Abstract
A 62-year-old man underwent pancreaticoduodenectomy (PD) for intraductal papillary mucinous carcinoma (IPMC) in 2006. No signs of adenocarcinoma at the resection margin were found by intraoperative pathological examination of frozen sections. The postoperative pathological diagnosis was invasive carcinoma derived from IPMC and moderately differentiated tubular adenocarcinoma. A blood analysis in 2011 showed serum (CA19-9) to be increased since the initial resection. Imaging test showed a recurrent tumour at the site of the pancreaticogastrostomy (PG) in the remnant pancreas. We conducted total remnant pancreatectomy for recurrent IPMC and partial gastrectomy. Because both lesions had a histopathological resemblance, the pathological diagnosis was recurrent invasive IPMC. Based on this experience, it is important to facilitate early detection by annual check-up. And also, we recommend PG as a reconstructive intervention in patients at high risk of IPMC recurrence in the remnant pancreas following PD as it is grossly visible on upper gastrointestinal endoscopy.
Background
Intraductal papillary mucinous neoplasms (IPMNs) were defined by the WHO in 2000 as intraductal mucin-producing neoplasms that can involve the main pancreatic duct but may also affect the side branches, or a combination of the two.1 WHO also established four categories of IPMNs: IPMNs with slight or no dysplasia as adenoma (IPMA), IPMNs with moderate dysplasia as borderline neoplasm (IPMB), IPMNs with severe dysplastic epithelial change without invasion as carcinoma in situ (CIS) and invasive carcinoma derived from IPMN (invasive IPMC).2
According to the absence or presence of neoplastic cells invading the pancreatic tissue surrounding the involved ducts, IPMC are separated into invasive and non-invasive types. Here we present a case of recurrent IPMC that arose in the remnant pancreas of PD for IPMC.
Case presentation
A 62-year-old man presented at our hospital in December 2005 to undergo a medical check-up. His medical history included diabetes mellitus, but he had no symptoms. A medical check-up in April 1999 had revealed a 15-mm pancreatic cyst which was diagnosed as benign, so he was followed closely without treatment. Since then, he underwent annual medical check-ups. The size of the cyst grew to 30 mm, and abdominal ultrasonography (AUS) showed dilation of the main pancreatic duct at the patient's annual medical check-up in 2006. AUS revealed that the pancreatic duct had enlarged to 9 mm in diameter, with an irregular outline of the duct lumen and a 30-mm tumour in the pancreatic head. Similarly, endoscopic ultrasonography (EUS) also showed a 30-mm tumour in the pancreatic head. Abdominal enhanced CT (figure 1) showed a high-density area in the head of the pancreas and dilatation of the main pancreatic duct (9 mm). The 30-mm tumour in the pancreatic head showed low signal intensity on T1-weighted MRI and high intensity on T2-weighted MRI.
Figure 1.

Contrast-enhanced CT showed a high-density area in the head of the pancreas and dilation of the main pancreatic duct (9 mm).
Since IPMC or main duct IPMN was suspected, we performed pylorus-preserving pancreaticoduodenectomy (PD) in March 2006. Histological examination showed invasive carcinoma derived from intraductal papillary carcinoma in the pancreatic head (T2, ly1, v0, ne0, mpd-, s0, rp-, ch-, du0, pv0, a0, pw-, bdw-, ew-, N0, M0) (figure 2). Hyperplastic change was found at the cut margin of the pancreas, but the cut margin was negative. The patient recovered without any serious complications and continued to undergo routine follow-up examination such as ultrasonography every 6 months and annual dynamic CT scan at the outpatient clinic.
Figure 2.

Histological examination showed invasive carcinoma derived from intraductal papillary carcinoma in the pancreatic head (T2, ly1, v0, ne0, mpd-, s0, rp-, ch-, du0, pv0, a0, pw-, bdw-, ew-, N0, M0).
Five years later (February 2011), (CA19-9) values had gradually elevated to 114 IU/ml. Positron emission tomography (PET) lead to an abnormal accumulation of fluorodeoxyglucose in the remnant pancreas (SUV max 3.5→3.8) (figure 3). AUS and EUS showed a 22-mm tumour in the remnant pancreas. Similarly, abdominal enhanced CT showed a 22-mm high-density area in the remnant pancreas (figure 4). The 22-mm tumour in the remnant pancreas and the anastomosis site of pancreaticogastrostomy (PG) showed low intensity on T1-weighted MRI, high intensity on T2-weighted MRI and high intensity on diffusion-weighted MRI. Upper gastrointestinal endoscopy and magnified endoscopy with narrow band imaging showed redness and an easily bleeding elevation lesion at the anastomosis site of PG (figure 5). As IPMC recurrence was suspected, total pancreatectomy of the remnant pancreas and partial gastrectomy was performed in May 2011 (figure 6). Histological examination showed invasive IPMC in the remnant pancreas (T2, ly0, v0, ne0, mpd+, s0, rp1, a0, pl-, ew-, N0, M0) (figure 7).
Figure 3.

Positron emission tomography (PET) lead to an abnormal accumulation of fluorodeoxyglucose in the remnant pancreas (SUV max 3.5→3.8).
Figure 4.

Contrast-enhanced CT showed a 22-mm high-density area in the remnant pancreas.
Figure 5.
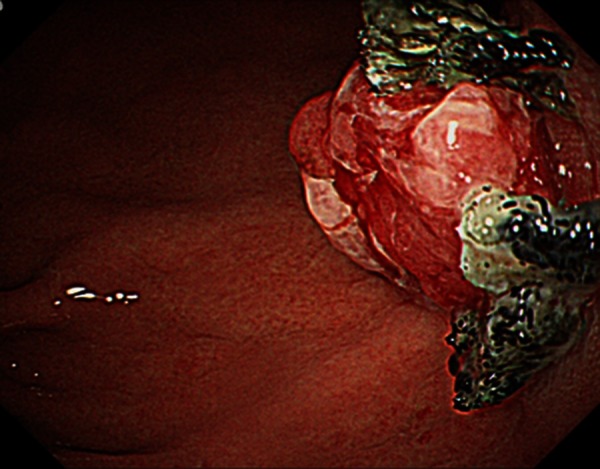
Upper gastrointestinal endoscopy showed redness and an easily bleeding elevation lesion at the anastomosis site of pancreaticogastrostomy (PG).
Figure 6.
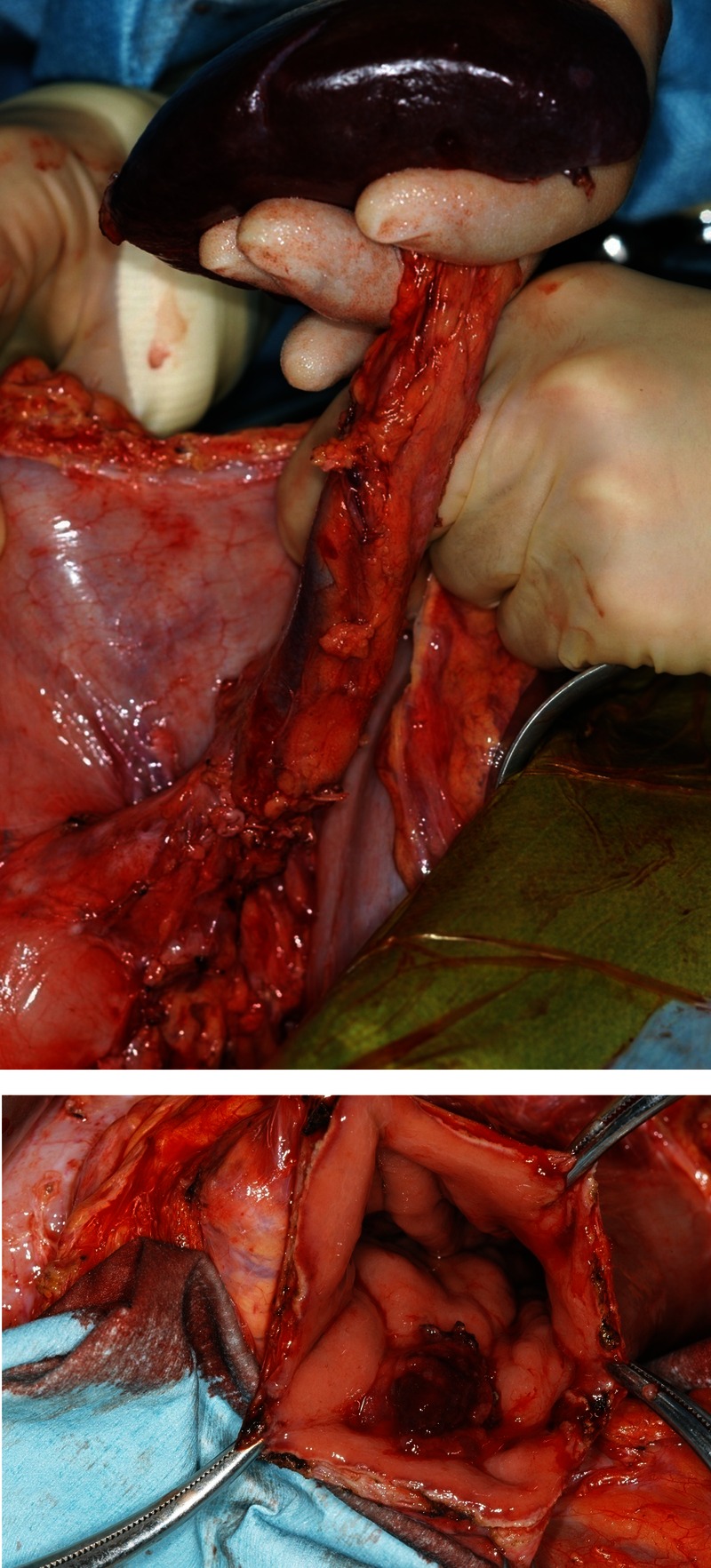
Total pancreatectomy of the remnant pancreas and partial gastrectomy was performed.
Figure 7.

Histological examination showed invasive intraductal papillary mucinous carcinoma in the remnant pancreas (T2, ly0, v0, ne0, mpd+, s0, rp1, a0, pl-, ew-, N0, M0). The main pancreatic duct became dilated, and immunochemistry was performed (MUC1(+), MUC2(–), MUC5AC(–), MUC6(+)).
The main pancreatic duct was dilated, and immunochemistry was performed (MUC1(+), MUC2(−), MUC5AC(−), and MUC6(+)). As both pathological findings were very similar, we regarded the second lesion as recurrent IPMC.
Brian M Alexander3 reported that adjuvant chemoradiotherapy was associated with improved overall and cancer-specific survival for patients with advanced disease. The patient is given adjuvant chemotherapy after second operation.
Since the second operation, the patient is alive with no signs of cancer recurrence.
Discussion
IPMN is characterised by the dilation of the main or branch ducts of the pancreas that contain a thick mucoid secretion, and this condition is classified as main-duct or branch-duct IPMN, based on imaging and histology modalities.4 IPMN shows various types of histological changes, such as hyperplasia, adenoma, adenocarcinoma and invasive cancer. PD remains the standard procedure for dealing with tumours arising in the pancreatic head, and in this procedure, the cut end of the pancreas is usually anastomosed to either the jejunum (pancreaticojejunostomy: PJ) or the stomach (PG).5
Recently, several cases of IPMC relapse have been reported following radical surgery for IPMN or IPMC (6–17). The incidence of disease recurrence in the pancreatic remnants or pancreatic beds following surgical removal of IPMC has been reported as 12% (n=13/113) by Chari et al,6 8% (n=4/51) by Falconi et al,7 26% (n=16/62) by Maire et al,8 12% (n=10/85) by Partelli et al,9 7% (n=8/114) by Salvia et al,10 and 50% (n=6/12) by Sho et al.11
The excision ratio has been reported as 15% (n=2/13) by Chari et al,6 100% (n=4/4) by Falconi et al,7 31% (n=5/16) by Maire et al,8 63% (n=5/8) by Salvia et al10 and 17% (n=1/6) by Sho al11 Averaging these results shows that recurrent tumours can be excised in only 36% (n=17/47) of cases. For the other 64% (n=30/47) of cases, excision was not possible when discovering the recurrence of IPMC because it had already progressed. If recurrent IPMC can be found earlier, it can be excised, and radical surgery is increasingly likely. To discover recurrent IPMC earlier, routine medical check-ups must be performed after the operation. Chiari et al6 reported that the mean interval between resection and diagnosis of recurrence was 18±3 months; 70% of recurrence cases occurred within 2 years and 91% within 3 years of resection. Sho et al11 reported that the median postoperative disease-free interval was 38 months. According to multiple reports, recurrence in the majority of cases is within 3 years after operation. In addition, in many cases, tumour recurrence is discovered by AUS because it is possible to do so easily, and the procedure has low-invasiveness.
Presently, PD is a standard therapy for IPMC of the pancreas head. According to multiple reports on surgery for IPMC of the pancreas head, PJ was performed in many cases. Makni et al12 reported no significant differences between PJ and PG in the overall postoperative complication rate, incidence of postoperative haemorrhage, biliary fistula, acute pancreatitis and delayed gastric emptying. However, Tomimaru et al5 recommend PG for all patients deemed to be at high risk for the recurrence of cancer in the pancreatic remnants following PD for IPMC of the pancreatic head. In the present case, we decided to proceed with PG rather than PJ to facilitate easier postoperative examination, because it was grossly visible on upper gastrointestinal endoscopy. If we perform PG after PD, a tumour at the site of PG can be seen, which can increase the chance of detection in postoperative routine medical check-ups.
In conclusion, we completely cured a recurrent IPMC that arose in the remnant pancreas following PD for IPMC, so earlier detection by means of annual check-ups, periodical upper gastrointestinal endoscopy, CT, PET-CT and AUS is important. Furthermore, we recommend PG as a reconstructive modality for patients at high risk of IPMC recurrence in the remnant pancreas following PD as it is grossly visible on upper gastrointestinal endoscopy.
Learning points.
Intraductal papillary mucinous carcinoma
Pancreaticoduodenectomy
Recurrence
Pancreaticogastrostomy
Footnotes
Contributors: YO analysed and interpreted the data; drafted the article; critically revised the article for important intellectual content; and final approval of the article. YO, KS, MM and MH were involved in provision of study materials or patients.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Klöppel G, Lüttges J. WHO-classification 2000: exocrine pancreatic tumors. Verh Dtsch Ges Pathol 2001;2013:219–28 [PubMed] [Google Scholar]
- 2.Longnecker DS, Adler G, Hruban RH, et al. Intraductal papillary-mucinous neoplasms of the pancreas. In: Hamilton SA, Aaltonen LA, eds. World Health Organization classification ot tumours: pathology and genetics of tumours of the digestive system. Lyon (France): IARC Press; 2000:219.2013 [Google Scholar]
- 3.Alexander BM, Femandez-del Castillo C, Ryan DP, et al. Intraductal papillary mucinous adenocarcinoma of the pancreas: clinical outcomes, prognostic factors, and the role of adjuvant therapy. Gastrointest Cancer Res 2011;2013:116–21 [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006;2013:17–32 [DOI] [PubMed] [Google Scholar]
- 5.Tomimaru Y, Ishikawa O, Ohigashi H, et al. Advantage of pancreaticogastrostomy in detecting recurrent intraductal papillary mucinous carcinoma in the remnant pancreas: a case of successful re-resection after pancreaticoduodenectomy. J Surg Oncol 2006;2013:511–15 [DOI] [PubMed] [Google Scholar]
- 6.Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology 2002;2013:1500–7 [DOI] [PubMed] [Google Scholar]
- 7.Falconi M, Salvia R, Bassi C, et al. Clinicopathological features and treatment of intraductal papillary mucinous tumour of the pancreas. Br J Surg 2001;2013: 376–81 [DOI] [PubMed] [Google Scholar]
- 8.Maire F, Hammel P, Terris B, et al. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut 2002;2013:717–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Partelli S, Fernandez-Del Castillo C, Bassi C, et al. Invasive intraductal papillary mucinous carcinomas of the pancreas: predictors of survival and the role of lymph node ratio. Ann Surg 2010;2013:477–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvia R, Fernández-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg 2004;2013:678–85; discussion 685–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sho M, Nakajima Y, Kanehiro H, et al. Pattern of recurrence after resection for intraductal papillary mucinous tumors of the pancreas. World J Surg 1998;2013:874–8 [DOI] [PubMed] [Google Scholar]
- 12.Makni A, Bedioui H, Jouini M, et al. Pancreaticojejunostomy vs. pancreaticogastrostomy following pancreaticoduodenectomy: results of comparative study. Minerva Chir 2011;2013:295–302 [PubMed] [Google Scholar]


