Abstract
Vitamin D deficiency, once thought to be eradicated, is becoming a frequent occurence in children, caused mainly by dietary insufficiency. The classical manifestation is rickets, but in infants severe hypocalcaemia may present as stridor, tetany, seizures or, rarely, heart disease. Here, we describe four infants who presented with complications of severe hypocalcaemia secondary to nutritional vitamin D deficiency. (1) Female, 4 months old, several spasms. (2) Male, 8 days old, generalised tonic-clonic seizure. (3) Male, 9 months old, tetany. (4) Male, 4 months old, cardiogenic shock. The cases highlight the importance of child vitamin D supplementation from birth and throughout childhood. We also note that the vitamin D state should be evaluated by the 25(OH)-D value and not the 1,25(OH)2-D.
Background
In the past, nutritional vitamin D deficiency almost disappeared in industrialised countries, once it was discovered that exposure to sunlight and easily available supplements could prevent and treat rickets. However, in the last few years a reappearance of this disease has been observed. Dietary insufficiency is the main cause, especially in those who are dark-skinned or exclusively breastfed infants or those born to mothers who have been vitamin D deficient throughout pregnancy, or in premature infants. Other important aetiological factors are low sun exposure (especially in winter months and in some ethnic minorities where women wear traditional clothing covering their entire skin) and the generalised use of sunscreen.1–8
Vitamin D has been recognised to be important not only on calcium/phosphorus metabolism, but also as an immunomodulator, playing a major role in the prevention of autoimmune diseases, cancer and also psychiatric conditions.3 5 8–11
In children, the classic manifestation of vitamin D deficiency is rickets. However, in infancy, because of increased growth velocity and calcium demands, there is a higher risk of severe hypocalcaemia, that may present as stridor, tetany, seizures or, rarely, cardiomyopathy.4 5 7 12–14
Current recommendations consider plasma levels of 25-hydroxyvitamin D (25(OH)-D) ≤10 ng/dl (25 nmol/l) to be deficient, 10–20 ng/dl (25–50 nmol/l) insufficient and ≥20 ng/dl (50 nmol/l) sufficient. Cases of deficiency also reveal normal or low plasma calcium, normal or low plasma phosphate, elevated parathyroid hormone (PTH), due to secondary hyperparathyroidism and elevated alkaline phosphatase (ALP), caused by calcium mobilisation from bone. Levels of 1,25(OH)2-Vitamin D (1,25(OH)2-D) may become elevated as PTH levels rise, stimulating 1α-hidroxilase activity. Therefore, levels of 1,25(OH)2-D are not a good indicator of vitamin D status. Here we report four cases of severe hypocalcaemia in infants due to hypovitaminosis D, which show the importance of vitamin D supplementation and the need of awareness for this ‘old new’ disease that can be fatal but can be prevented.3 5–8 13 15–17
Case presentation
Case 1: Female infant, 4 months old, from a healthy unrelated-Pakistani family. The mother wore traditional clothing covering her entire skin during the pregnancy. Her pregnancy was not monitored by a health professional or supplemented with any vitamins. The baby was born in Lisbon, full-term, normal delivery, APGAR score (AS) 9/10 and she was an appropriate weight for gestational age. The baby was exclusively breastfed in the first month of life and afterwards fed with low-fat cow's milk, and was not given any supplements. At the age of 4 months, she was brought to the emergency department with infantile spasms (several per minute) and a fixed gaze.
Case 2: Male infant, 8 days of age, also from a healthy unrelated-Pakistani family. The mother also wore traditional clothing covering her entire skin throughout the pregnancy. The pregnancy was monitored in Pakistan and the baby was not given any vitamins. He was born in Lisbon, full-term, distocic (forceps) delivery, AS 9/10 and he was an appropriate weight for gestational age. He was fed with breast milk and formula from day 1, without any supplements. By day 8, he presented with a generalised tonic-clonic seizure.
Case 3: Male infant, 9 months old, from a deaf HbAS carrier mother and drepanocitic father, both from Guinea-Bissau and dark-skinned. The pregancy was monitored at a Lisbon hospital, but also not supplemented with any vitamins. The male baby was born in the same hospital, full-term, normal delivery, AS 9/10 and was large for gestational age (53 cm and 4.2 kg/9.2 lb). He was exclusively breastfed for 5 months, starting complementary feeding at that age; he did not receive any supplements. At the time he was 9 months old, he presented with tetany (with positive Chvostek sign), showing an evident widening of both wrists (figure 1).
Figure 1.
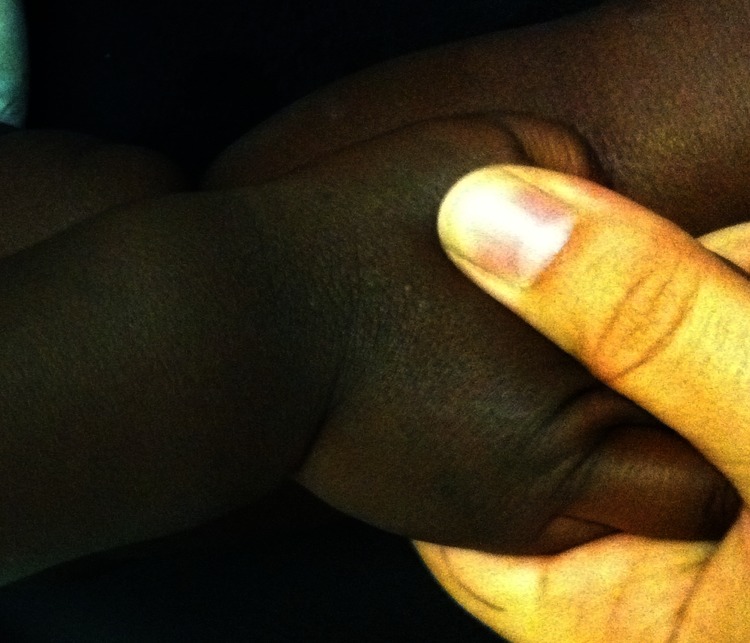
Widening of the wrist (case 3).
Case 4: Male infant, 4 months old, from a healthy unrelated family, natural from Guinea-Bissau, dark-skinned. Pregnancy was monitored in Lisbon and not supplemented with vitamins. He was born in Lisbon, full-term, normal delivery, AS 9/10 and he was an appropriate weight for gestational age. He was exclusively breastfed and was given no supplements. At the age of 3 months, he presented with cardiogenic shock.
Investigations
Case 1: Very low total calcium, normal phosphate, elevated PTH, elevated ALP, low 25(OH)-D and normal 1,25(OH)2-D (table 1). Plasma sodium (138 mmol/l), magnesium (1.8 mg/dl), potassium (4.7 mmol/l) and glycaemia (103 mg/dl) were normal. C reactive protein, complete blood count and blood gases were also in the reference ranges.
Table 1.
Laboratory evaluation
| Initial evaluation | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Ca T (8.3–11.4 mg/dl) | 4.8 | 6.0 | 6.4 | 5.6 |
| P (3.1–6.8 mg/dl) | 6.6 | 7.8 | 3.1 | 6.2 |
| PTH (11–67 pg/ml) | 325 | 87 | 314 | 232 |
| ALP (82–383 UI/l) | 920 | 371 | 1222 | 422 |
| 25OH-D (30–80 ng/dl) | 9.8 | 8.0 | 8.9 | 5.9 |
| 1,25OH-D (20–46 ng/dl) | 42.9 | 24.2 | 97.2 | 18.6 |
| After 1–2 months of vitamin D | ||||
| Ca T | 10.5 | 10.5 | 10.4 | 9.1 |
| PTH | 39.7 | 27.6 | 152 | 61.6 |
| ALP | 262 | 330 | 330 | 107 |
| 25OH-D | 68.5 | 30.9 | 23.3 | 38.61 |
ALP, alkaline phosphatase; PTH, parathyroid hormone.
Skeletal x-rays showed widening and cupping of the metaphyses of long bones (figure 2). EEG was normal according to age.
Figure 2.
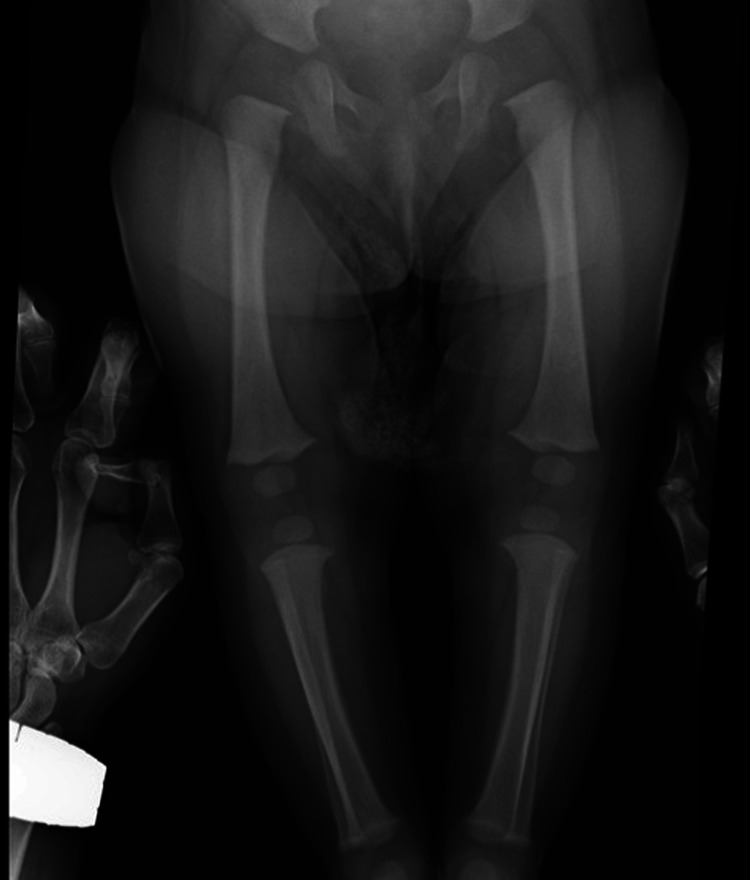
Widening and cupping of long bones (case 1).
Case 2: Very low total calcium, elevated phosphate, elevated PTH, normal ALP, low 25(OH)-D and normal 1,25(OH)2-D (table 1). Plasma sodium (139 mmol/l), magnesium (1,4 mg/dl), potassium (5 mmol/l) and glycaemia (94 mg/dl) were normal. C reactive protein, complete blood count, blood gases and lumbar puncture were also in the reference ranges.
The first EEG showed paroxistic bilateral frontal activity with normal baseline. Skeletal x-rays, cranial ultrasound and cranioencephalic MRI were normal.
Case 3: Very low total calcium, normal phosphate, elevated PTH, elevated ALP, low 25(OH)-D and elevated 1,25(OH)2-D (table 1).
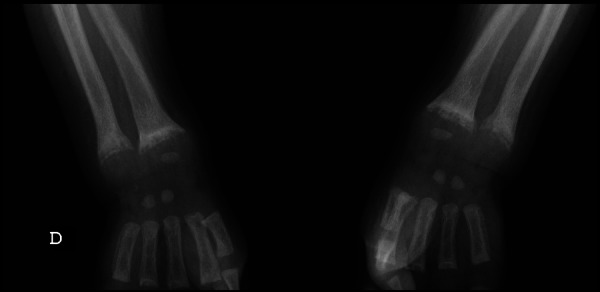
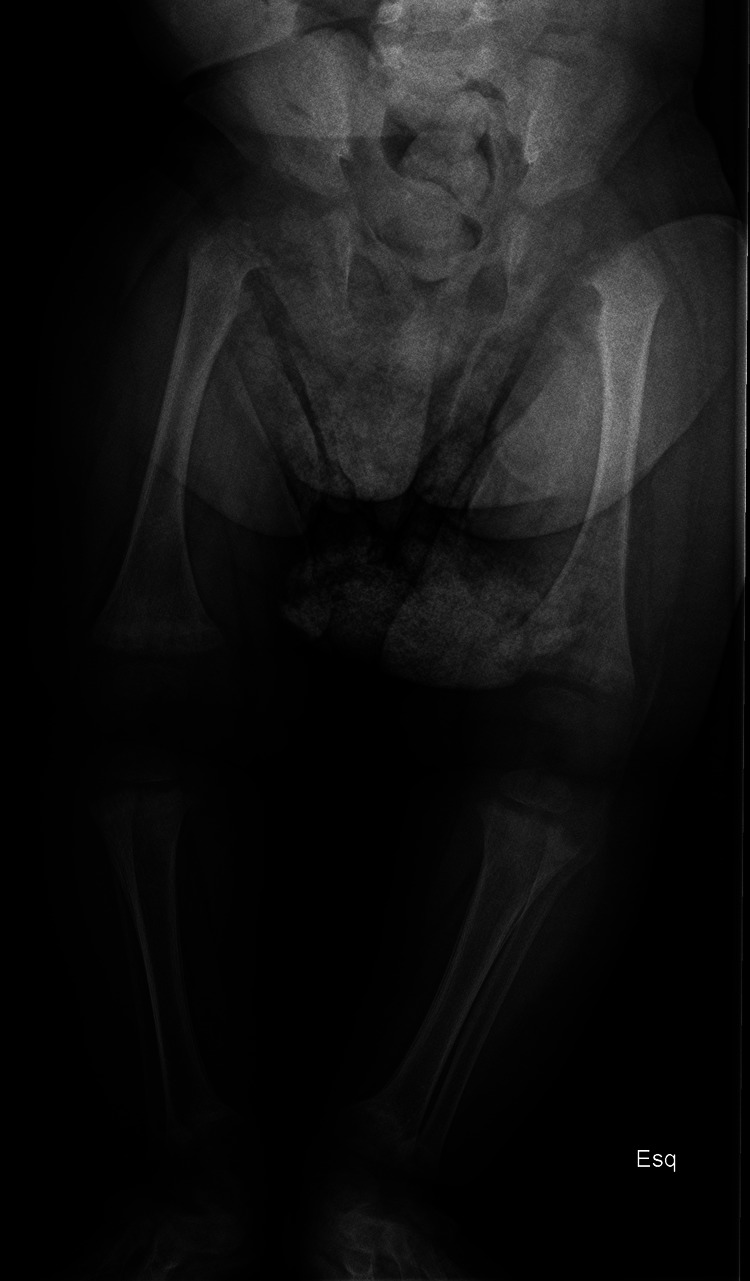
Skeletal x-rays showed widening, fraying, cupping and demineralisation of the metaphyses of long bones (figures 3 and 4).
Figure 3.
Widening, fraying, cupping and demineralisation of the metaphyses of long bones (case 3).
Figure 4.
Widening, fraying, cupping and demineralisation of the metaphyses of long bones (case 3).
Case 4: Very low total calcium, normal phosphate, elevated PTH, elevated ALP, low 25(OH)-D and low 1,25 (OH)2-D (table 1). Severe metabolic acidosis.
Cardiomegaly was evident on the x-ray (figure 5) and the echocardiography revealed severe dilation of the left ventricle and systolic insufficiency with an ejection fraction of 5%.
Figure 5.
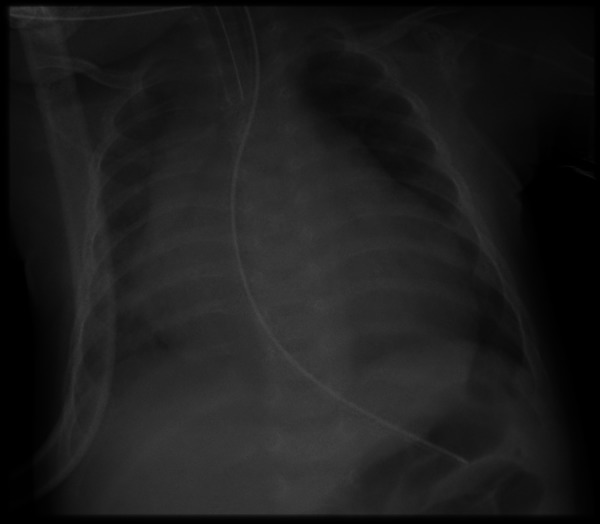
Cardiomegaly (case 4).
The reverse-transcription PCR for enterovirus was positive in the infant's faeces (initial evaluation). Cytogenetic study for DiGeorge Syndrome and metabolic study were normal.
Vitamin D levels in the mothers were not investigated.
Treatment
Case 1: Calcium administered intravenously (10% calcium gluconate 7 ml/kg/day) for 10 days. On day 8 the baby was started on oral vitamin D (cholecalciferol 1800 U/day). No anticonvulsant was used.
Case 2: Fenobarbital administered intravenously (20 mg/kg/day) for 3 days and then oral (11.5 mg/kg/day). Calcium administered intravenously (10% calcium gluconate 5 ml/kg/day) during 8 days. On day 6 the baby was started on oral calcium (10% calcium gluconate 1 ml/day) and vitamin D (calcitriol (25 μg/day)).
Case 3: Calcium administered intravenously (10% calcium gluconate 6 ml/kg/day) for 7 days. On day 5 the baby was started on oral calcium (calcium glubionate/calcium lactobionate 1000 mg/day) and vitamin D (cholecalciferol 1800 U/day).
Case 4: Supportive measures, inotropic drugs, calcium administered intravenously (10% calcium gluconate 8 ml/kg/day) for 17 days. On day 16 the baby was started on oral vitamin D (calcitriol 25 mcg/day). He was discharged with oral calcium (calcium glubionate/lactobionate 2000 mg/day), calcitriol, captopril, carvedilol, digoxin, furosemide and spironolactone.
Outcome and follow-up
Case 1: As soon as the baby started treatment, symptoms remitted and, after 1–2 months, laboratory evaluation was normal (table 1). During 2 years of follow-up, her psychomotor development was always normal.
Case 2: As soon as he started treatment, symptoms remitted and, after 1–2 months, laboratory evaluation was normal (table 1). After 4 months, his psychomotor development and the EEG were normal, and anticonvulsant could be stopped.
Case 3: As soon as he started treatment, symptoms remitted and, after 1–2 months, laboratory evaluation was almost normal (table 1). Six months later, the widening of the wrists was no longer evident.
Case 4: Initially, heart function was difficult to compensate (ejection fraction of 5%), even with calcium administered intravenously. However, it was rapidly restored soon after vitamin D treatment was started (ejection fraction of 25% by day 26). Two months later, heart function was normal (ejection fraction of 32%).
Discussion
Vitamin D deficiency reports are increasing worldwide. Its prevalence in the paediatric age has ranged from 6.6% in Greek preschool children to 95% in American children from 1 to 11 years old. In Portugal, prevalence in children and adolescents between 2 and 16 years old was 26%. Last American review revealed 56.4% prevalence in 14-year-old to 18-year-old teenagers. In infants no published data are available.1 3 5 7 17–22
The main cause is the lack of food enrichment and vitamin D supplementation not only in pregnant and lactating women, but also in infants during the first days of life. The risk is higher in exclusively breastfed infants, born to vitamin D deficient mothers and prematures.2–8 12 13 18 23 24 Decreased ultraviolet B (UVB) exposure and the excessive use of sunscreen and clothes to reduce the risk of skin cancer, or in face of cultural beliefs, are also very important aetiological factors. UV radiation is required for the production of vitamin D in the skin and this process accounts for more than 90% of its sources in humans (less than 10% is derived from diet). This production is, therefore, fundamental for achieving healthy amounts of vitamin D. Dark-skinned individuals have an increased risk because melanin reduces the absorption of UVB and, consequently, production of vitamin D.1 2 13 23
Current recommendations on supplementation with vitamin D vary between 400 and 1000 U/day. The American Academy of Paediatrics recommends 400 U/day during the first days of life until adolescence is completed (2008), especially during months of low sun exposure WHO recommends 400–600 U/day (or weekly dose for the oldest) during the entire year for all children and adolescents (2010). British guidelines include the pregnant woman and breastfeeding mother. Unfortunately, none of these recommendations take skin pigmentation into account.2–6 8 12 13 18 23–26
In spite of this, in Portugal, in the last decade, many infants have not been given vitamin D supplements, nor have pregnant or breastfeeding women. The reason for discontinuing supplementation was the belief that the population had enough sun exposure and sufficient dietary supplies.
These case reports highlight the severity of hypovitaminosis D presentation in infants. In the last case, prolonged hypocalcaemia probably resulted in dilated cardiomyopathy, which in face of an acute viral infection (positive enterovirus PCR), led to cardiac failure. This situation was very difficult to compensate, even with high dosage of intravenously administered calcium, until the child was started on vitamin D.
A high index of suspicion for vitamin D deficiency should be maintained, especially if the infant/child is premature, dark-skinned, exclusively breastfed and has been born to a vitamin D deficient mother.
Learning points.
Vitamin D supplementation of pregnant and lactating women, and to infants is essential to prevent vitamin D deficiency, particularly in dark-skinned individuals.
Low exposure to sunlight and excessive use of clothes and sunscreen should be avoided.
Infants may present with seizures, tetany or cardiomyopathy in case of severe hypocalcaemia.
Levels of 25(OH)-D should be used to evaluate vitamin D status and not 1,25(OH)2-D.
Levels of 25(OH)-D ≤10 ng/dl are considered deficient, 10–20 ng/dl insufficient and ≥20 ng/dl sufficient.
Footnotes
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Schoor N, Lips P. Worldwide vitamin D status. Best Pract Res Clin Endocrinol Metab 2011;2013:671–80 [DOI] [PubMed] [Google Scholar]
- 2.Henry H. Regulation of vitamin D metabolism. Best Pract Res Clin Endocrinol Metab 2011;2013:531–41 [DOI] [PubMed] [Google Scholar]
- 3.Binkley N, Ramamurthy R, Krueger D. Low vitamin D status: definition, prevalence, consequences and correction. Endocrinol Metab Clin N Am 2010;2013:287–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thandrayen K, Pettifor J. Maternal vitamin D status: implications for the development of infantile nutritional Rickets. Endocrinol Metab Clin N Am 2010;2013:303–20 [DOI] [PubMed] [Google Scholar]
- 5.Misra M, Pacaud D, Petryk A, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics 2008;2013:398–417 [DOI] [PubMed] [Google Scholar]
- 6.Greer F. 25-Hydroxyvitamin D: functional outcomes in infants and young children. Am J ClinNutr 2008;2013(Suppl):529S–33S [DOI] [PubMed] [Google Scholar]
- 7.Mithal A, Wahl D, Bonjour J, et al. Global vitamin D status and determinantes of hypovitaminosis D. Osteoporos Int 2009;2013:1807–20 [DOI] [PubMed] [Google Scholar]
- 8.Balk S, AAP Ultraviolet radiation: a hazard to children and adolescents. Pediatrics 2011;2013:791–817 [DOI] [PubMed] [Google Scholar]
- 9.Belle T, Gysemans C, Mathieu C. Vitamin D in autoimmune, infectious and allergic diseases: a vital player? Best Pract Res Clin Endocrinol Metab 2011;2013:617–32 [DOI] [PubMed] [Google Scholar]
- 10.Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin N Am 2010;2013:365–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker V, Modlin R. The vitamin D connection to pediatric infections and immune function. Pediatr Res 2009;2013:106R–13R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettifor J, Prentice A. The role of vitamin D in paediatric bone health. Best Pract Res Clin Endocrinol Metab 2011;2013:573–84 [DOI] [PubMed] [Google Scholar]
- 13.Vieth R. Why the minimum desirable serum 25-hydroxyvitamin D level should be 75 nmol/L (30 ng/dL). Best Pract Res Clin Endocrinol Metab 2011;2013:681–91 [DOI] [PubMed] [Google Scholar]
- 14.Kumar M, Saikia D, Kumar V, et al. Vitamin D deficiency presenting with cardiogenic shock in an infant. Ann Pediatr Cardiol 2011;2013:207–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holick M. Vitamin D: extraskeletal Health. Endocrinol Metab Clin N Am 2010;2013:381–400 [DOI] [PubMed] [Google Scholar]
- 16.Hollis B. Assessment and interpretation of circulating 25-hydroxyvitamin D and 1,25-hydroxyvitamin D in the clinical environment. Endocrinol Metab Clin N Am 2010;2013:271–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rovner A, O'Brien K. Hypovitaminosis D among healthy children in the United States. Arch Pediatr Adolesc Med 2008;2013:513–19 [DOI] [PubMed] [Google Scholar]
- 18.Bouillon R. Why modest but widespread improvement of vitamin D status is the best strategy? Best Pract Res Clin Endocrinol Metab 2011;2013:603–702 [DOI] [PubMed] [Google Scholar]
- 19.Nicolaidou P, Papadopoulou A, Kaleyias J, et al. Vitamin D status in mother-newborn pairs in Greece. Calcif Tissue Int 2006;2013:337–42 [DOI] [PubMed] [Google Scholar]
- 20.Mansbach JM, Ginde AA, Camargo CA., Jr Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics 2009;2013:1404–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Severo M, Lopes C, Lucas R, et al. Development of a tool for the assessment of calcium and vitamin D intakes in clinical settings. Osteoporos Int 2009;2013:231–7 [DOI] [PubMed] [Google Scholar]
- 22.Dong Y, Pollock N, Stallmann-Jorgensen IS, et al. Low 25-hydroxyvitamin D levels in adolescents: race, season, adiposity, physical activity and fitness. Pediatrics 2010;2013:1104–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christakos S, Ajibade D, Dhawan P, et al. Vitamin D: metabolism. Endocrinol Metab Clin N Am 2010;2013:243–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee Prevention of rickets and vitamin D deficiency in infants, children and adolescents. Pediatrics 2008;2013:1142–52 [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization, Standing Committee of European Doctors . Vitamin D nutrition policy in Europe. CPME 2009/179 Final EN.
- 26.http://www.sunsmart.org.uk/prod_consump/groups/cr_common/@nre/@sun/documents/generalcontent/cr_052628.pdf (Vitamin D position statement, British Association of Dermatologists, Cancer Research UK, Diabetes UK, the Multiple Sclerosis Society, the National Heart Forum, the National Osteoporosis Society and the Primary Care Dermatology Society, 2010). (accessed 4 Apr 2013).


