Aug;90(2):339–350. doi: 10.1083/jcb.90.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M. O., Sperling L., Cassio D., Levilliers J., Sala-Trepat J., Weiss M. C. Undermethylation at the 5' end of the albumin gene is necessary but not sufficient for albumin production by rat hepatoma cells in culture. Cell. 1982 Oct;30(3):825–833. doi: 10.1016/0092-8674(82)90287-2. [DOI] [PubMed] [Google Scholar]
- Ott M. O., Sperling L., Herbomel P., Yaniv M., Weiss M. C. Tissue-specific expression is conferred by a sequence from the 5' end of the rat albumin gene. EMBO J. 1984 Nov;3(11):2505–2510. doi: 10.1002/j.1460-2075.1984.tb02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITOT H. C., PERAINO C., MORSE P. A., Jr, POTTER V. R. HEPATOMAS IN TISSUE CULTURE COMPARED WITH ADAPTING LIVER IN VIVO. Natl Cancer Inst Monogr. 1964 Apr;13:229–245. [PubMed] [Google Scholar]
- Peterson J. A., Weiss M. C. Expression of differentiated functions in hepatoma cell hybrids: induction of mouse albumin production in rat hepatoma-mouse fibroblast hybrids. Proc Natl Acad Sci U S A. 1972 Mar;69(3):571–575. doi: 10.1073/pnas.69.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D. J., Friedman J. M., Oulette A. J., Krauter K. S., Darnell J. E., Jr Transcriptional and post-transcriptional control of specific messenger RNAs in adult and embryonic liver. J Mol Biol. 1984 Oct 15;179(1):21–35. doi: 10.1016/0022-2836(84)90304-8. [DOI] [PubMed] [Google Scholar]
- REUBER M. D. A transplantable bile-secreting hepatocellular carcinoma in the rat. J Natl Cancer Inst. 1961 Apr;26:891–899. [PubMed] [Google Scholar]
- Robson K. J., Chandra T., MacGillivray R. T., Woo S. L. Polysome immunoprecipitation of phenylalanine hydroxylase mRNA from rat liver and cloning of its cDNA. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4701–4705. doi: 10.1073/pnas.79.15.4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Jagodzinski L. L., Yang M., Bonner J. Fine structure and evolution of the rat serum albumin gene. Mol Cell Biol. 1981 Oct;1(10):871–883. doi: 10.1128/mcb.1.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellem C. H., Cassio D., Sala-Trepat J. M., Weiss M. C. Coordinate secretion of rat and mouse albumin by mouse hepatoma x rat hepatoma hybrid cells directly reflects the intracellular concentration of the corresponding mRNAs. J Cell Physiol. 1983 May;115(2):175–178. doi: 10.1002/jcp.1041150211. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo-Warren H., Monahan J. E., Short J., Short H., Bruzel A., Wynshaw-Boris A., Meisner H. M., Samols D., Hanson R. W. Isolation and characterization of the gene coding for cytosolic phosphoenolpyruvate carboxykinase (GTP) from the rat. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3656–3660. doi: 10.1073/pnas.80.12.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]