Abstract
Secondary hypertension is the most common form of hypertension in childhood, particularly in the young age group: parenchymal disease and lesions of the renal artery account for the majority of such cases. Renal artery aneurysms (RAA) are rare and are usually diagnosed by Doppler ultrasonography or angiography performed in the investigation of specific clinical symptoms. We report herein a case of severe arterial hypertension in an 8-year-old girl arising from a large saccular RAA. Intravenous antihypertensive drugs were necessary to achieve blood pressure control and the final diagnosis was obtained from angio-CT scan and selective angiography that demonstrated a large saccular aneurysm of the left renal artery with parietal calcification. After confirmation of inexistent function of the entire left kidney by Tc99m-MAG3 renal isotope scan, nephrectomy was performed. The child's blood pressure further normalised and, 1 month after surgery, she had ceased any antihypertensive therapy.
Background
Secondary hypertension is the most common form of hypertension in childhood, particularly in the young age group: parenchymal disease and lesions of the renal artery account for the majority of such cases.1 Renal artery aneurysms (RAA) are rare but when present are commonly associated with hypertension, abdominal pain and haematuria and can also cause hypokalaemia from excess aldosterone secretion.2 RAA are usually diagnosed by Doppler ultrasonography or angiography performed in the investigation of specific clinical symptoms.3 Conservative treatment seldom results in normalisation of hypertension secondary to RAA and the majority of patients require intervention.2 Few data exist on the management of RAAs in children.1 We review the presentation, diagnosis and treatment of an 8-year-old girl with renovascular hypertension and unilateral RAA.
Case presentation
A previously healthy 8-year-old Caucasian girl was admitted to the paediatric department for treatment of an acute pyelonephritis. After admission she was noted to have an elevated blood pressure of 180/132 mm Hg and was subsequently transferred to the paediatric intensive care unit for treatment and further evaluation. Some improvement was achieved with oral administration of nifedipine but was not long-lasting. Bolus intravenous administration of propranolol was tried but with no significant effect, so a labetalol intravenous perfusion was established with a partial blood pressure control after achieving a maximum dose of 3 mg/kg/h. On the 4th day of admission, due to difficulty in maintaining acceptable blood pressure levels, a second perfusion with sodium nitroprusside was started. Oral administration of amlodipine and captopril was also added on the 5th day as a means of weaning off the intravenous perfusions of nitroprusside and labetalol that were finally discontinued at the 8th and 11th day, respectively.
During the investigations for hypertension, the haemogram showed normocytic normochromic anaemia (9 g/dl) with a normal blood chemistry including renal function and sedimentation rate. Urinalysis demonstrated a nephrotic proteinuria and there was an increased activity of the plasmatic renin (360μl/ml). She showed no signs suggesting systemic vasculitis, namely cutaneous or articular manifestations or history of previous renal infection. Renal sonogram and Doppler examination revealed size-discrepant kidneys with a larger right kidney measuring 104 mm with an abnormal echostructure and reduced cortical–parenchymal differentiation and a normal left kidney measuring 88 mm—compatible with acute pyelonephritis of the right kidney. The study also showed an aneurysm of the left renal artery measuring 32×19.8 mm with distal haemodynamic repercussions (figure 1). This finding was confirmed by angio-CT scan (figures 2–4) and selective angiography (figure 5) that demonstrated a large saccular aneurysm of the left renal artery measuring 20×21×24 mm with parietal calcification and no signs of rupture confirmed it. The Tc99m-MAG3 renal isotope scan was also performed and revealed a completely functional right kidney with inexistent function of the entire left kidney (figure 6). During admission, cardiac ultrasound showed a normal ventricular function but with a concentric hypertrophied left ventricle, neurological examination remained normal and proteinuria returned to normal values. She was released after 18 days, completely asymptomatic, medicated with amlodipine (0.2 mg/kg/day) and captopril (3 mg/kg/day) but maintaining elevated blood pressure albeit stabilised with fewer fluctuations (120–135/62–95 mm Hg).
Figure 1.
Renal sonogram showing an aneurysm of the left renal artery measuring 32×19.8 mm.
Figure 2.

Angio-CT scan (horizontal plane): large saccular aneurysm of the left renal artery measuring 20×21×24 mm with parietal calcification.
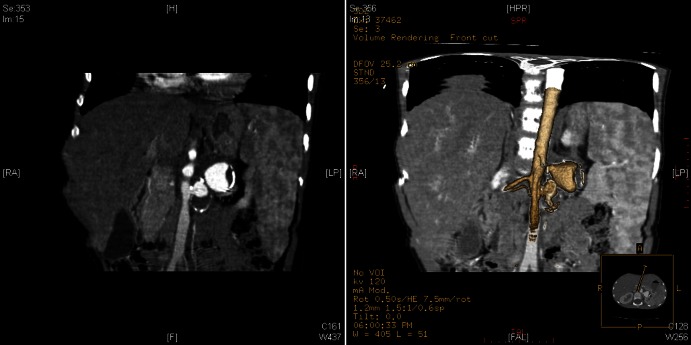
Figure 3.
Angio-CT scan (coronal plane): large saccular aneurysm of the left renal artery measuring 20×21×24 mm with parietal calcification.
Figure 4.

Three-dimensional angio-CT scan: large saccular aneurysm of the left renal artery with parietal calcification.
Figure 5.

Selective angiography revealing aneurysm of the left renal artery.
Figure 6.

Tc99m-MAG3 renal isotope scan: functional right kidney with inexistent function of the entire left kidney.
Since the aneurysm had large dimensions and the left kidney showed no function in the renal scan, angioplasty was felt to be inappropriate and thus a left nephrectomy was performed without removing the calcified aneurysm. The histological examination showed ‘morphological features consistent with atrophy, with glomerular hypoplasia and multifocal areas of congestion and stromal haemorrhage and of the glomerular component. These aspects are consistent with ischaemia’.
Outcome and follow-up
The child's blood pressure further normalised within a few days after surgery but with continuation of medication (amlodipine and enalapril replacing captopril). One month after surgery, she had ceased any antihypertensive therapy and her ambulatory blood pressure report revealed that 26% of all systolic blood pressure and 23% of all diastolic blood pressure exceeded the specific thresholds of 135/85 mm Hg while awake and 120/70 mm Hg while asleep, which represents a significant improvement for our patient.
Discussion
In children, hypertension is usually secondary to either renal parenchymal disease or renal artery lesions. Renal artery dilation as a cause of secondary hypertension is mostly due to diseases such as fibromuscular dysplasia or vasculitis.1 2 4 5 True aneurysms in childhood usually accompany connective tissue disease such as Marfan's syndrome, Ehlers-Danlos syndrome (EDS), Kawasaki disease, Takayasu's disease, coarctation of the aorta or arteritis.6 Idiopathic aneurysms are less common but can be multiple and are associates with aortic aneurysms.7
RAA are thought to develop secondary to hypertension with the weakness of the arterial wall close to bifurcations favouring the development of aneurysms at this site. However, RAA have been reported in children without hypertension6 suggesting that it might not always be a prerequisite for their development. Thus, the pathogenesis of renovascular hypertension in patients with RAA may be multifactorial and can coexist with other conditions such as renal artery stenosis. In children without stenosis, the most likely cause is altered renal flow from kinking or torsion, although distal parenchymal embolisation should be considered as a possible mechanism.3
Since RAA may develop into a larger size rapidly and potentially rupture, diagnosis and treatment should be undertaken as soon as possible.1 Renal Doppler ultrasonography is a good non-invasive method for evaluation of renal size and flow, but angiography remains the goldstandard for diagnosing aneurysms within the renal arterial system.8 Fortunately, rupture is unusual during childhood, but it represents a serious threat in adult life.1
The current trend is to manage RAA conservatively and endovascular treatment is the preferred method to preserve healthy renal tissue in hypertensive patients.3 Nevertheless, surgical intervention is indicated in refractory hypertension for which conservative treatment was unsuccessful. In the presented case, even with optimal therapy with amlodipine and captopril, the renovascular hypertension was not fully controlled and, after carrying out a MAG3 renal isotope scan that demonstrated a complete lack of renal function in the left kidney, a unilateral nephrectomy was performed, since the loss of renal tissue was inevitable.
A complete cure of arterial hypertension is obtained in up to 82% of patients submitted to nephrectomy therefore, surgery is still critical in the treatment of refractory renovascular hypertension in children, showing a favourable outcome and prognosis.9
Learning points.
Secondary hypertension is the most common form of hypertension in childhood.
Renal artery aneurysms (RAA) are rare.
RAA are commonly associated with hypertension, abdominal pain and haematuria.
When investigating secondary hypertension, Doppler ultrasonography or angiography should be performed.
Majority of patients with RAA require intervention since conservative treatment seldom results in normalisation of hypertension.
Acknowledgments
Dr Pedro Nunes and Dr Clara Abadesso, both from the Paediatric Intensive Care Unit of the Hospital Prof. Doutor Fernando Fonseca, also participated in the stabilization and follow-up of the patient as well as contributing in the daily discussion on therapeutic options and patient handling.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Robitaille P, Lord H, Dubois J, et al. A large unilateral renal artery aneurysm in a young child. Pediatr Radiol 2004;2013:253–5 [DOI] [PubMed] [Google Scholar]
- 2.Hobbs DJ, Barletta GM, Mowry JA, et al. Renovascular hypertension and intrarenal artery aneurysms in a preschool child. Pediatr Radiol 2009;2013:988–90 [DOI] [PubMed] [Google Scholar]
- 3.Gumustas S, Ciftci E, Bircan Z. Renal artery aneurysm in a hypertensive child treated by percutaneous coil embolization. Pediatr Radiol 2010;2013:1285–7 [DOI] [PubMed] [Google Scholar]
- 4.Oguzkurt L, Cekirge S, Balkanci F. Inferior suprarenal artery aneurysm in polyarteritis nodosa. Pediatr Radiol 1997;2013:234–5 [DOI] [PubMed] [Google Scholar]
- 5.McCulloch M, Andronikou S, Goddard E, et al. Angiographic features of 26 children with Takayasu's arteritis. Pediatr Radiol 2003;2013:230–5 [DOI] [PubMed] [Google Scholar]
- 6.Checinski P, Henschke J, Pawlak B, et al. Multiple aneurysms in childhood—case report and review of the literature. Eur J Vasc Endovasc Surg 2000;2013:108–10 [DOI] [PubMed] [Google Scholar]
- 7.Callicutt CS, Rush B, Eubanks T, et al. Idiopathic renal artery and infrarenal aortic aneurysms in a 6-year-old child: case report and literature review. J Vasc Surg 2005;2013:893–6 [DOI] [PubMed] [Google Scholar]
- 8.Bunchman TE, Walker HS, III, Joyce PF, et al. Sonographic evaluation of renal artery aneurysm in childhood. Pediatr Radiol 1991;2013:312–13 [DOI] [PubMed] [Google Scholar]
- 9.Lacombe M. Role of surgery in the treatment of renovascular hypertension in the child. Bull Acad Natl Med 2003;2013:1081–93; discussion 93–4 [PubMed] [Google Scholar]