Summary
Few animal models have been reported to evaluate and compare mechanical endovascular thrombectomy (MET) devices used to treat human ischemic stroke. These models may contribute to the understanding of arterial injury induced by a MET device and potentially by extrapolation to human intracranial arteries. We have developed a novel swine model for MET that allows visualization of the thrombus/device interaction and characterization of mechanical impact on the vessel wall. Twenty superficial femoral arteries were occluded with radiopaque thrombus, and 20 without thrombus were treated with thrombectomy devices. Acute histopathological changes were evaluated. The swine femoral artery, which is comparable in size to the human middle cerebral artery or basilar artery, may offer a useful animal model for the study of histologic alterations induced by MET.
Key words: ischemic stroke, mechanical endovascular thrombectomy, swine model
Introduction
Mechanical endovascular thrombectomy (MET) is being increasingly used for revascularization in case of acute ischemic stroke with proximal arterial occlusion 1. To date, few animal models have been reported to analyze MET in human stroke 2. However in vivo models are essential to allow analyses of MET-induced histopathological damage to evaluate and improve MET devices.
Previously, a model was developed in swine, using the superficial cervical artery to evaluate arterial structural changes induced by MET devices 3. However, the normal histological properties of the superficial cervical artery are not well-described in swine and this model requires a long and difficult surgical exploration, which may be a source of mortality. Therefore new models are needed to further understand arterial structural damages caused by devices used for MET in order to improve their safety.
We report here a novel animal model using the swine superficial femoral artery (SFA). This artery presents several advantages, such as well-known histological properties and easy surgical access.
Technique
All procedures were performed at Haute-Vienne Research and Analysis Department (Establishment for Animal Experimentation) according to international guidelines and approved by the responsible local authorities. Experiments were done in 20 male swine, three to four months of age, weighing 20-25 kg, under general anesthesia.
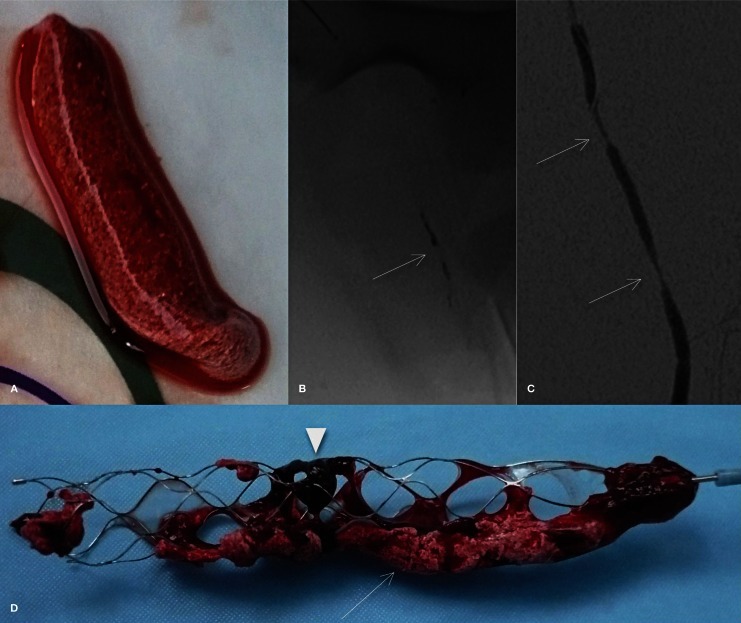
Radiopaque clots were prepared in vitro, as described by Kan et al. 4. In brief, 20 mL of swine whole blood were mixed with 2 g of barium sulfate powder to obtain radiopacity. This mixture was incubated at room temperature for 120 minutes in a syringe to obtain two phases (serum and solid component). The serum was then eliminated and a piece of clot (25 mm in length) was resected. Each prepared thrombus was inserted into a silicone tube (Cole-Parmer; Vernon Hills, IL, USA) with saline and prepared for injection (Figure 1).
Both SFAs of each swine were dissected to approximately 5 cm in length (Figure 2A). The 20 right SFA samples were not subjected to any kind of intervention (control samples). The 20 left SFA samples were randomly submitted to thrombectomy. An angiogram of the left SFA was performed on a biplane angiography system (Integris; Philips, Best, The Netherlands) (Figure 2B). Rotational angiography, followed by three-dimensional reconstruction of the native projections, was performed just before the thrombectomy procedure. The diameters of the vessels were measured using the three-dimensional reconstruction images. For selective thromboembolization, the silicone tube containing a prepared clot was connected to the 5F guiding catheter (Chaperon; MicroVention, Tustin, CA, USA), and 2 mL of saline were injected to propel the clot into the SFA.
Arterial occlusion was confirmed by angiogram and the guiding catheter was then retrieved. Arterial occlusion was maintained for one hour (± 10 minutes) before mechanical retrieval was attempted (Figure 2C,D). MET of the SFAs was performed with Merci Retrieval System (Concentric Medical; Mountain View, CA, USA), Catch thromboembolectomy system (Balt Extrusion; Montmorency, France), Solitaire FR Revascularization Devices (EV3; Plymouth, MN, USA), and Penumbra System (Penumbra; Alameda, CA, USA). The properties are summarized in Table 1. For each revascularization device used, all recanalization attempts were conducted according to the requirements of the manufacturers. One hour after the end of the last retrieval attempt, the animals were euthanized with an intravenous injection of 20 mmol potassium chloride.
Table 1.
Mechanical thrombectomy devices.
| Descriptions | Solitaire FR 4 mm |
Solitaire FR 6 mm |
Catch | Merci Retriever V 2.5 Firm |
|---|---|---|---|---|
| Design | Wall-contact | Wall-contact | Wall-contact | Wall-contact |
| Outer Diameter (mm) | 4 | 6 | 4 | 2.5 |
| Length (mm) | 20 | 20 | 18 | 6 |
| Recommended vessel diameter (mm) |
2.0-4.0 | 3.0-5.5 | ≤ 4 | 2.0-3.5 |
Both of the SFAs were dissected away from the surrounding tissue and each was cut transversely into six individual pieces, numbered proximally to distally, with regard to arterial blood flow. The most proximal and distal pieces were 1 cm in diameter and were excluded to avoid ligature sites occurring during the surgery. The vessel sections from each artery were embedded in paraffin according to standard laboratory operating procedures. They were subsequently sectioned at 5-7 μm and stained with three histological stains (hematoxylin-eosin, Masson's trichrome, and orcein). The sections were examined by a board-certified pathologist (C.Y.) with an optical microscope using magnification ranging from 40 to 400 times.
A grading system was developed to evaluate the acute mural response to the MET devices. The following were assessed: 1) amount of endothelial denudation (percentage of surface area); 2) presence of mural thrombus (vascular occlusion percentage); 3) intimal layer edema (inner intima circumference percentage); 4) medial layer edema (inner media circumference percentage); and 5) internal elastic lamina (IEL) fractured (percentage).
Figure 1.
A) Photograph shows radiopaque clot. B) Nonsubtracted image shows the injected thrombus (arrow) in the left superficial femoral artery (SFA). C) Anteroposterior angiogram of the left SFA immediately after thrombectomy demonstrates stenosis (arrows). D) Photograph after thrombectomy (Solitaire FR Revascularization device 4 mm) shows a barium sulfate–marked (arrow) thrombus (arrowhead).
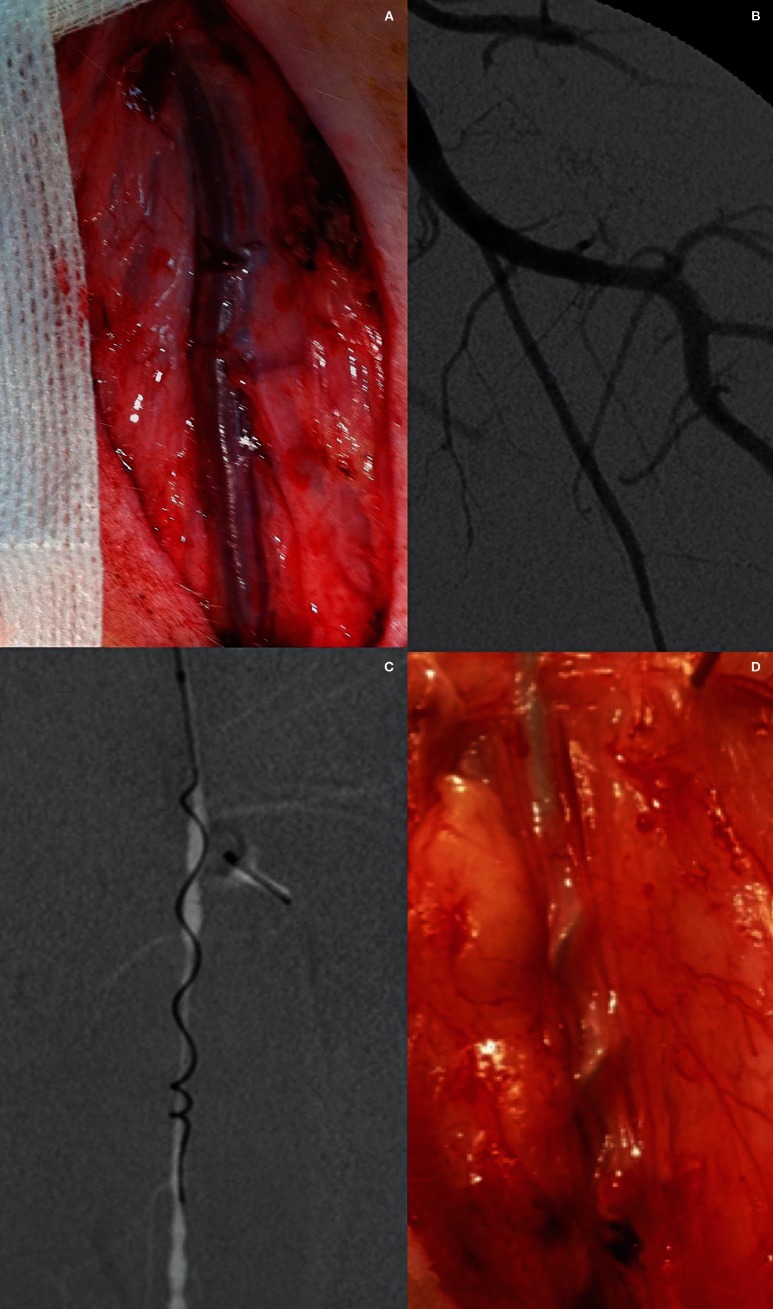
Figure 2.
Mechanical endovascular thrombectomy in the left SFA using the Merci Retrieval System. A) Photograph of a surgically exposed left SFA. Note the superficial position allowing easy resection. B) Anteroposterior angiogram of the left femoral artery show the SFA. Note the size (inner diameter 2.7-2.9 mm) of this artery, which reproduces the characteristics of proximal intracranial arteries, such middle cerebral artery or basilar artery. C) Anteroposterior angiogram of the left SFA shows the Merci device during retrieval. D) Intraprocedural photograph of the left SFA. Note the extension of the Merci device inserted into the vessel lumen.
Results
All experimental procedures were carried out without complication. No animal died during the experiment before planned euthanasia.
Forty SFAs from 20 swine were analyzed. All 40 angiograms showed well-developed bilateral SFAs. The porcine SFA originates at an angle of 95° from the common femoral artery in lateral orientations and at an angle of 30° in anteroposterior orientations. With a diameter of about 2.0-3.5 mm, and a length of 105-120 mm, the vessel size of the model allowed insertion and navigation of standard-sized devices used in humans.
The study was strictly designed to assess the degree of vascular damage. The mean arterial damage for each device is summarized in Table 2. At histologic examination, all devices caused vascular injuries extending into the medial layer (Figure 3). In the device samples versus the control samples, these were characterized by significant endothelial denudation and medial layer edema. Intimal layer edema and fracture of the IEL were also found in all of the device samples, but with insignificant differences for all device samples combined compared to the control samples. No other lesions were found deeper in the external elastic lamina or adventitia, nor were there perforations or dissections in any samples.
Table 2.
Comparison of arterial damage in the device and control samples.
| Solitaire 4 (n = 4) |
Solitaire 6 (n = 4) |
Catch (n = 4) |
Merci (n = 4) |
Penumbra (n = 4) |
Wall- Contact† (n = 16) |
All Device (n = 20) |
Control (n = 20) |
P Value* | |
|---|---|---|---|---|---|---|---|---|---|
| Endothelial denudation |
85.8 ± 22.9 | 76.2 ± 12.8 | 76.1 ± 21.2 | 85.9 ± 12.1 | 40.1 ± 47.6 | 81.0 ± 16.8 | 72.8 ± 29.4 | 0.9 ± 1.9 | 0.0001 |
| Mural thrombus |
14.1 ± 28.1 | 0 | 9.4 ± 15.6 | 3.1 ± 6.2 | 0 | 6.6 ± 15.7 | 5.3 ± 14.2 | 0 | 0.05 |
| Intimal layer edema |
43.8 ± 51.5 | 81.3 ± 65.7 | 43.8 ± 37.5 | 66.5 ± 47.1 | 100 ± 89.1 | 58.8 ± 48.9 | 67 ± 56.2 | 44.6 ± 43.6 | 0.25 |
| IEL fractured† |
12.5 ± 14.4 | 25 ± 35.4 | 18.8 ± 23.9 | 50 ± 100 | 12.5 ± 14.4 | 26.6 ± 51.2 | 23.7 ± 46.2 | 20.6 ± 33.3 | 0.78 |
| Medial layer edema |
25 ± 0 | 37.5 ± 43.3 | 81.3 ± 23.9 | 41.5 ± 35.2 | 75 ± 35.4 | 46.3 ± 34.8 | 52 ± 35.9 | 18.1 ± 27.8 | .004 |
| EEL fractured |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| Adventitia edema |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| Note: Data are the means (%) ± standard deviation.(%) IEL = Internal elastic lamina; EEL = External elastic lamina; NA = Non applicable; † Wall-contact devices including Merci retriever, Catch thromboembolectomy system and Solitaire FR revascularization devices 4 and 6 mm; * Mann-Whitney Test comparing all device samples combined with control samples. | |||||||||
There were no statistically significant differences in the arterial damage among the five device groups. However, the aspiration device was associated with more intimal and medial layer edema compared with the wall-contact devices except for the Catch thromboembolectomy system.
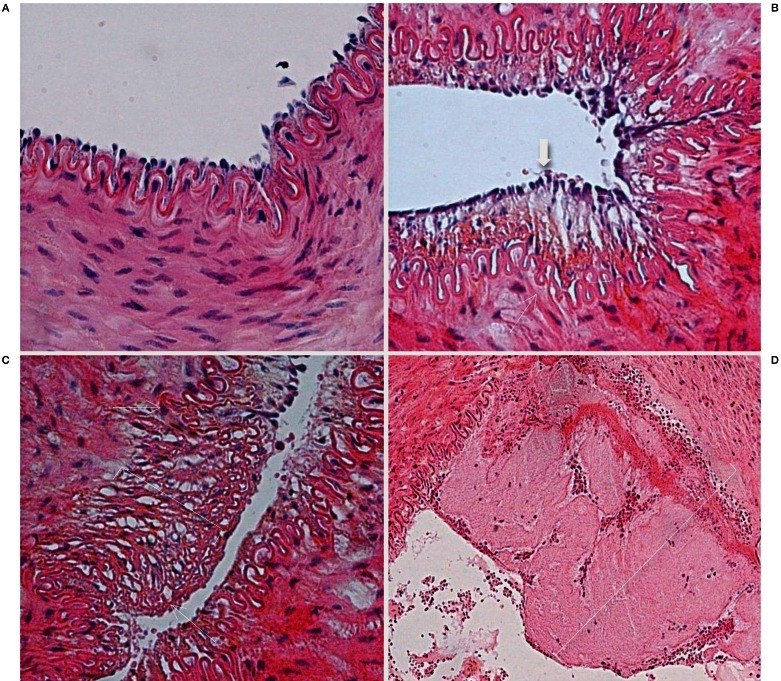
Figure 3.
Histopathological findings after mechanical thrombectomy. A) Microscopic view of a right SFA (control sample) shows all layers preserved (hematoxylin-eosin [H&E] staining, original magnification ×400). B,C) Microscopic view of an artery treated with the aspiration device demonstrate the intact internal elastic lamina (thin arrow) and a single layer of endothelial cells (thick arrow). The parentheses show edema of the subendothelial layer in B and the media layer in C. There is no thrombus (H&E staining, original magnification ×400). D) Microscopic view of an artery treated with the Catch thromboembolectomy system shows a mural clot (parenthesis) and the internal elastic lamina fractured. (H&E staining, original magnification ×200).
Discussion
Animal models are necessary to characterize the efficacy and safety of new mechanical devices before their clinical use. However, few animal models have been proposed to evaluate neurovascular devices used in clinical practice. The aim of this study was to develop a new animal model to evaluate and compare arterial wall damage induced by various devices used for MET in acute ischemic stroke.
The aspiration device was responsible for more intimal and medial layer edema. However, these differences were not statistically significant, although this could be due to the small animal sample. These results are probably related to the fact that the designs and mechanisms of these two types of devices are completely different. The aspiration device applies aspiration force to the proximal base of the thrombus 5. As opposed to this suction device, wall-contact devices capture the clot by exerting continuous radial force against the vessel wall, which may injure the endothelium in the process 6.
The similarities between the porcine and human cardiovascular systems make the pig a useful animal for the study of vascular biology 7. The size of the swine SFA is comparable to the middle cerebral artery or basilar artery which are the target vessels for MET. In our study, the mean diameter of the SFA was 2.7 mm (2.0-3.5 mm), which is similar to the outer diameter of the proximal middle cerebral artery in humans (2.4 mm).
Compared to the model described by Yuki et al. 3, surgical access to swine SFA is not technically difficult, due to its superficial position. The normal histological characteristics of porcine femoral artery have been reported and raise the possibility of evaluating pathological lesions 7. In addition, our model also allows direct visualization of the interaction between the surfaces of the vessel wall and the retrieval device.
Our new model does not represent a counterpart to the entire phenomenon seen in patients with stroke. Nevertheless, we believe that it may expand the analysis of the safety profile and offer the possibility of an experimental model, which is potentially useful for the future development and evaluation of mechanical devices in preclinical studies. Further studies at subacute and chronic stages are necessary to identify the evolution of different types of histological lesions.
References
- 1.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 2.Gralla J, Schroth G, Remonda L, et al. A dedicated animal model for mechanical thrombectomy in acute stroke. Am J Neuroradiol. 2006;27:1357–1361. [PMC free article] [PubMed] [Google Scholar]
- 3.Yuki A, Kan I, Golshan A, et al. A swine model to analyze arterial structural changes induced by mechanical thrombectomy. Am J Neuroradiol. 2012 doi: 10.3174/ajnr.A3221. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kan I, Yuki I, Murayama Y, et al. A novel method of thrombus preparation for use in a swine model for evaluation of thrombectomy devices. Am J Neuroradiol. 2010;31:1741–1743. doi: 10.3174/ajnr.A1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bose A, Henkes H, Alfke K, et al. The Penumbra System: a mechanical device for the treatment of acute stroke due to thromboembolism. Am J Neuroradiol. 2008;29:1409–1413. doi: 10.3174/ajnr.A1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gralla J, Schroth G, Remonda L, et al. Mechanical thrombectomy for acute ischemic stroke, thrombus, device interaction, efficiency, and complications in vivo. Stroke. 2006;37:3019–3024. doi: 10.1161/01.STR.0000248457.55493.85. [DOI] [PubMed] [Google Scholar]
- 7.Solanes N, Rigol M, Ramirez J, et al. Histological basis of the porcine femoral artery for vascular research. Anat Histol Embryol. 2005;34:105–111. doi: 10.1111/j.1439-0264.2004.00580.x. [DOI] [PubMed] [Google Scholar]