Summary
Polyarteritis nodosa (PAN) is a rare multisystem disease characterized by systemic necrotizing arteritis of small and medium size arteries. The skin, joints, kidneys, gastrointestinal tract and peripheral nerves are most commonly involved. Although aneurysms are commonly seen in the visceral vessels, intracranial aneurysms are rare with 15 reported cases. The intracranial aneurysms are usually multiple and located in supra- as well as infra-tentorial compartments. Most of the cases presented with subarachnoid or parenchymal hemorrhage. The aneurysms were usually small, although large cavernous aneurysms were reported in one case. Treatment guidelines are not clear regarding the management of these cases. Most patients were treated conservatively by medical management with surgical excision performed in only two cases and coiling done in one patient with cavernous aneurysms. Repeat hemorrhages or re-bleed in spite of medical treatment have also been reported.
We describe the case of a 22-year-old woman, a known case of PAN who presented with subarachnoid hemorrhage. Cerebral angiogram showed a ruptured right middle cerebral artery bifurcation aneurysm along with unruptured left middle cerebral, right posterior communicating and left posterior inferior cerebellar artery aneurysms. Her previous abdominal angiogram had revealed multiple aneurysms in visceral arteries. Successful coil embolization of the ruptured right MCA bifurcation aneurysm was performed with preservation of the parent vessel. The patient made a complete recovery and was placed on medical treatment for PAN. Follow-up MR angiography at three months revealed stable occlusion of the embolized aneurysm with no change in the unruptured aneurysms.
Although rare, PAN can be associated with intracranial aneurysms which can cause subarachnoid or parenchymal hemorrhage. Selected cases can be treated safely by coil embolization.
Key words: polyarteritis nodosa, aneurysm, embolization
Introduction
Polyarteritis nodosa (PAN) is a rare multisystem disease characterized by systemic necrotizing arteritis of small- and medium-sized arteries. The skin, joints, kidneys, gastrointestinal tract and peripheral nerves are most commonly involved. Although aneurysms are commonly seen in the visceral vessels, intracranial aneurysms are rare with 15 reported cases. Treatment guidelines are not clear regarding the management of these cases.
Clinical History
A 22-year-old woman, a known case of polyarteritis nodosa (PAN), presented with acute onset severe headache associated with vomiting. CT scan revealed acute subarachnoid hemorrhage in the right sylvian fissure (Figure 1A). Digital subtraction angiography (DSA) (Axiom Artis Zee; Siemens, Erlangen, Germany) revealed a small lobulated right middle cerebral artery (MCA) bifurcation aneurysm (Figure 1B). An irregular projection was seen at the fundus, which was probably the point of rupture. There were associated small broad-based aneurysmal bulges distally in the superior division of the MCA, right posterior communicating artery origin (not shown), left MCA bifurcation (Figure 1C) and at the left posterior inferior cerebellar artery (PICA) origin (Figure 1D). She had been diagnosed with PAN one year earlier, when she had presented with recurrent abdominal pain, bleeding per rectum along with generalized malaise, weakness and weight loss. There was no family history of multiple intracranial aneurysms and no clinical features to suggest other associations of multiple intracranial aneurysms. She was also found to be hypertensive. Her renal function tests were normal, Erythrocyte sedimentation rate was 38 at the end of one hour and C Reactive Protein was 15 mg/dl. Viral markers including hepatitis B surface antigen were negative. Immunological profile, including ANA, ANCA, anti-cardiolipin antibodies, was negative. Her upper gastrointestinal endoscopy was negative for any lesion. Visceral angiogram showed focal aneurysmal dilatations of small arterial branches within splenic, pancreatic, hepatic and renal parenchyma as well as along the mucosal surface of the distal jejunum and proximal ileal loops (Figure 2). On the basis of these findings, she was diagnosed as PAN based on the criteria established by American College of Rheumatology 1. Since then she had been placed on a treatment protocol of steroid and cyclophosphamide pulse therapy. In view of the distribution of blood on CT scan and the morphology of the right MCA bifurcation aneurysm, it was considered to be the one which had bled. After discussion of the management options, endovascular coiling was planned. The procedure was performed under general anesthesia. A guiding catheter (6F, Envoy, Codman Neurovascular, Raynham, MA, USA) was positioned in the cervical segment of the right internal cerebral artery (ICA). Intravenous bolus of 3000 IU of heparin was given at the start of procedure and activated clotting time was maintained at twice the level of baseline during the procedure. Under roadmap guidance, a microcatheter (Excelsior SL-10, Boston Scientific, Target Therapeutics, Fremont, CA) was placed in the aneurysm with help of microguidewire (Transcend-14, Boston Scientific, Target Therapeutics, Fremont, CA). The aneurysm was embolized using multiple detachable coils. Post-procedure angiogram showed small neck remnant with near total aneurysm occlusion- Raymond grade II 2 (Figure 3) with preservation of the normal vasculature. Follow-up MR angiogram (Magnetom Verio 3T, Siemens, Erlangen, Germany) after 3- months showed minimal bulge at right MCA bifurcation with no significant aneurysm recanalization (Figure 3F). Other unruptured aneurysms were unchanged. Patient refused for DSA and at 9-month follow-up she was clinically stable on medical therapy.
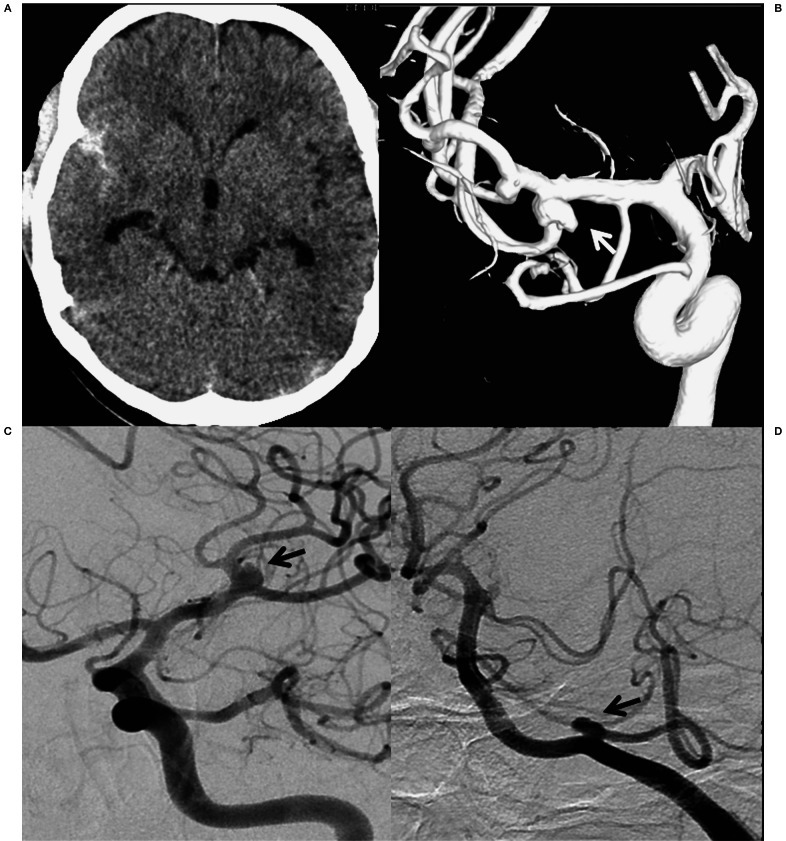
Figure 1.
A) CT scan shows SAH in the right sylvian fissure. B) 3D angiogram of the right ICA delineating a right MCA aneurysm with an irregular projection from the fundus (arrow). A small M2 segment saccular aneurysm is also seen arising from the superior division of the right MCA. C) Left ICA angiogram showing a small left MCA aneurysm (arrow). D) Left vertebral artery angiogram showing a small PICA aneurysm (arrow).
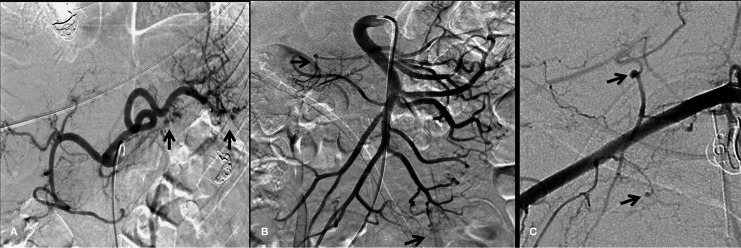
Figure 2.
Visceral angiograms. A) Selective injection of the celiac trunk showing small aneurysmal dilatations of splenic and pancreatic branches (arrows). B) Superior mesenteric artery angiogram shows small aneurysms in the inferior pancreaticoduodenal branch and jejunal branches (arrows). C) Selective injection of the right subclavian artery showing small aneurysmal dilatations in muscular branches (arrows).
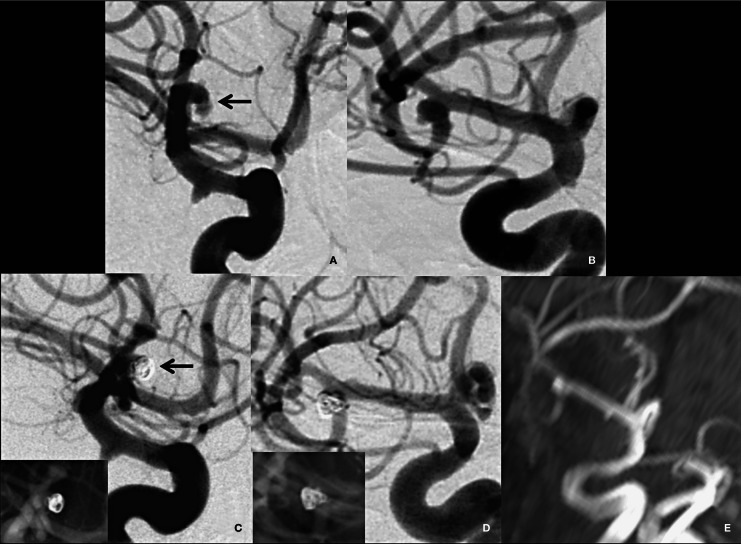
Figure 3.
Embolization procedure. A) Pre-embolization angiogram in lateral projection. B) Preembolization angiogram in a similar projection to the 3D image in Figure 1B. C,D) Post-embolization images showing near complete occlusion of the aneurysm with a small neck remnant (arrow in C). The in set in C and D shows the coil mass. E) Follow-up MR angiogram after 3 months showing a minimal bulge at the right MCA bifurcation with no significant aneurysm recanalization.
Discussion
Polyarteritis nodosa (PAN) is the prototypic necrotizing vasculitis that usually affects small to medium size vessels in multiple organs 3. The most common sites affected are skin, joints, kidney, gastrointestinal tract and peripheral nerves 3. Angiographic findings include aneurysms and segmental ectasia which were seen in 61% of cases in the study by Stanson et al. 4. Most of the aneurysms were seen in abdominal vessels. In their study, occlusive arterial lesions like luminal irregularity, stenosis or occlusion were seen in 39% of cases. The prevalence of CNS involvement ranges as widely as 15 to 45% 5 and does not usually occur until late in the course of the illnes 6. The most commonly reported CNS manifestation is diffuse encephalopathy, followed in frequency by focal deficits and seizures 5,6. Typical neuroimaging findings are small peripheral cerebral infarctions secondary to probable vasculitis in intracranial arteries 7. Reports of cerebral angiographic findings are few and have shown either alternating segments of narrowing and widening of small and medium size intracranial arteries or occlusion of small arteries 7. Although aneurysm of the visceral arteries is a common finding in patients with PAN, the association between PAN and intracranial aneurysms has rarely been reported. Our review of the literature revealed 15 reported cases of PAN associated with intracranial aneurysms (Table 1). Eleven of the 15 cases presented with subarachnoid or intracerebral hemorrhage. As in our case, most of the reported cases were already diagnosed as PAN before presenting with cerebral symptomatology. It is very unusual for a case of PAN to present with intracranial aneurysms as the initial presentation. Simonetti et al. 11 reported a case of PAN presenting with subarachnoid hemorrhage (SAH) as the initial manifestation. Sarkar et al. 21 reported a case of distal anterior inferior cerebellar artery (AICA) aneurysm in a 60-year-old man presenting with cranial nerve palsy, who was subsequently diagnosed as PAN. Eight of the 15 reported cases had more than one intracranial aneurysm. Aneurysms were widespread involving supra- and infra-tentorial compartments. Multiple small peripheral aneurysms reported as “miliary aneurysms” were seen in two cases 9,17. Large aneurysms involving cavernous carotid segments were seen in one patient 19. Rumboldt et al. 17 presented a case of PAN with multiple intracranial aneurysms along with dural arteriovenous fistula (AVF). The authors proposed that the AVF was probably secondary to PAN-associated venous sinus thrombosis. Our case also had multiple aneurysms in the supra- as well as infra-tentorial compartments at the bifurcations and branch points. The tendency of the vasculitis process to involve these locations has been reported in the literature where weakening of the arterial wall may cause aneurysm formation 7.
Table 1.
Review of cases of PAN with intracranial aneurysms reported in literature.
| No | Reference | Presentation | Age | Gender | Number of intracranial aneurysms |
Location of aneurysm |
Management |
|---|---|---|---|---|---|---|---|
| 1 | Gherardi et al.8 | SAH | 26 | F | 1 | ACA | ND |
| 2 | Leonhardt et al.9 | No hemorrgahic stroke |
16 | M | Multiple | Multiple | ND |
| 3 | Travers et al.10 | SAH | ND | ND | 2 | MCA, ICA | ND |
| 4 | Simonetti et al.11 | SAH | 49 | M | ND | ND | ND |
| 5 | Beattie et al.12 | SAH | 50 | M | 2 | ACOM, ICA | ND |
| 6 | Munn et al.13 | SAH | 20 | M | 4 | SCA, VA, MCA, ICA |
ND |
| 7 | Oran et al.14 | ICH | 10 | M | 2 | AICA, SCA | ND |
| 8 | Takahashi et al.15 | SAH | 70 | F | 1 | ACA | Trapping |
| 9 | Thompson et al.16 | SAH | 38 | F | 1 | VA | ND |
| 10 | Rumboldt et al.17 | SAH | 34 | F | 7 | Multiple | ND |
| 11 | Oomura et al.18 | SAH | 65 | M | NK | NK ? VA | ND, Died |
| 12 | Oh et al.19 | Headache, diplopia |
24 | M | 2 | Bilateral cavernous ICA |
Coiling. |
| 13 | Sharma et al.20 (report of two cases) |
Case 1-ICH, Case 2- asymptomatic |
13, 32 | M,M | Multiple | Distal branches, PCA |
ND |
| 14 | Sarkar et al.21 | Hearing loss and facial palsy |
60 | M | 1 | Distal AICA | Trapping |
| 15 | This report | SAH | 22 | F | 4 | Bilateral MCA,PCOM, PICA |
Coiling |
| ACA, anterior cerebral artery; ACOM, anterior communicating; AICA, anterior inferior cerebellar artery; F, female; ICA, internal carotid artery; M, male; MCA, middle cerebral artery; ND, not described; NK, not known; PCA, posterior cerebellar artery; PICA, posterior inferior cerebellar artery; SAH, subarachnoid hemorrhage; SCA, superior cerebellar artery; VA, vertebral artery. | |||||||
High-dose cyclophosphamide and prednisolone therapy has been shown to cause regression of aneurysms in patients with PAN. 22. However, ruptured intracranial aneurysms have a high incidence of rebleeding which can be clinically devastating. Oomura et al. 18 reported a case of PAN in which repeat SAH occurred in spite of intravenous steroid therapy and proved to be fatal for the patient. We are aware of only three case reports regarding operative or interventional management of cerebral aneurysms in these patients 15,19,21. Takahashi et al. 15 operated on a ruptured anterior cerebral artery aneurysm in a known case of PAN. The aneurysm was trapped and resected, however the post-operative course was complicated by intracranial hemorrhage secondary to consumption coagulopathy. Oh et al. 19 presented a case of PAN presenting as bilateral ophthalmoplegia due to bilateral large ICA aneurysms in the cavernous segment. Endovascular coil embolization was done for both the aneurysms. Sarkar et al. 21 reported a case of distal AICA aneurysm in a 60-year-old man who presented with seventh and eighth cranial nerve palsy. Trapping and thrombectomy of the aneurysm was performed which lead to a symptomatic infarction in the middle cerebellar peduncle.
We decided to treat our case because there was a risk of repeat hemorrhage in spite of medical therapy for vasculitis 18. The aneurysm morphology with an irregular projection also indicated a high risk of repeat bleeding. Persistence of untreated aneurysms on follow-up MR indicates that the ruptured aneurysm was unlikely to have regressed and supported our decision to occlude the aneurysm. In view of the possible ongoing vasculitis and high rate of complications in previously operated cases 15,21, we opted for a minimally invasive approach of endovascular coiling. Our case is the first reported case in which coiling has been performed in ruptured aneurysm associated with PAN. Our case is also unique in that it is the only one with clinical and imaging follow-up in this rare condition.
Conclusion
Although rare, PAN can be associated with intracranial aneurysms which can cause subarachnoid or parenchymal hemorrhage. These aneurysms are frequently multiple, vary in size and location, and can involve the circle of Willis or small arteries. Selected cases can be treated safely by coil embolization.
References
- 1.Lightfoot RW Jr, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum. 1990;33:1088–1093. doi: 10.1002/art.1780330805. [DOI] [PubMed] [Google Scholar]
- 2.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32:1998–2004. doi: 10.1161/hs0901.095600. [DOI] [PubMed] [Google Scholar]
- 3.Conn DL. Polyarteritis. Rheum Dis Clin North Am. 1990;16:341–362. [PubMed] [Google Scholar]
- 4.Stanson AW, Friese JL, Johnson CM, et al. Polyarteritis nodosa: spectrum of angiographic findings. Radiographics. 2001;21:151–159. doi: 10.1148/radiographics.21.1.g01ja16151. [DOI] [PubMed] [Google Scholar]
- 5.Ford RG, Siekert RG. Central nervous system manifestations of periarteritis nodosa. Neurology. 1965;15:114–122. doi: 10.1212/wnl.15.2.114. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg MR, Parshley M, Gibson S, et al. Central nervous system polyarteritis nodosa. West J Med. 1990;153:553–556. [PMC free article] [PubMed] [Google Scholar]
- 7.Provenzale JM, Allen NB. Neuroradiologic findings in polyarteritis nodosa. Am J Neuroradiol. 1996;17:1119–1126. [PMC free article] [PubMed] [Google Scholar]
- 8.Gherardi GJ, Lee HY. Localized dissecting hemorrhage and arteritis. Renal and cerebral manifestations. JAMA. 1967;199:219–220. [PubMed] [Google Scholar]
- 9.Leonhardt ET, Jakobson H, Ringqvist OT. Angiographic and clinicophysiologic investigation of a case of polyarteritis nodosa. Am J Med. 1972;53:242–256. doi: 10.1016/0002-9343(72)90133-7. [DOI] [PubMed] [Google Scholar]
- 10.Travers RL, Allison DJ, Brettle RP, et al. Polyarteritis nodosa: a clinical and angiographic analysis of 17 cases. Semin Arthritis Rheum. 1979;8:184–199. doi: 10.1016/s0049-0172(79)80007-4. [DOI] [PubMed] [Google Scholar]
- 11.Simonetti C, Bechini F. Subarachnoid hemorrhage as initial manifestation of polyarteritis nodosa. Riv Neurol. 1988;58(5):180–182. [PubMed] [Google Scholar]
- 12.Beattie DK, Hellier WP, Powell MP. Stroke-induced cardiovascular changes: a rare cause of death from polyarteritis nodosa. Br J Neurosurg. 1995;9:223–226. doi: 10.1080/02688699550041601. [DOI] [PubMed] [Google Scholar]
- 13.Munn EJ, Alloway JA, Diffin DC, et al. Polyarteritis with symptomatic intracerebral aneurysms at initial presentation. J Rheumatol. 1998;25:2022–2025. [PubMed] [Google Scholar]
- 14.Oran I, Memis A, Parildar M, et al. Multiple intracranial aneurysms in polyarteritis nodosa: MRI and angiography. Neuroradiology. 1999;41:436–439. doi: 10.1007/s002340050779. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi JC, Sakai N, Iihara K, et al. Subarachnoid hemorrhage from a ruptured anterior cerebral artery aneurysm caused by polyarteritis nodosa. Case report. J Neurosurg. 2002;96:132–134. doi: 10.3171/jns.2002.96.1.0132. [DOI] [PubMed] [Google Scholar]
- 16.Thompson B, Burns A. Subarachnoid hemorrhages in vasculitis. Am J Kidney Dis. 2003;42:582–585. doi: 10.1016/s0272-6386(03)00791-1. [DOI] [PubMed] [Google Scholar]
- 17.Rumboldt Z, Beros V, Klanfar Z. Multiple cerebral aneurysms and a dural arteriovenous fistula in a patient with polyarteritis nodosa. Case illustration. J Neurosurg. 2003;98:434. doi: 10.3171/jns.2003.98.2.0434. [DOI] [PubMed] [Google Scholar]
- 18.Oomura M, Yamawaki T, Naritomi H, et al. Polyarteritis nodosa in association with subarachnoid hemorrhage. Intern Med. 2006;45:655–658. doi: 10.2169/internalmedicine.45.1632. [DOI] [PubMed] [Google Scholar]
- 19.Oh MS, Kim MH, Chu MK, et al. Polyarteritis nodosa presenting with bilateral cavernous internal carotid artery aneurysms. Neurology. 2008;70:405. doi: 10.1212/01.wnl.0000298726.31304.be. [DOI] [PubMed] [Google Scholar]
- 20.Sharma S, Kumar S, Mishra NK, et al. Cerebral miliary micro aneurysms in polyarteritis nodosa: report of two cases. Neurol India. 2010;58:457–459. doi: 10.4103/0028-3886.65840. [DOI] [PubMed] [Google Scholar]
- 21.Sarkar A, Link MJ. Distal anterior inferior cerebellar artery aneurysm masquerading as a cerebellopontine angle tumor: case report and review of literature. Skull Base. 2004;14:101–106. doi: 10.1055/s-2004-828703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raza K, Carruthers DM, Exley AR, et al. Dramatic aneurysm regression in polyarteritis nodosa following high dose pulse cyclophosphamide. J Rheumatol. 2000;27:1320–1321. [PubMed] [Google Scholar]