Summary
Vertebral artery arteriovenous fistula (VAVF) is mostly known as a post-traumatic and/or iatrogenic arteriovenous complication. However, spontaneous high-flow VAVF associated with flow reversal in the basilar artery has not been reported in children. We describe a unique asymptomatic presentation of a spontaneous high-flow VAVF associated with flow reversal in the basilar artery in a pediatric patient. The literature for classification, pathophysiology, treatment strategies, and post-procedural complications is also reviewed.
Key words: spontaneous vertebral artery arteriovenous fistula, endovascular embolization, reversed basilar artery flow, pediatric
Introduction
Vertebral artery arteriovenous fistula (VAVF), a sub-type of extradural arteriovenous fistula (AVF), is a simple or complex direct communication between the extracranial portion of the vertebral artery and neighboring venous plexus (epidural and paravertebral veins) most prominently at the C1-C2 and C6-C7 levels 1,2. VAVFs are relatively rare and have been reported in association with connective tissue diseases including fibromuscular dysplasia, neurofibromatosis, Ehler-Danlos syndrome, and Marfan's syndrome 3-5. Although VAVFs are commonly encountered in adults secondary to post- traumatic and/or iatrogenic arteriovenous complications, spontaneous and congenital VAVFs have rarely been described in the pediatric population 2,6,7.
Reversal of flow in the basilar artery is exceptionally uncommon and has been described in rare complicated cases of intracranial vertebral artery dissections 8, vertebrobasilar occlusions 9, giant cell arteritis 10, bilateral subclavian steal phenomenon 11, and post-traumatic/iatrogenic VAVFs in adults 12. We present a unique pediatric case of a high-flow VAVF causing basilar flow reversal requiring endovascular treatment with coil embolization.
Case Report
History and examination. An eight-year-old boy presented to the emergency department with fever and lethargy. During routine physical examination, a prominent pulse was palpable and a bruit was auscultated on the right side of the neck. The patient denied headache, neck pain, tinnitus, vertigo, hoarseness, or focal neurological deficits. His neurological, respiratory, and cardiac examinations were normal. No history of trauma to the head and neck area or family history of connective tissue disorders was noted.
On MRI head and neck studies an extradural AVF was suspected at the level of the distal V3 segment draining into the adjacent right vertebral venous plexus (Figure 1). Digital subtraction angiography (DSA) demonstrated a high-flow AVF between the distal portion of the right cervical vertebral artery at the level of the proximal transverse foramen of C2, draining into a very large recipient paraspinous venous pouch (Figure 2). Significant enlargement of the right vertebral artery was observed at and above the level of the shunt with retrograde supply across the hypertrophied left vertebral artery and vertebrobasilar junction into the right vertebral artery V4 segment. Selective DSA also demonstrated an enlarged right deep cervical artery supplying the AVF and an enlarged left costocervical trunk augmenting the collateral supply to the left vertebral artery. There was no antegrade visualization of the basilar artery from either the vertebral or costocervical/thyrocervical injections due to the significant steal towards the AVF. Internal carotid DSA demonstrated a large right posterior communicating artery opacifying the basilar artery, with reversed flow caudally toward the AVF. Venous drainage from the recipient C2 varix was predominantly via the paraspinous, right external jugular, and epidural veins.
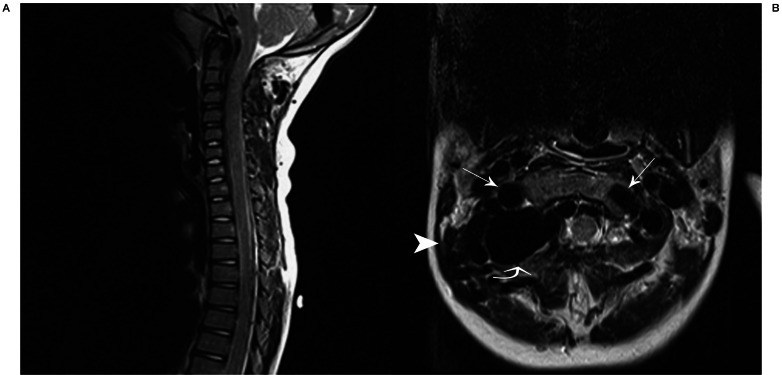
Figure 1.
T2W sagittal (A) and axial (B) images demonstrate enlarged flow-voids of the distal V3 segment of the vertebral arteries, bilaterally, R>L (arrows) with a large flow void dorsal to the distal cervical right vertebral artery representing the recipient venous pouch of the vertebral artery arteriovenous fistula (curved arrow). Prominent flow-voids of bilateral paraspinal and epidural veins are demonstrated (arrowhead).
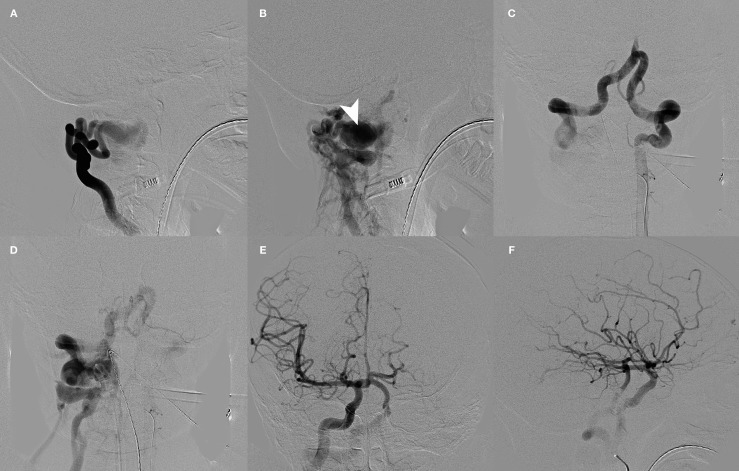
Figure 2.
A-F) Pre-embolization angiography. A,B) Right costocervical trunk lateral views demonstrate extremely high flow arteriovenous fistula at the level of the proximal transverse foramen of C2 draining into a very large recipient paraspinous venous pouch (arrowhead). Late venous phase demonstrates drainage of venous pouch into the paraspinous, right external jugular, and epidural veins. C,D) Left vertebral artery angiography AP views demonstrate significant enlargement of the left vertebral artery and both V4 segments. There is no antegrade opacification of the basilar artery. AP (E) and lateral (F) right internal carotid artery injections demonstrate reverse flow in the basilar artery via a large right posterior communicating artery.
Treatment. Under general anesthesia and utilizing a standard transfemoral approach, the left vertebral artery was selectively accessed with a guide sheath placed into the distal cervical segment. A microcatheter was advanced across the vertebrobasilar junction into the right vertebral artery in retrograde fashion, with its tip positioned in the recipient venous varix. Occlusion of the venous varix was performed using detachable platinum (Cosmos, Hydrocoil, Microvention, Tustin, CA, USA) and pushable fibered (Nester, Cook, Bloomington, IN, USA) coils and was continued retrograde into the adjacent dilated and patulous segment of the right vertebral artery (Figure 3). Multiple control DSA images demonstrated no evidence for a residual VAVF.
Postoperative course. Following embolization, the patient was left intubated and sedated for 18 hours in the pediatric intensive care unit (PICU) for strict hemodynamic control and prophylactic heparin infusion. Jugular pulsation and blood pressure were strictly monitored with nicardipine infusion to maintain a 25% reduced mean arterial pressure (MAP) of 50-60 mmHg. Subsequently, the patient was extubated and weaned off the nicardipine, remaining normotensive. The patient complained of visual disturbance soon after the sedation was terminated. However, the visual impairment completely resolved within 36 hours following embolization. The patient's hospital course was uncomplicated and he was discharged on post-embolization day 2. In follow-up visits, no abnormal sign or symptom was noted. Follow-up DSA and time-resolved MR angiography after embolization confirmed the reconstitution of the right vertebral artery with antegrade flow into the basilar artery and complete resolution of the fistula (Figure 4).
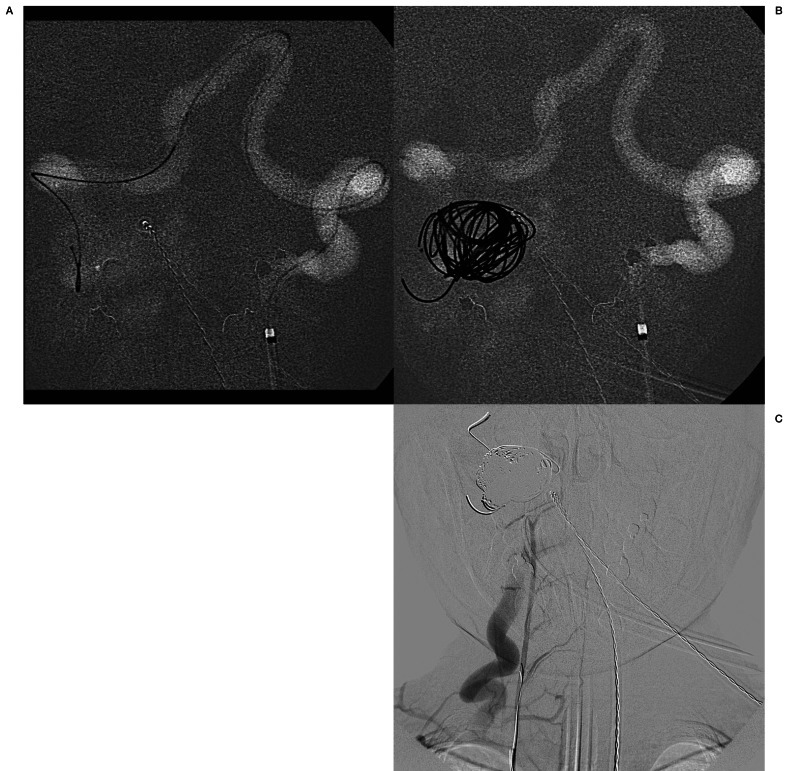
Figure 3.
Images obtained during treatment. A) Roadmap image demonstrating retrograde access to the lesion by navigating the microcatheter via the left vertebral artery into the right vertebral artery and placing the tip within the recipient venous pouch. B) AP roadmap store monitor image demonstrating coils deployed into the venous pouch. C) Unsubtracted image from an AP final control angiogram via the right costocervical trunk demonstrates complete occlusion of the fistula.
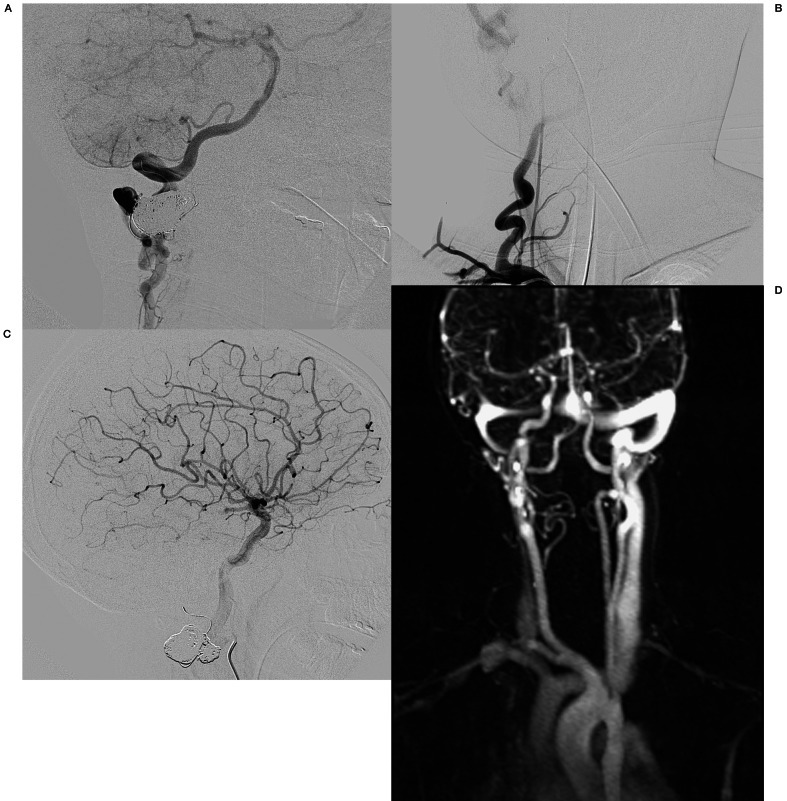
Figure 4.
A-C) Follow-up angiography 6 weeks after embolization. A) Right deep cervical artery angiography lateral view demonstrates a decrease in size of the previously enlarged deep cervical artery with reconstitution of the right vertebral artery at the C2 level. Collateral branches fill the distal V3 and V4 segments of the right vertebral artery in antegrade fashion and there is antegrade flow within the basilar artery with a normal posterior circulation. B) Right subclavian artery AP angiogram demonstrates opacification of the diminutive proximal cervical vertebral artery extending just proximal to the level of the treated arteriovenous fistula. There is no evidence for any supply to the arteriovenous fistula. C) Lateral view of the right internal carotid artery angiography reveals normal distal cervical and intracranial segments of the artery without opacification of the basilar artery consistent with antegrade flow within the basilar artery. D) Follow-up time-resolved MR angiography 15 months after embolization demonstrates no early venous enhancement consistent with the resolution of the arteriovenous fistula.
Discussion
Reversal of basilar artery flow is an uncommonly described entity, and indicates both very high-flow AV shunting and patent posterior communicating arteries to compensate for vertebrobasilar insufficiency 8-11,13. Although reversed basilar flow has been associated with significant neurological symptoms in patients suffering from subclavian steal phenomenon 11, it may be asymptomatic as in our case and emphasizes the robust collateral network often present in children. Furthermore, we have not been able to identify a prior pediatric case documenting a high grade VAVF with basilar artery flow reversal in the available medical literature. VAVFs commonly develop secondary to traumatic or iatrogenic etiologies 2,7. In rare instances, in the absence of an identifiable traumatic or iatrogenic cause, VAVFs may be congenital, detected immediately after birth in association with significant clinical symptoms 2,7,14, versus spontaneous diagnosis in older age groups especially in the pediatric population and may be asymptomatic 4,6,15. Congenital and spontaneous VAVFs in the pediatric population most often occur at the C1-C2 level and the embryonic proatlantal system has been implicated in the evolution of these lesions 1. Lower cervical spontaneous VAVFs occur in an older population, are usually low-flow lesions, and are more common in patients with underlying connective tissue disorders 3-5. In a review of 47 cases reported by Kondoh et al., VAVFs were classified into three types based on their hemodynamic features: 1) lesions supplied from the parent vertebral artery with antegrade flow; 2) lesions with retrograde supply from the contralateral vertebral artery; 3) lesions supplied from the contralateral vertebral artery and concurrent steal from the internal carotid arteries via basilar artery retrograde flow into the parent vertebral artery which have been rarely described 12. Although 30% of patients are symptom-free at the time of diagnosis, a pulsatile bruit over the neck with tinnitus comprise the most common findings in patients with VAVFs 16.Congenital or spontaneous high-flow VAVFs can be associated with steal phenomenon and vertebrobasilar insufficiency resulting in transient ischemic attacks of diplopia, vertigo, and ataxia 16,17. Cardiac decompensation leading to high-output congestive heart failure resulting from high-flow VAVF is more common in the congenital and pediatric population 2,6,14,18. Additionally, as a subtype of spinal extradural AVF, VAVFs may cause local mass effect with radiculopathy, venous congestion with spinal cord/medullary ischemia, or subarachnoid hemorrhage particularly in adults.
Conservative management has been described in antegrade-flow, minor VAVF in the absence of disabling clinical symptoms 20. High-flow lesions are most commonly associated with retrograde flow in the parent vertebral artery, gradually worsening and leading to symptomatic presentations, particularly in children 17. The ultimate goal of various treatment strategies is the permanent occlusion of the fistula and preservation of the ipsilateral vertebral artery which is a considerable challenge in surgical approaches particularly in high-flow lesions and in patients with underlying vascular dysplasia. In addition, a wide surgical dissection is usually required to achieve adequate proximal and distal access, especially if the lesion occurs at the C1-C2 level 21. Endovascular embolization has been accepted as the primary treatment for VAVFs 16.Using latex detachable balloons and detachable coils, complete resolution of the lesion preserving the vertebral artery involved has been achieved in 25%-95% of VAVFs in multiple reported case series 16,20,22. In addition, stent grafts 18, liquid embolic agents including N-butyl-cyanoacrylate 7,14,21 and Onyx 23 have been utilized for endovascular embolization of VAVFs in recent years. Endovascular embolization utilizing detachable coils and latex detachable balloons via the ipsilateral and/or contralateral vertebral artery has been the most described and feasible technique for occlusion of the high-flow VAVFs, particularly in the pediatric population 2,6,24. Using platinum detachable coils, compact and stable occlusion of the recipient venous varix can be achieved with excellent results. Detachable platinum coils avoid the risks of unintended distal migration of a liquid embolic agent into the distal draining veins of high-flow lesions or embolic reflux into the vertebral-basilar system 24. Cardiac decompensation may develop during the evolution of the high-flow VAVFs in children. Cardiovascular complications due to the sudden hemodynamic changes in vertebral blood flow may be encountered following treatment; thus strict hemodynamic and blood pressure monitoring is mandatory in the neurointensive care unit following treatment. Nakstad et al. reported a remarkable cardiovascular reaction in a seven-year-old girl following the endovascular embolization of a high-flow VAVF with steal from the contralateral vertebral artery 6. They encountered a considerable rise in systolic blood pressure (from 110 to 180 mmHg) and heart rate (200 beats per minute) lasting for a week that was eventually controlled by administration of various vasodilator agents. Additionally, neurological dysfunction is possible in longstanding VAVFs with chronic steal from the vertebrobasilar system, posing risks for autoregulatory dysfunction and/or reperfusion injury 12. Adverse neurovascular ischemic events following abrupt occlusion of several VAVFs have been described by Halbach et al., which were resolved by staged closure of the fistulas 25. However, phrenic nerve paresis following staged coil embolization of a VAVF leading to severe pneumonia and death has also been reported 19. In addition, immediate and delayed spinal cord ischemia following obliteration of the VAVF have been described 4,26. According to the “normal perfusion pressure break-through” (NPPB) phenomenon described by Spetzler et al. in 1978, the vascular autoregulation mechanism in the chronically hypoperfused cerebral tissue downstream to an arteriovenous shunt with significant steal is impaired. This leads to the failure of regional CBF adjustment and excessive perfusion pressures following sudden restoration of the normal blood flow causing cerebral edema or even hemorrhage 27.
In 1993 Al-Rodhan et al. challenged this hypothesis, proposing the “occlusive hyperemia” theory 28. They suggested edema and/or hemorrhage may develop secondary to passive hyperemia caused by venous outflow obstruction in the cerebral tissue adjacent to an AVM in addition to stagnant blood flow in the previous fistula's arterial feeders. Subsequently, Young et al. demonstrated the normal function of the cerebral autoregulatory system in patients with NPPB-like complications 29. In addition, it has been demonstrated that increased CBF following treatment is not limited to the region adjacent to the lesion 30. In this regard, Kondoh et al. described massive multifocal edema and hemorrhage immediately after endovascular occlusion of a high-flow VAVF leading to the patient's death 12. Leftward adaptive displacement of the autoregulatory curve in which the normal pressure exceeds the upper limits of shifted autoregulatory capacity proposed by Young et al. seems to be the most evidence-based explanation for these post-treatment complications 30. Although we did not encounter any critical neurovascular or cardiovascular complication immediately following restoration of normal blood flow in the vertebral-basilar arteries, the patient was kept intubated and sedated in the PICU for close hemodynamic monitoring and tight blood pressure control aimed at maintaining the MAP at 50-60 mmHg. The patient's transient visual complaints appeared to be related to his recent sedation; however, they may theoretically have been due to transient posterior circulation hyperemia and delayed completion of autoregulation.
Conclusion
A methodical angiographic and interventional technique, an understanding of complex AVF anatomy, and post-procedure management play a pivotal role in obtaining technical success and minimizing complications in the treatment of high-flow VAVFs with significant steal.
References
- 1.Van Dijk JM CB, Steinberg GK. Spinal arteriovenous fistulas. In: Kim BR, Huhn S, Newton P, editors. Surgery of the pediatric spine. New York: Thieme; 2008. pp. 345–347. [Google Scholar]
- 2.Shownkeen H, Bova D, Chenelle AG, et al. Pediatric congenital vertebral artery arteriovenous malformation. Pediatric Radiol. 2003;33:354–356. doi: 10.1007/s00247-003-0866-0. [DOI] [PubMed] [Google Scholar]
- 3.Bahar S, Chiras J, Carpena JP, et al. Spontaneous vertebro-vertebral arterio-venous fistula associated with fibro-muscular dysplasia. Report of two cases. Neuroradiology. 1984;26:45–49. doi: 10.1007/BF00328203. [DOI] [PubMed] [Google Scholar]
- 4.Paolini S, Colonnese C, Galasso V, et al. Extradural arteriovenous fistulas involving the vertebral artery in neurofibromatosis Type 1. Spine. 2008;8:181–185. doi: 10.3171/SPI/2008/8/2/181. [DOI] [PubMed] [Google Scholar]
- 5.Baltacioğlu F. Endovascular treatment of a vertebral arteriovenous fistula: case report. Marmara Med J. 2009;22:248–251. [Google Scholar]
- 6.Nakstad PH, Haakonsen M, Magnaes B, et al. Combined endovascular and surgical treatment in vertebral arteriovenous fistula. A case report. Acta Radiol. 1997;38:25–29. doi: 10.1080/02841859709171237. [DOI] [PubMed] [Google Scholar]
- 7.Nunez F, Martinez-Costa C, Soler F, et al. Arteriovenous fistula of the vertebral artery in a female infant with hypotonia and cephalocorporal disproportion. Acta Paediatr. 2010;99:1434–1436. doi: 10.1111/j.1651-2227.2010.01831.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee SI, Yang HD, Son IH, et al. Recovery of reversed basilar artery flow as seen by transcranial sonography and MRA source images for vertebral dissection. J Neuroimaging. 2008;18:451–453. doi: 10.1111/j.1552-6569.2007.00201.x. [DOI] [PubMed] [Google Scholar]
- 9.Sharma VK, Teoh HL, Chan BP, et al. Reversed flow in the basilar artery in acute vertebrobasilar ischemia. J Clin Neurosci. 2009;16:1493–1495. doi: 10.1016/j.jocn.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Boettinger M, Sebastian S, Gamulescu MA, et al. Bilateral vertebral artery occlusion with retrograde basilary flow in three cases of giant cell arteritis. BMJ Case Rep. 2009:2009. doi: 10.1136/bcr.07.2008.0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Antelo MJ, Puy-Nunez A, Ayo-Martin O, et al. Relevance of basilar artery study in patients with subclavian steal phenomenon. Open Neurol J. 2011;5:34–36. doi: 10.2174/1874205X01105010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kondoh T, Tamaki N, Takeda N, et al. Fatal intracranial hemorrhage after balloon occlusion of an extracranial vertebral arteriovenous fistula. Case report. J Neurosurg. 1988;69:945–948. doi: 10.3171/jns.1988.69.6.0945. [DOI] [PubMed] [Google Scholar]
- 13.Ribo M, Garami Z, Uchino K, et al. Detection of reversed basilar flow with power-motion Doppler after acute occlusion predicts favorable outcome. Stroke. 2004;35:79–82. doi: 10.1161/01.STR.0000106760.25228.2C. [DOI] [PubMed] [Google Scholar]
- 14.Nakano S, Agid R, Klurfan P, et al. Limitations and technical considerations of endovascular treatment in neonates with high-flow arteriovenous shunts presenting with congestive heart failure: report of two cases. Childs Nerv Syst. 2006;22:13–17. doi: 10.1007/s00381-005-1237-y. [DOI] [PubMed] [Google Scholar]
- 15.Modi M, Bapuraj JR, Lal A, et al. Vertebral arteriovenous fistula presenting as cervical myelopathy: a rapid recovery with balloon embolization. Cardiovasc Intervent Radiol. 2010;33:1253–1256. doi: 10.1007/s00270-009-9708-2. [DOI] [PubMed] [Google Scholar]
- 16.Beaujeux RL, Reizine DC, Casasco A, et al. Endovascular treatment of vertebral arteriovenous fistula. Radiology. 1992;183:361–367. doi: 10.1148/radiology.183.2.1561336. [DOI] [PubMed] [Google Scholar]
- 17.Sadasivan B, Mehta B, Dujovny M, et al. Balloon embolization of nontraumatic vertebral arteriovenous fistulae in children. Surgical Neurol. 1989;32:126–130. doi: 10.1016/0090-3019(89)90200-0. [DOI] [PubMed] [Google Scholar]
- 18.Saket RR, Razavi MK, Sze DY, et al. Stent-graft treatment of extracranial carotid and vertebral arterial lesions. J Vasc Interv Radiol. 2004;15:1151–1156. doi: 10.1097/01.rvi.0000134496.71252. [DOI] [PubMed] [Google Scholar]
- 19.Bostroem A, Hans FJ, Moeller-Hartmann W, et al. Spontaneous vertebral arteriovenous fistula simulating a cervical spine tumour. Minim Invasive Neurosurg. 2008;51:54–56. doi: 10.1055/s-2007-1004549. [DOI] [PubMed] [Google Scholar]
- 20.Goyal M, Willinsky R, Montanera W, et al. Spontaneous vertebrovertebral arteriovenous fistulae clinical features, angioarchitecture and management of twelve patients. Interv Neuroradiol. 1999;5:219–224. doi: 10.1177/159101999900500304. [DOI] [PubMed] [Google Scholar]
- 21.Jayaraman MV, Do HM, Marks MP. Treatment of traumatic cervical arteriovenous fistulas with N-butyl-2-cyanoacrylate. Am J Neuroradiol. 2007;28:352–354. [PMC free article] [PubMed] [Google Scholar]
- 22.Vinchon M, Laurian C, George B, et al. Vertebral arteriovenous fistulas: a study of 49 cases and review of the literature. Cardiovasc Surg. 1994:359–369. [PubMed] [Google Scholar]
- 23.Gordhan A. Onyx embolization of high-flow spontaneous cervical vertebral arteriovenous fistula. Vasc Endovascular Surg. 2012;46:484–486. doi: 10.1177/1538574412452156. [DOI] [PubMed] [Google Scholar]
- 24.De Keukeleire K, Defreyne L, Vanlangenhove P, et al. Asymptomatic congenital extradural vertebral arteriovenous fistula: treatment with electrolytically detachable coils. Cardiovasc Intervent Radiol. 2006;29:311–314. doi: 10.1007/s00270-004-9197-2. [DOI] [PubMed] [Google Scholar]
- 25.Halbach VV, Higashida RT, Hieshima GB, et al. Normal perfusion pressure breakthrough occurring during treatment of carotid fistulas. Am J Neuroradiol. 1987;8:751–756. [PMC free article] [PubMed] [Google Scholar]
- 26.Dublin AB, Latchaw RE, Herrera DA, et al. Delayed complication after embolotherapy of a vertebral arteriovenous fistula: spinal cord ischemia. J Vasc Interv Radiol. 2010;21:392–393. doi: 10.1016/j.jvir.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Spetzler RF, Wilson CB, Weinstein P, et al. Normal perfusion pressure breakthrough theory. Clinical Neurosurg. 1978;25:651–672. doi: 10.1093/neurosurgery/25.cn_suppl_1.651. [DOI] [PubMed] [Google Scholar]
- 28.al-Rodhan NR, Sundt TM, Jr., Piepgras DG, et al. Occlusive hyperemia: a theory for the hemodynamic complications following resection of intracerebral arteriovenous malformations. J Neurosurg. 1993;78:167–175. doi: 10.3171/jns.1993.78.2.0167. [DOI] [PubMed] [Google Scholar]
- 29.Young WL, Kader A, Prohovnik I, et al. Pressure autoregulation is intact after arteriovenous malformation resection. Neurosurgery. 1993;32:491–496. doi: 10.1227/00006123-199304000-00001. discussion 496-497. [DOI] [PubMed] [Google Scholar]
- 30.Young WL, Pile-Spellman J, Prohovnik I, et al. Evidence for adaptive autoregulatory displacement in hypotensive cortical territories adjacent to arteriovenous malformations. Columbia University AVM Study Project. Neurosurgery. 1994;34:601–610. doi: 10.1227/00006123-199404000-00006. discussion 610-611. [DOI] [PubMed] [Google Scholar]