Abstract
A 14-year-old asymptomatic girl without relevant medical history was referred to our department for heart murmur evaluation. The echocardiogram showed cardiac chambers with normal size and function. Noteworthy was the presence of an apparently fibrous tissue joining the ventricular surfaces of the aortic non-coronary and right coronary leaflets with the anterior mitral leaflet. Both valves were slightly thickened and there was a mild anterior mitral valve ‘billowing’ causing an eccentric mild-to-moderate regurgitant jet. During systole, tethering of this tissue caused the incomplete opening of both mentioned aortic leaflets, causing a turbulent flow with no significant gradient across the valve. During diastole, moderate eccentric aortic regurgitation jet was noted, probably related to incomplete coaptation at the insertion point of this anomalous tissue. We speculate that this finding may represent the remnant of some tissue during heart development that abnormally persisted in this young lady.
Background
The cardiac embryonic development is still to date not fully understood. We report a rare case of a young girl with a string-like tissue that connects the mitral and aortic valves. To our knowledge this finding has never been reported before. We can only speculate that this tissue is related to the heart valves development.1–5 Maybe this case provides feedback to know other similar cases, so we can understand this finding.
Case presentation
Systolic heart murmur. No medical, social or familial history of interest.
Investigations
Parents have normal echocardiograms without similar findings.
Differential diagnosis
Intraventricular chordae tendinae.
Endocarditis
Treatment
The patient is asymptomatic and there is no relevant associated valve disease; therefore, only follow up was advised. Endocarditis prophylaxis was not advised.
Outcome and follow-up
Serial echocardiograms were advised annually.
Discussion
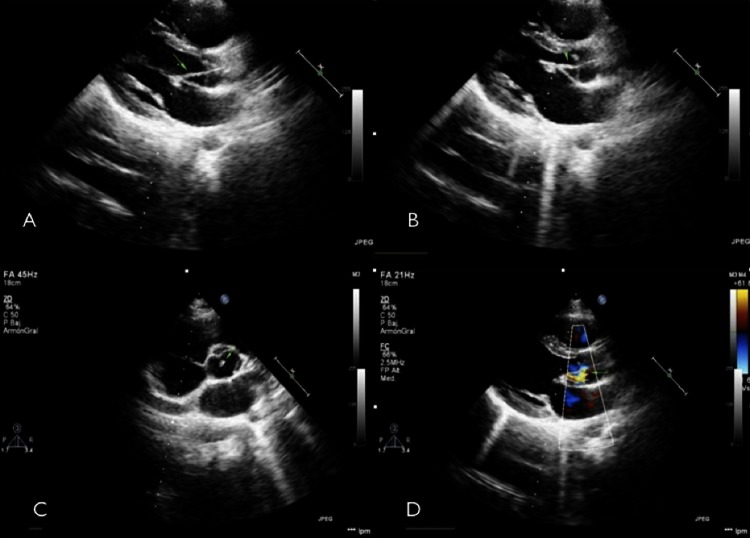
This case could be misinterpreted as intraventricular chordae tendinae. Sometimes this tendinae depart from the intraventricular cavity and reach the outflow tract (myocardium to myocardium), but this tissue joins clearly both left-sided valves without reaching the myocardium (figure 1).
Figure 1.
(A) Long axis view end-diastole. (B) Long axis view diastole. (C) Short axis view. (D) Colour flow diastole. String-like tissue (arrows).
One interesting question is how is it possible to have moderate anterior mitral valve billowing when there was strain from the fibrous tissue? First, we must define how current guidelines define billowing: it is the situation when only a part of the mitral valve body protrudes into the left atrium and coaptation remains preserved6 in contrast to ‘prolapse’ where the coaptation line is behind the annular plane. Therefore we speculate that mitral valve billowing could have been more severe (or even prolapsed) instead of being mild, since this fibrous tissue strains the A2 scallop of the mitral valve during systole. In systole aortic valve tethering is clear, so it is unlikely that straining in the mitral valve is softer than in the aortic valve because strain must be equal at both sides.
About follow up, European Society of Cardiology Guidelines state that asymptomatic patients with moderate valvular disease and preserved left ventricular function can be followed up on a yearly basis and echocardiogram should be performed every 2 years.7 However this is a very rare finding and progression of the disease is unpredictable. For this reason we decided to follow her up on a yearly basis, including echocardiogram at each visit while she remains asymptomatic and without severe valve disease.
Even though recent endocarditis guidelines do not recommend prophylaxis in this sort of valve disease, attention must be paid on this possibility. Also, it seems unlikely that embolisation of a part or the whole chord during follow up occurs.
We wondered also whether we could avoid valve disease progression with an early surgery or not? That will remain unanswered because performing cardiac surgery (even minimally invasive) does not seem advisable. This patient may persist asymptomatic and without further disease progression during her entire life. Although turbulent flow and the young age of presentation could lead to further deterioration of both valves (see online supplementary material), especially the aortic valve since this one has a double mechanism of progression, moderate regurgitation and turbulent systolic flow.
Learning points.
Cardiac development is not fully understood.
Aortic tethering is possible.
Footnotes
Contributors : LA-A constructed the hypothesis and contributed to data collection, interpretation, the literature review and the drafting of the manuscript; ES-V reviewed the interpretation of the results and gave expert opinion, MF-L reviewed the data interpretation, gave expert opinion and reviewed the manuscript. MF-V helped with the image analysis and preparation and also gave expert opinion about the findings.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Snarr B, Kern C, Wessels A. Origin and fate of cardiac mesenchyme. Dev dyn 2008;2013:2804–19 [DOI] [PubMed] [Google Scholar]
- 2.Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res 2009;2013:408–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoen FJ. Evolving concepts of cardiac valve dynamics. Circulation 2008;2013:1864–80 [DOI] [PubMed] [Google Scholar]
- 4.Hinton RB, Lincoln J, Deutsch GH, et al. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res 2006;2013:1431–8 [DOI] [PubMed] [Google Scholar]
- 5.Moorman A, Webb S, Brown NA, et al. Development of the heart: formation of the cardiac chambers and arterial trunks. Heart 2003;2013:806–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Patrizio Lancellotti (Chair), Luis Moura, Luc A Pierard, Eustachio Agricola, Bogdan A Popescu, Christophe Tribouilloy, Andreas Hagendorff, Jean-Luc Monin, Luigi Badano, and Jose L Zamorano on behalf of the European Association of Echocardiography. [DOI] [PubMed]
- 7. Guidelines on the management of valvular heart disease (version 2012) The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) (1) European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Patrizio Lancellotti (Chair), Luis Moura, Luc A Pierard, Eustachio Agricola, Bogdan A Popescu, Christophe Tribouilloy, Andreas Hagendorff, Jean-Luc Monin, Luigi Badano and Jose L Zamorano on behalf of the European Association of Echocardiography.