Abstract
A 29-year-old Caucasian man presented for the evaluation of a new onset of shortness of breath associated with cough and wheeze for 1 day. The history was significant for a recent travel of 20 h duration to Houston, a new onset of cigarette smoking for 2 weeks and marijuana smoking. The patient was afebrile and did not have any leg swelling; initial diagnosis of community-acquired pneumonia was made and the patient was started on antibiotics. Despite being on antibiotics, his medical condition continued to deteriorate and extensive diagnostic workup for infectious and autoimmune aetiology including bronchoalveolar lavage was completed and was inconclusive. Ultimately, the patient underwent video-assisted thoracoscopic lung biopsy which led to the diagnosis of acute eosinophilic pneumonia. Steroids were started with a good treatment response. The patient was discharged on a tapering dose of steroids; a follow-up chest x ray at 6 weeks was within normal limits.
Background
Acute eosinophilic pneumonia (AEP) is a rare condition. The exact aetiology for AEP is not known, a new onset of cigarette smoking or a change in the cigarette smoking pattern has been suggested to be associated with AEP. AEP owing to concomitant marijuana use and cigarette smoking has been described only once in the medical literature. We describe an interesting case of a young man who developed AEP due to concomitant marijuana and cigarette smoking.
Case presentation
A 29-year-old Caucasian man with a medical history of anxiety disorder was admitted to the hospital for the evaluation of shortness of breath (SOB) associated with non-productive cough and wheezing for 1 day. SOB was sudden in the onset and progressively got worse. There was no history of fever, chills, recent upper respiratory tract infection, rash, pedal oedema or chest pain. The rest of review of symptoms was positive only for generalised body aches for 1 day. The patient had a negative HIV test 1 month prior to the presentation. The social history was significant for the history of smoking about one pack every 3 days for the last 2 weeks (first time smoker), smoking marijuana on two occasions (smoked once 3 months ago and once 1 day prior to the presentation) and social drinking. The patient was a heterosexual male with no history of any sexually transmitted diseases in the past. The patient did not have any exposure to inhalational toxins (works as a waiter in a restaurant). The other significant history included a recent travel of 20 h duration to Houston on a car, 3 days prior to admission. At the time of presentation, the patient was in mild distress; the vital signs were blood pressure of 119/55 mm Hg, pulse 93 bpm, oxygen saturation was 85% on room air (improved to 95% on 3 l/min oxygen through nasal cannula, initial arterial blood gas (ABG) was not performed), temperature 98.6F (37°C) and respiratory rate of 18 breaths/min. Physical examination findings were positive for a slightly diminished air movement over the right lung fields without any crackles or wheezes. The patient was 5 feet 11 inches tall and weighed about 190 pounds. Initial diagnostic work up revealed a leucocytosis of 20.9 K/mm3 (4.2–11 K/mm3) with predominant neurtrophilia; the chest x-ray was positive for the possible right upper pulmonary lobe infiltrates (figure 1A). The patient was presumed to have community-acquired pneumonia; blood cultures and sputum cultures were sent, and intravenous levofloxacin and therapeutic fondaparinux were started for concerns of pulmonary embolism. Initial urine toxicology screen was positive for cannabinoids and opiates. A CT scan of the chest with intravenous contrast was negative for any pulmonary embolism (figure 1C); therapeutic anticoagulation was switched to prophylactic dosage. By the second day of admission, the leucocyte count increased to 29.4 K/mm3, piperacillin-tazobactam to broaden the antimicrobial coverage (one time dose given only), and trimethoprim-sulfamethoxazole (TMP-SX) along with methylprednisolone (one time dose given for each) for concerns of Pneumocystis jirovecii (PJP) were started, but were discontinued immediately by the infectious disease team as the patient was deemed not to be at risk for PJP infection. During evening of day 2, the patient's medical condition worsened, an ABG obtained on 5-liters of oxygen flow through nasal cannula revealed a PH of 7.33, pCO2 of 42, pO2 of 123.3 and HCO3 of 22. On the night of day 3, the patient was found to be tachyponeic with a respiratory rate of 28 per minute and with oxygen saturation in lower 80s, despite being on 5 l of oxygen through nasal cannula. The patient was placed on a 100% non-rebreather (NRB) mask and was transferred to the intensive care unit for closer monitoring. An arterial blood gas was obtained while on 100% NRB with an oxygen flow rate of 15 l/min and showed PO2 of 80 mm Hg, PCO2 32 mm Hg, PH 7.45 and HCO3 22 meq; vancomycin and oseltamivir were added along with continued levofloxacin treatment to broaden the antimicrobial coverage. Diagnostic bronchoalveolar lavage (BAL) was attempted on day 3, but had to be discontinued prematurely due to a marked drop in oxygen saturation to upper 70s. The BAL fluid obtained was hazy in colour, had a white blood cell count of 494/mm3 (<300/mm3) with only 6% eosinophils and with no malignant cells were seen on cytology. The BAL fluid bacterial, fungal and acid-fast bacilli (AFB) cultures showed no growth. Transthoracic echocardiogram was done and was negative for any intracardiac shunt or any other obvious cardiac pathology. A comprehensive diagnostic workup, including rapid influenza/respiratory syncytial virus screen, influenza by PCR, legionella urine antigen, Chlamydia pneumoniae by PCR, sputum AFB and fungal cultures, blood cultures, serum mycoplasma and cytomegalovirus titres, HIV PCR, coxsackie antibodies, parvovirus B19 PCR, cryptococcal serum antigen, urine blastomyces and histoplasma antigen tests, erythrocyte sedimentation rate, C reactive protein, antineutrophil antibody, rheumatoid factor, myeloperoxidase antibody, anti-neutrophil-cytoplasmic antibodies targeting proteinase 3, was carried out and did not yield any specific infectious or autoimmune diagnosis. A repeat CT scan of the chest at day 7 showed large bilateral pleural effusions (figure 1E), diagnostic thoracentesis was carried out at day 8. Pleural fluid analysis results were consistent with exudative effusion; no specific diagnosis could be established on pleural fluid analysis and cultures. The patient underwent a diagnostic video-assisted thoracoscopic lung biopsy at day 8. Histological examination of the biopsy specimen showed acute bronchopneumonia and interstitial pneumonitis with marked eosinophilic infiltrates in both alveolar spaces as well as in interstitium confirming the diagnosis of AEP (figure 2). AFB smear and culture, bacterial culture and fungal culture of the biopsy specimen did not yield any infective aetiology. Methylprednisolone 60 mg intravenous twice daily dose was started and the antibiotics were discontinued. The patient had a good treatment response and was discharged at day 15 on a tapering dose of steroid. A follow-up chest x-ray 6 weeks later showed a complete resolution of the lung infiltrates and pleural effusions (figure 1F). Six months after the episode of AEP, the patient had restarted cigarette smoking without any further recurrence of AEP. The patient was strictly advised to quit smoking and an educational material on the hazards of cigarette smoking and help for cigarette smoking cessation was offered, but the patient refused to quit.
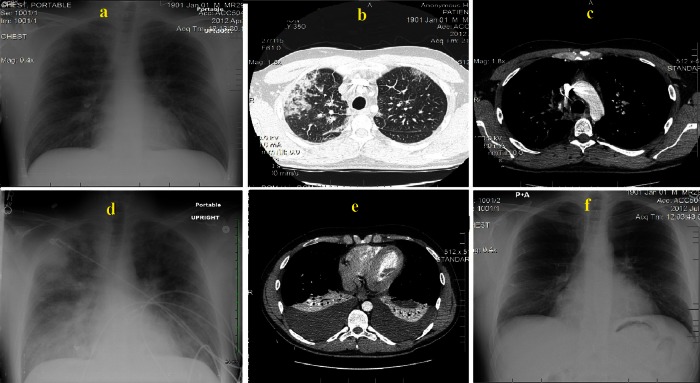
Figure 1.
(A) Portable chest x-ray (CXR) at time of presentation and shows possible right upper lobe pneumonia. (B and C) CT chest with intravenous contrast at day of presentation, (B) infiltrates in right upper lung, (C) absence of pulmonary emolism. (D) Portable CXR 3 days after admission and shows diffuse bilateral alveolar infiltrates, (E) CT scan done at day 7 and shows massive bilateral pleural effusions. (F) A normal follow-up posterio-anterior CXR done at 6 weeks.
Figure 2.

Alveolar and interstitial eosinophilic infiltrates, features consistent with acute eosinophilic pneumonia are shown.
Discussion
AEP is a rare condition with a reported incidence rate of 9.1 cases per 100 000 person-years.1 It is usually seen in an age group of 13–49 years2 with men being affected more commonly.1 The exact aetiology of AEP is unknown; a relationship between cigarette smoking and AEP has been suggested.1–5 In the study carried out by Uchiyama et al,4 around 70% of the patients who developed AEP just began smoking and AEP was induced by cigarette smoking provocation test in 100% of the cases.
AEP caused due to concomitant smoking with marijuana is very rare and to the best of our knowledge there has been only one other case reported so far.5 Sauvaget et al described AEP in a teenager with concomitant cigarette and cannabis smoking (cannabis smoked once 3 months ago and once few hours before the onset of respiratory symptoms). Our patient also smoked marijuana twice, the first time 3 months ago and the second time 1 day prior to the presentation. However, whether marijuana alone or concomitantly with cigarette smoking-induced AEP in our patient is difficult to establish.
Cigarette smoke has been proposed to stimulate lung macrophages by cytokine-mediated inflammatory response and lead to AEP.6 Additionally, high levels of interlukin-1and interlukin-5 have been found in BAL of patients with AEP. Shintani et al7 suggested that patients develop tolerance to further cigarette smoking-associated AEP after an initial episode and our case confirms their finding. Our patient at a follow-up visit at 6 months had restarted cigarette smoking for over a month without any further recurrence of AEP symptoms.
The main characteristic features of AEP include the acute onset of febrile respiratory illness with duration usually less than 7 days; hypoxaemia—diffuse bilateral infiltrates with peripheral predominance and pleural effusions; pulmonary eosinophilia with more than 25% eosinophils in BAL fluid or eosinophilic pneumonia at lung biopsy; absence of exposure to drugs or any other known causes of eosinophilic lung disease.3 CT scan findings of AEP are characterised by bilateral patchy ground glass or reticular opacities with a peripheral predominance.8 In our patient, the diagnosis of AEP was delayed for a week; there is a slight possibility that AEP might have been secondary to medications/radio-contrast given during the course of stay, but the classic-appearing radiographic findings of interlobular septal thickening, ground glass opacification and predominant peripheral patchy areas of consolidation seen on CT chest at day 1 (figure 1B) strongly argue against that and make a strong case of considering AEP in differentials when the aforementioned radiographic findings are seen in the appropriate clinical context.
Histopathological features of AEP include marked infiltration of eosinophils in the interstitium and alveolar spaces. Additional features include diffuse alveolar damage with hyaline membranes, fibroblast proliferation, fibrinous exudates and inflammatory cells.9
AEP often resolves spontaneously, rapidly progressing cases require corticosteroid therapy.10 Corticosteroid therapy is the mainstay in treating AEP.11 Jantz et al12 recommend the use of intravenous methylprednisolone at an initial dose of 60–125 mg every 6 h and then switching to oral prednisone at a dose of 40–60 mg a day, tapered over 2–6 weeks period depending on the clinical course.
Recovery in cases of AEP is usually complete with no long-term pulmonary symptoms and no residual radiological features. Relapse after steroid taper is extremely rare.10
Learning points.
Acute eosinophilic pneumonia (AEP) should be suspected in new onset cigarette smokers or in patients with a recent change in cigarette smoking habits who present with a new onset of shortness of breath.
Steroid should be considered early in the suspected cases of AEP.
Marijuana smoking alone or concomitant with cigarette smoking may cause AEP.
Diagnosis of AEP should be considered in differentials when there is a radiographic evidence of interlobular septal thickening, ground glass opacification with predominant peripheral patchy areas of consolidation in an appropriate clinical context.
Footnotes
Contributors: All authors contributed equally to this manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Shorr AF, Scoville SL, Cersovsky SB, et al. Acute eosinophilic pneumonia among US Military personnel deployed in or near Iraq. JAMA 2004;2013:2997–3005 [DOI] [PubMed] [Google Scholar]
- 2.Philit F, Etienne-Mastroianni B, Parrot A, et al. Idiopathic acute eosinophilic pneumonia: a study of 22 patients. Am J Respir Crit Care Med 2002;2013:1235–9 [DOI] [PubMed] [Google Scholar]
- 3.Pope-Harman AL, Davis WB, Allen ED, et al. Acute eosinophilic pneumonia. A summary of 15 cases and review the literature. Medicine 1996;2013:334–42 [DOI] [PubMed] [Google Scholar]
- 4.Uchiyama H, Suda T, Nakamura Y, et al. Alterations in smoking habits are associated with acute eosinophilic pneumonia. Chest 2008;2013:1174–80 [DOI] [PubMed] [Google Scholar]
- 5.Sauvaget E, Dellamonica J, Arlaud K, et al. Idiopathic acute eosinophilic pneumonia requiring ECMO in a teenager smoking tobacco and cannabis. Pediatr Pulmonol 2010;2013:1246–9 [DOI] [PubMed] [Google Scholar]
- 6.Tappia PS, Troughton KL, Langley-Evans SC, et al. Cigarette smoking influences cytokine production and antioxidant defences. Clin Sci (Lond) 1995;2013:485–9 [DOI] [PubMed] [Google Scholar]
- 7.Shintani H, Fujimura M, Yasui M, et al. Acute eosinophilic pneumonia caused by cigarette smoking. Intern Med 2000;2013:66–8 [DOI] [PubMed] [Google Scholar]
- 8.King MA, Pope-Harman AL, Allen JN, et al. Acute eosinophilic pneumonia: radiologic and clinical features. Radiology 1997;2013:715–19 [DOI] [PubMed] [Google Scholar]
- 9.Tzaelaar HD, Linz LJ, Colby TV, et al. Acute eosinophilic pneumonia: histopathological findings in nine patients. Am J Respir Crit Care 1997;2013:296–302 [DOI] [PubMed] [Google Scholar]
- 10.Umeki S. Reevaluation of eosinophilic pneumonia and its diagnostic criteria. Arch Intern Med 1992;2013:1913–19 [PubMed] [Google Scholar]
- 11.Allen J. Acute eosinophilic pneumonia. Semin Respir Crit Care Med 2006;2013:142–7 [DOI] [PubMed] [Google Scholar]
- 12.Jantz MA, Sahn SA. Corticosteroids in acute respiratory failure. Am J Respir Crit Care Med 1999;2013:1079–100 [DOI] [PubMed] [Google Scholar]