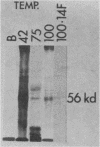
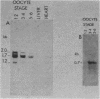
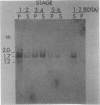
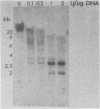
Abstract
Xenopus laevis oocytes contain a unique group of proteins which decrease during oogenesis, bind poly(A) RNA, and possibly play a role in the regulation of translation. A monoclonal antibody generated against one of these proteins was used to screen an expression vector cDNA library. A cDNA clone was isolated and confirmed to code for the binding protein by in vitro translation of hybrid-selected RNA followed by immunoprecipitation. This cDNA, when used in RNA gel blots, hybridized to four transcripts of 2.0, 1.7 (two transcripts of similar size), and 1.2 kilobases. All of the transcripts decreased in amount during oogenesis and were not evident in somatic cells. In addition, the fraction of the transcripts associated with polysomes decreased during oogenesis. Digestion of the cDNA insert with PstI generated two fragments of 220 and 480 base pairs which, when used as probes in an RNA gel blot, hybridized to unique as well as common transcripts. Genomic Southern blots suggested the presence of a single gene, indicating that these transcripts arose by alternative processing.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. M., Richter J. D., Chamberlin M. E., Price D. H., Britten R. J., Smith L. D., Davidson E. H. Sequence organization of the poly(A) RNA synthesized and accumulated in lampbrush chromosome stage Xenopus laevis oocytes. J Mol Biol. 1982 Mar 5;155(3):281–309. doi: 10.1016/0022-2836(82)90006-7. [DOI] [PubMed] [Google Scholar]
- Anderson D. M., Smith L. D. Patterns of synthesis and accumulation of heterogeneous RNA in lampbrush stage oocytes of Xenopus laevis (Daudin). Dev Biol. 1978 Dec;67(2):274–285. doi: 10.1016/0012-1606(78)90199-9. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bisbee C. A., Baker M. A., Wilson A. C., Haji-Azimi I., Fischberg M. Albumin phylogeny for clawed frogs (Xenopus). Science. 1977 Feb 25;195(4280):785–787. doi: 10.1126/science.65013. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capetanaki Y. G., Ngai J., Flytzanis C. N., Lazarides E. Tissue-specific expression of two mRNA species transcribed from a single vimentin gene. Cell. 1983 Dec;35(2 Pt 1):411–420. doi: 10.1016/0092-8674(83)90174-5. [DOI] [PubMed] [Google Scholar]
- Darnbrough C. H., Ford P. J. Identification in Xenopus laevis of a class of oocyte-specific proteins bound to messenger RNA. Eur J Biochem. 1981 Jan;113(3):415–424. doi: 10.1111/j.1432-1033.1981.tb05081.x. [DOI] [PubMed] [Google Scholar]
- Dixon L. K., Ford P. J. Regulation of protein synthesis and accumulation during oogenesis in Xenopus laevis. Dev Biol. 1982 Oct;93(2):478–497. doi: 10.1016/0012-1606(82)90136-1. [DOI] [PubMed] [Google Scholar]
- Dolecki G. J., Smith L. D. Poly(A)+ RNA metabolism during oogenesis in Xenopus laevis. Dev Biol. 1979 Mar;69(1):217–236. doi: 10.1016/0012-1606(79)90287-2. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Dworkin-Rastl E., Shrutkowski A., Dworkin M. B. Multiple ubiquitin mRNAs during Xenopus laevis development contain tandem repeats of the 76 amino acid coding sequence. Cell. 1984 Dec;39(2 Pt 1):321–325. doi: 10.1016/0092-8674(84)90010-2. [DOI] [PubMed] [Google Scholar]
- Falkenthal S., Parker V. P., Davidson N. Developmental variations in the splicing pattern of transcripts from the Drosophila gene encoding myosin alkali light chain result in different carboxyl-terminal amino acid sequences. Proc Natl Acad Sci U S A. 1985 Jan;82(2):449–453. doi: 10.1073/pnas.82.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford P. J., Mathieson T., Rosbash M. Very long-lived messenger RNA in ovaries of Xenopus laevis. Dev Biol. 1977 Jun;57(2):417–426. doi: 10.1016/0012-1606(77)90226-3. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Bond B. J., Hershey N. D., Mixter K. S., Davidson N. The actin genes of Drosophila: protein coding regions are highly conserved but intron positions are not. Cell. 1981 Apr;24(1):107–116. doi: 10.1016/0092-8674(81)90506-7. [DOI] [PubMed] [Google Scholar]
- Garcia R., Paz-Aliaga B., Ernst S. G., Crain W. R., Jr Three sea urchin actin genes show different patterns of expression: muscle specific, embryo specific, and constitutive. Mol Cell Biol. 1984 May;4(5):840–845. doi: 10.1128/mcb.4.5.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg A. M., King B. O., Roeder R. G. Xenopus 5S gene transcription factor, TFIIIA: characterization of a cDNA clone and measurement of RNA levels throughout development. Cell. 1984 Dec;39(3 Pt 2):479–489. doi: 10.1016/0092-8674(84)90455-0. [DOI] [PubMed] [Google Scholar]
- Golden L., Schafer U., Rosbash M. Accumulation of individual pA+ RNAs during oogenesis of Xenopus laevis. Cell. 1980 Dec;22(3):835–844. doi: 10.1016/0092-8674(80)90560-7. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlik C. C., Mahaffey J. W., Coutu M. D., Fyrberg E. A. Organization of contractile protein genes within the 88F subdivision of the D. melanogaster third chromosome. Cell. 1984 Jun;37(2):469–481. doi: 10.1016/0092-8674(84)90377-5. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D., Gurdon J. B., Partington G. A. Protein synthesis in oocytes of Xenopus laevis is not regulated by the supply of messenger RNA. Cell. 1977 Jun;11(2):345–351. doi: 10.1016/0092-8674(77)90051-4. [DOI] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E., Forbes D. J. The two embryonic U1 small nuclear RNAs of Xenopus laevis are encoded by a major family of tandemly repeated genes. Mol Cell Biol. 1984 Dec;4(12):2580–2586. doi: 10.1128/mcb.4.12.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- Richter J. D., Evers D. C. A monoclonal antibody to an oocyte-specific poly(A) RNA-binding protein. J Biol Chem. 1984 Feb 25;259(4):2190–2194. [PubMed] [Google Scholar]
- Richter J. D., Smith L. D., Anderson D. M., Davidson E. H. Interspersed poly(A) RNAs of amphibian oocytes are not translatable. J Mol Biol. 1984 Feb 25;173(2):227–241. doi: 10.1016/0022-2836(84)90191-8. [DOI] [PubMed] [Google Scholar]
- Richter J. D., Smith L. D. Developmentally regulated RNA binding proteins during oogenesis in Xenopus laevis. J Biol Chem. 1983 Apr 25;258(8):4864–4869. [PubMed] [Google Scholar]
- Richter J. D., Smith L. D. Differential capacity for translation and lack of competition between mRNAs that segregate to free and membrane-bound polysomes. Cell. 1981 Nov;27(1 Pt 2):183–191. doi: 10.1016/0092-8674(81)90372-x. [DOI] [PubMed] [Google Scholar]
- Richter J. D., Smith L. D. Reversible inhibition of translation by Xenopus oocyte-specific proteins. Nature. 1984 May 24;309(5966):378–380. doi: 10.1038/309378a0. [DOI] [PubMed] [Google Scholar]
- Rosbash M. Polyadenylic acid-containing RNA in Xenopus laevis oocytes. J Mol Biol. 1974 May 5;85(1):87–101. doi: 10.1016/0022-2836(74)90131-4. [DOI] [PubMed] [Google Scholar]
- Shastry B. S., Honda B. M., Roeder R. G. Altered levels of a 5 S gene-specific transcription factor (TFIIIA) during oogenesis and embryonic development of Xenopus laevis. J Biol Chem. 1984 Sep 25;259(18):11373–11382. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thiébaud C. H., Fischberg M. DNA content in the genus Xenopus. Chromosoma. 1977 Feb 3;59(3):253–257. doi: 10.1007/BF00292781. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wegnez M., Denis H. Biochemical research on oogenesis. Transfer RNA is fully charged in the 42-S storage particles of Xenopus laevis oocytes. Eur J Biochem. 1979 Jul;98(1):67–75. doi: 10.1111/j.1432-1033.1979.tb13161.x. [DOI] [PubMed] [Google Scholar]
- Woodland H. R. Changes in the polysome content of developing Xenopus laevis embryos. Dev Biol. 1974 Sep;40(1):90–101. doi: 10.1016/0012-1606(74)90111-0. [DOI] [PubMed] [Google Scholar]