Summary
Haemorrhagic complications can occur following aneurysm treatment with flow diverters (FD), but the underlying mechanism remains unknown. We describe a case where deformation of the device may have contributed to the complication.
A patient with a giant, previously unruptured cavernous aneurysm that extended intracranially to cause oedema of the internal capsule was treated with flow diversion. Treatment was followed by multiple episodes of peri-aneurysmal haemorrhages within eight days. A deformation of the device which occurred where it curved to cross the aneurysm neck created residual flows which, in the presence of a stent stenosis immediately beyond the neck, may have contributed to the observed ruptures. Following multiple haemorrhages the patient subsequently died.
Autopsy demonstrated early red thrombus partially bridging the struts of the flow diverter, and intra-aneurysmal thrombus of various ages. Microscopic pathology showed an aneurysm wall consisting of collagen infiltrated with neutrophils, but the wall was absent near the cerebral peduncle, adjacent to the brain haemorrhage.
Radiographs of the extracted specimen confirmed deformation of the FD construct, located at the transition zone of the stent, leading to increased pore size and porosity. The site of the deformation correlated with the angiographic presence of a continued blood inflow jet into the aneurysm.
Stent deformation at the transition zone may promote persistent blood entry into the aneurysm, and in turn potentially contribute to haemorrhagic complications.
Key words: flow diverter, giant aneurysm, unruptured aneurysm, complication, stent deformation
Introduction
Flow diverters represent a fundamentally different approach to endovascular aneurysm treatment. Unlike endosaccular approaches such as coiling, where the immediate goal of therapy is occlusion of the aneurysm with coils and secondary thrombus, flow diversion (FD) intends to ‘reconstruct' the parent vessel bearing the aneurysm by preferentially directing blood flow within the new tubular metallic construct.
Anticipated effects of FD on the aneurysm are delayed intra-aneurysmal thrombus formation, relying on the porous nature of the device to spare jailed branch vessels. Case series of FDs have generally shown promising results 1,2, but FDs can also lead to devastating hemorrhagic complications 3-5.
The braided, self-expendable construction of FDs makes them susceptible to deformations after deployment that can be studied both in animal models and in vitro 6-8. In the presence of certain anatomical features, such as a curve or a wide defect in the parent vessel, or both, these deformations can create focal high porosity areas, where leaks can occur. These leaks can perhaps explain failures and, given certain circumstances, aneurysmal ruptures. Here, we analyse the clinico-pathological features of an unruptured aneurysm patient whose treatment with a two flow-diverter construct eventually led to multiple aneurysm hemorrhages and death.
Clinical Case
A 69-year-old woman presented with progressive left-sided paraesthesias and mild hemiparesis. Pre-procedural investigations included CT, CTA, and a diagnostic angiogram, which demonstrated a giant 60 mm right cavernous aneurysm with intradural extension and oedema of the internal capsule (Figure 1). The neck was estimated to be 16 mm; a 50% stenosis of the carotid artery distal to the aneurysm neck was noted. The circle of Willis was incomplete, with aplasia of the right P1 and hypoplasia of the A1 segment. Given the progression of symptoms and the risk of subarachnoid haemorrhage, reconstructive endovascular treatment was elected. Informed consent for treatment with a flow diversion strategy was obtained, and the patient was prepared with 325 mg ASA and 75 mg clopidogrel daily for seven days prior to intervention. Under general anaesthesia, a Vasco+21 microcatheter (Balt, Montmorency, France) was navigated distal to the cavernous aneurysm and a 3.5 × 35 mm Silk flow diverter (FD) (Balt) was deployed from the ICA distal to the ophthalmic artery to the petrous segment, spanning the blown-out aneurysm neck. As the device was unsheathed it expanded to a maximal unconstrained diameter across the blown-out neck, leading to a shorter than anticipated proximal landing zone. Angiography showed contrast stagnation within the aneurysm. The carotid stenosis distal to the aneurysm was suspected to be restricting distal blood flow, and an attempt was made to cross the Silk with a 3.25 × 15 mm Gateway balloon catheter (Boston Scientific). Passing the balloon pushed the proximal portion of the FD into the aneurysm. After multiple unsuccessful attempts to re-catheterize or to retrieve the device, the lumen of the FD was eventually re-crossed with a wire, the stenosis dilated with the balloon, followed by exchange with another Vasco+21. A second telescoping 4.0 × 35 mm Silk was deployed from inside the first stent distal to the dilatation site, to the petrous carotid artery, with a 10 mm long proximal landing zone (Figure 2). The stenosis within the devices remained significant (60-70%), but further attempts to dilate the segment were not undertaken for fear of prolapsing the construct again. A persistent leak through the FD construct was seen where the devices turned 90 degrees and opened at the level of the transition zone (Figure 3), between the segment constrained by the carotid artery (4 mm) and where the device expands within the blown-out neck (at least 7 mm in diameter), with severe stagnation of contrast within the aneurysm.
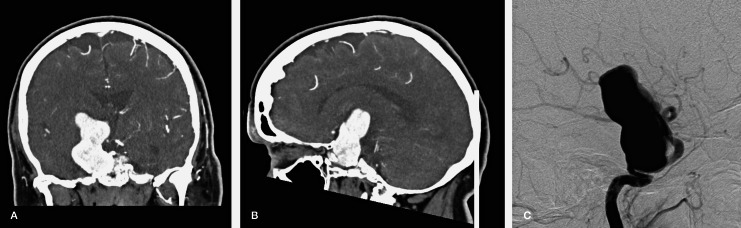
Figure 1.
Pre-treatment CT (A,B) and lateral subtraction angiographic images (C) of the 60 mm unruptured giant right cavernous carotid artery aneurysm.
Figure 2.
Flow-limiting stenosis within the distal portion of the device (A). After recatheterization of the device and balloon angioplasty (B) the intra-stent stenosis persisted (C).
Figure 3.
A, B) Contrast leak through the stent construct seen to occur at the first major curve, within the transition zone (A; open arrow). Magnified photograph of the curved transition zone of the flow diverter (C; open arrow) which occurs as the device begins to expand as it is released from the constraints of the parent vessel (arrow). Radiograph of the pathological specimen showing increased porosity at the transition zone (D).
The patient woke up moving all four extremities well following her intervention, but was kept intubated overnight due to the prolonged anaesthetic time. Four hours after extubation the next day, the patient suddenly developed expressive dysphasia with left-sided weakness, followed by an abruptly decreasing level of consciousness requiring urgent re-intubation and ventilation. CT scan showed an intra-aneurysmal clot localized to the medial portion of the aneurysm, with a small intraparenchymal haemorrhage, against the cerebral peduncle. A CT angiogram showed a patent carotid artery and partial opacification of the aneurysm. Due to concerns regarding thrombosis of the FD construct, neither ASA or clopidogrel were discontinued. Over the next few days, the patient's neurological status further deteriorated, in a step-wise fashion, with further decreases in her Glasgow Coma Scale score. Serial CT scans following each observed deterioration demonstrated increased amounts of peri-aneurysmal intraparenchymal blood consistent with repeated haemorrhages (Figure 4). A control angiogram on day 6 was unchanged, with a patent but stenosed carotid artery and severe stagnation of contrast within the aneurysm. A control MRI of the brain on day 8 showed a partially thrombosed aneurysm, increased in size, and increased peri-aneurysmal blood and oedema. After discussing the various management options with the family, the decision was made for no further intervention, and active care was withdrawn on day 8 post-intervention. A neurological autopsy was performed, which required sectioning the aneurysm axially to remove the brain. The sphenoid, FD construct, and the base of the aneurysm were removed en-bloc. After decalcification for three months, the segment of the right carotid artery bearing the aneurysm was dissected, opened longitudinally, and photographed using an operating microscope (Figure 5). The giant aneurysm and peri-aneurysmal tissues were sectioned with the brain, stained with hematoxylin-eosin and Luxol Fast Blue, paying attention to the aneurysm wall. Magnified photography of the FD specimen showed early red thrombus bridging the struts of the flow diverter, and intra-aneurysmal thrombus of early as well as later age. Cross-sections showed fresh and old clot within the aneurysm, as well as blood outside the mural confines at the medial surface. The medial portion of the aneurysm wall consisted mainly of collagen infiltrated with neutrophils. The wall was absent near the cerebral peduncle, adjacent to the brain haemorrhage.
Figure 4.
Evidence of repeated intra-cerebral haemorrhages immediately adjacent to the aneurysm A) one, and B) two days following treatment. Day 8 MRI showing partially thrombosed aneurysm and significant peri-lesional oedema.
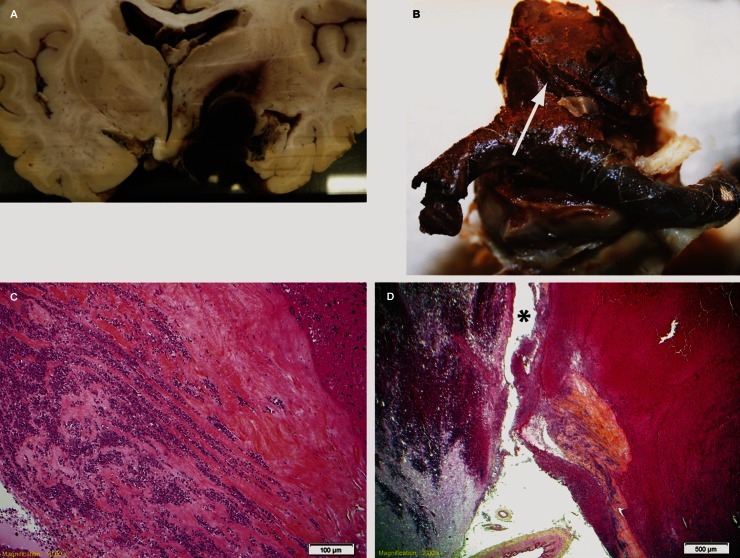
Figure 5.
A) Coronal pathological section showing aneurysm rupture point. B) Explanted flow diverter construct showing fresh thrombus covering the stent struts. C) Slide showing aneurysmal wall tissues, with collagenous wall infiltrated with abundant neutrophils. D) Deficient aneurysm wall at suspected rupture point (*).
Discussion
In a series of 13 European cases of rupture of previously unruptured aneurysms following treatment with flow diversion, Kulcsar et al. 3 proposed four factors that might predispose aneurysms to rupture: i) very large/giant size; ii) symptomatic aneurysms; iii) aspect ratio of >1.6; iv) inertia-driven flow. The patient presented here had all four criteria.
Mechanisms purported to play a role in the development of haemorrhage after flow diversion include an increase in intra-aneurysmal pressure after FD deployment 9-11, the presence of intra-aneurysmal thrombus with mural degradation 5, initially suggested in ruptured abdominal aortic aneurysms 12 and recanalizing endothelialized channels re-establishing flow between the degraded wall and the thrombus shown in an animal model 13. The case presented here showed pathological findings consistent with aneurysm rupture precipitated by altered but persistent blood flow to an aneurysm containing thrombus. The aneurysm wall in the vicinity of the thrombus was severely infiltrated with neutrophils, and in some areas, absent. In the present case the wall breakdown may have antedated the treatment, as suggested by the observed peri-aneurysmal oedema with accompanying symptoms 14. Treatment with flow diversion alone may have exacerbated this problem. Balt released a field notice in 2010 warning that haemorrhagic complications have resulted when giant aneurysms were treated with FDs alone, and recommended that coiling should be combined with flow diversion 15. Aneurysm coiling may promote stabilization of the thrombus on and around coils, which subsequently can become organized as the aneurysm heals 16. Coiling does not prevent ruptures in all cases, however 1,17. In the present case, it was felt that an extraordinary number of coils would have been needed to achieve even loose aneurysm packing.
The persistent leak occurred at the level of the 90 degree curve in the proximal cavernous segment. Computational fluid dynamics and in vitro studies have shown how curves transform shear-driven to inertia-driven flow, which is less affected by stents and flow diverters 18. In addition, braided devices show higher porosities and larger pores on the convexity of such curves 8. In the present case, the leak occurred at the ‘transition zone' between the constrained and the unconstrained portions of the device, where the parent vessel suddenly opened at the level of the neck of the aneurysm, allowing full expansion of the device. Bench studies have previously shown that in such circumstances, there is a FD transition zone that constantly remains of higher porosity than the fully expanded middle expansion or compaction zone, no matter how braided devices are deployed 8. Animal studies have also shown that leaks through the neointima that forms over FDs tend to occur at the level of the transition zone 6,7. The transition zone higher porosity is more conspicuous when FDs are oversized as compared to the parent vessel diameter 8, and can be identified radiographically by the presence of a fusiform deformation 6. Finally, in the presence of persistent leak at the level of the curved transition zone, it is tempting to speculate that the flow restriction caused by the stenosed carotid artery at the outlet further contributed to the complication that was observed in this particular case.
Tentatively, we propose that given a combination of risk factors (giant size, inertia driven flows, brain oedema and symptoms suggesting an already deficient aneurysm wall), the observation of a major persistent leak at the level of the transition zone (identified by a fusiform dilatation) may be an indication to attempt to seal the leak with another shorter flow diverter of a slightly smaller diameter, to prevent ruptures.
Future solutions may include the design of devices in such a way as to minimize the increase in porosity occurring at the transition zone.
Conclusion
Efforts to reconstruct parent vessels with flow diverters should bear in mind the predictably more porous nature of the transition zone of the device. The persistence of a major leak into FD-treated aneurysms may contribute to subsequent haemorrhage.
References
- 1.Byrne JV, Beltechi R, Yarnold JA, et al. Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: a multicentre prospective study. PLoS One. 2010;5:pii: e12492. doi: 10.1371/journal.pone.0012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiorella D, Kelly ME, Albuquerque FC, et al. Curative reconstruction of a giant midbasilar trunk aneurysm with the pipeline embolization device. Neurosurgery. 2009;64:212–217 discussion 217. doi: 10.1227/01.NEU.0000337576.98984.E4. [DOI] [PubMed] [Google Scholar]
- 3.Kulcsar Z, Houdart E, Bonafé A, et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. Am J Neuroradiol. 2011;32:20–25. doi: 10.3174/ajnr.A2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turowski B, Macht S, Kulcsar Z, et al. Early fatal hemorrhage after endovascular cerebral aneurysm treatment with a flow diverter (SILK-Stent): do we need to rethink our concepts? Neuroradiology. 2011;53:37–41. doi: 10.1007/s00234-010-0676-7. [DOI] [PubMed] [Google Scholar]
- 5.Hampton T, Walsh D, Tolias C, et al. Mural destabilization after aneurysm treatment with a flow-diverting device: a report of two cases. J NeuroIntervent Surg. 2011;3:167–171. doi: 10.1136/jnis.2010.002873. [DOI] [PubMed] [Google Scholar]
- 6.Bing F, Darsaut TE, Salazkin I, et al. Stents and flow-diverters in the treatment of aneurysms: device deformation in vivo and impact on porosity. Neuroradiology. 2013;55:85–92. doi: 10.1007/s00234-012-1082-0. [DOI] [PubMed] [Google Scholar]
- 7.Darsaut TE, Bing F, Salazkin I, et al. Flow diverters can occlude aneurysms and preserve arterial branches: a new experimental model. Am J Neuroradiol. 2012;33:2004–2009. doi: 10.3174/ajnr.A3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makoyeva A, Bing F, Darsaut TE, et al. The varying porosity of braided self-expanding stents and flow diverters: an experimental study. Am J Neuroradiol. 2012;34:596–602. doi: 10.3174/ajnr.A3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cebral JR, Mut F, Raschi M, et al. Aneurysm rupture following treatment with flow-diverting stents: computational hemodynamics analysis of treatment. Am J Neuroradiol. 2011;32:27–33. doi: 10.3174/ajnr.A2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan T, Ahmed YM, Hassan AA. The adverse effects of flow-diverter stent-like devices on the flow pattern of saccular intracranial aneurysm models: computational fluid dynamics study. Acta Neurochir (Wien) 2011;153:1633–1640. doi: 10.1007/s00701-011-1055-9. [DOI] [PubMed] [Google Scholar]
- 11.Schneiders JJ, Vanbavel E, Majoie CB, et al. A flow-diverting stent is not a pressure-diverting stent. Am J Neuroradiol. 2013;34:E1–4. doi: 10.3174/ajnr.A2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Touat Z, Ollivier V, Dai J, et al. Renewal of mural thrombus releases plasma markers and is involved in aortic abdominal aneurysm evolution. Am J Pathol. 2006;168:1022–1030. doi: 10.2353/ajpath.2006.050868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raymond J, Darsaut TE, Kotowski M, et al. Thrombosis heralding aneurysmal rupture: an exploration of potential mechanisms in a novel giant aneurysm model in swine. Am J Neuroradiol. 2013;34:346–353. doi: 10.3174/ajnr.A3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berge J, Tourdias T, Moreau JF, et al. Perianeurysmal brain inflammation after flow-diversion treatment. Am J Neuroradiol. 2011;32:1930–1934. doi: 10.3174/ajnr.A2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balt Extrusion Urgent Field Safety Notice: Intracranial stent ‘SILK’. Clarifications of the indications. Letter to the attention of Hospital Chief Executives, Medical Directors and Directors of Radiology. . Available at: http://www.mhra.gov.uk/home/groups/dts-bi/documents/fieldsafetynotice/con076110.pdf. Accessed on September 1st, 2010. [Google Scholar]
- 16.Raymond J, Darsaut T, Salazkin I, et al. Mechanisms of occlusion and recanalization in canine carotid bifurcation aneurysms embolized with platinum coils: an alternative concept. Am J Neuroradiol. 2008;29:745–752. doi: 10.3174/ajnr.A0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szikora I, Berentei Z, Kulcsar Z, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the pipeline embolization device. Am J Neuroradiol. 2010;31:1139–1147. doi: 10.3174/ajnr.A2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng H, Wang Z, Kim M, et al. Saccular aneurysms on straight and curved vessels are subject to different hemodynamics: implications of intravascular stenting. Am J Neuroradiol. 2006;27:1861–1865. [PMC free article] [PubMed] [Google Scholar]