Abstract
Type 1 diabetes mellitus (T1DM) is a common cause of end-stage renal disease (ESRD). Various factors contribute to wide fluctuations in blood glucose levels and exogenous insulin requirement in such patients even after renal transplantation (RT). Simultaneous pancreas–kidney transplantation is one of the therapies for these patients. Stem cell (SC) therapy for T1DM and for minimisation of immunosuppression after RT has shown encouraging results. We report a 30-year-old-man with T1DM since 15 years and ESRD since 2 years, who underwent living donor RT and co-infusion of in vitro generated insulin-making cells differentiated from donor adipose tissue derived mesenchymal stem cells and bone marrow -derived haematopoietic SC into subcutaneous tissue, portal and thymic circulation under non-myeloablative conditioning. Over follow-up of 13 months he has stable graft function with serum creatinine, 1.2 mg/dl, zero rejection and glycosylated haemoglobin level of 6.1% on calcineurin-inhibitor based therapy.
Background
End-stage renal disease (ESRD) is a common complication of type 1 diabetes mellitus (T1DM). In ESRD, both uraemia and dialysis can complicate glycaemic control by affecting the secretion, clearance and peripheral tissue sensitivity of insulin. Therapeutic options for such patients are simultaneous kidney–pancreas transplants or stem cell therapy (SCT) with renal transplantation (RT). We report a patient with T1DM with ESRD treated with SCT and RT.
Case presentation
A 30-year-old-man with T1DM for 15 years and ESRD since 2 years presented on 11 November with weakness, fatigue, weight loss, oedema, nausea and variable blood sugar levels. He was on exogenous insulin, 120 International units (IU)/day for DM before he developed ESRD and 50 IU/day thereafter. He was subjected to living donor RT (LDRT) with co-infusion of in vitro generated insulin-making cells differentiated from donor adipose tissue derived mesenchymal stem cells (ADMSC), undifferentiated ADMSC and bone marrow (BM)-derived haematopoietic SC (HSC).
Investigations
With a 48 kg body-weight (BW) and 162 cm height his clinical examination was unremarkable. Fasting and postprandial blood sugars (FBS/PPBS) were 63 mg/dl and 230 mg/dl. On admission, his serum creatinine (SCr) was 4.42 mg/dl, blood urea 88 mg/dl, glycosylated haemoglobin (HbA1c) 6.7%, S. C-peptide 0.01 ng/ml, urine sugar +4, urinary albumin +4 and S. acetone 30 ng/ml. Glutamic acid decarboxylase antibody and anti-islet cell antibody were absent and insulin antibody was 5 U/ml (normal range: <12 U/ml). His thyroid, liver functions and lipid profile were unremarkable.
Treatment
He was administered Bortezomib, 1.3 mg/m2 body surface area, on days 1, 4, 8 and 11 to delete auto-immune/ rejecting B-cells along with methylprednisone 125 mg, intravenously in 100 ml normal saline and 1.5 mg/kg BW rabbit anti-thymocyte globulin (r-ATG) on day 15, followed by SC infusion. Insulin-making cells, ADMSC and HSC were generated as per our previous protocol1 and infused into portal circulation (70 ml), thymic circulation (2 ml) by femoral catherisation under C-arm guidance and into abdominal subcutaneous tissue (30 ml). Thymus was selected to achieve central tolerance,2 liver because it is the most tolerogenic organ and subcutaneous tissue being immunologically privileged site will serve as back-up reservoir. At the end of SC infusion he developed B-cell flow-cross-match positivity of 326 median channel shift (MCS) (reference range: <50 MCS) while T-cell cross-match and standard CDCC technique cross-matching (by serology) were negative. He was therefore treated with four plasmapheresis sessions with intravenous Immunoglobulin, 10 g and mycofenolate mofetil, 1.5 gm per day. LDRT with father's kidney was performed after 5 weeks of SC infusion with favourable cross-match (figure 1). Informed patient consent forms and SC generation protocols were approved by the Institutional Review Board.
Figure 1.
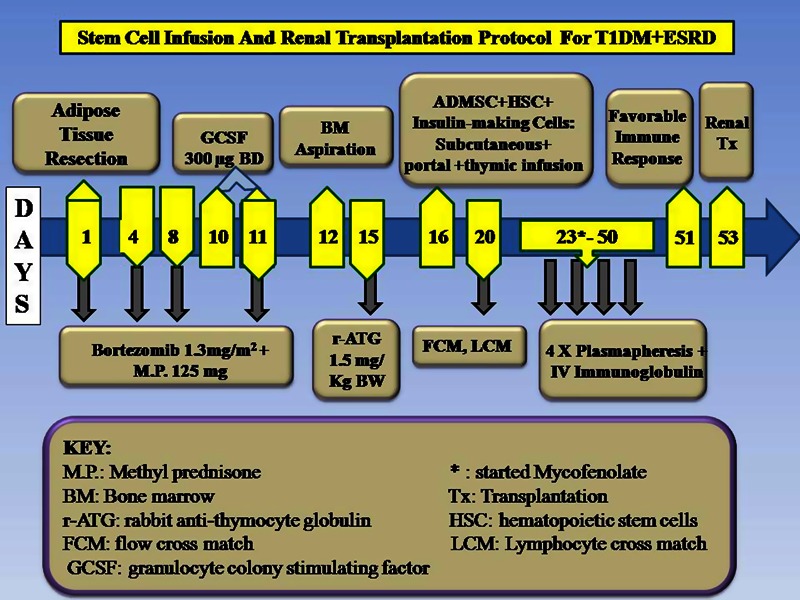
Stem cell infusion and renal transplantation protocol for type 1 diabetes mellitus+ end-stage renal disease.
Total nucleated cells infused are 34×106/kg BW, HSC CD34+, 5.4×104/kg BW, ADMSC CD 90+/73+, 1.1×104/kg BW and insulin-making cells 1.8×104/kg BW. SC infusion was uneventful.
Outcome and follow-up
Over the follow-up of 13 months, he weighed 47 kg, his FBS/PPBS was 92 mg/dl and 165 mg/dl, respectively, SCr, 1.22 mg/dl and HbA1c of 6.1%. His S. C-peptide is sustained at 0.52 ng/ml with sustained insulin requirement of 40 IU /day. His maintenance immunosuppression consists of Tacrolimus, 0.05 mg/kgBW/day, mycofenolate sodium, 360 mg twice a day and prednisone, 10 mg/day.
Disussion
Approximately 40% of the patients with T1DM eventually develop ESRD.3 In ESRD elevated blood urea nitrogen causes formation of carbamylated haemoglobin, which is indistinguishable from HbA1c by electrical-charge-based assays and can cause significant alteration in glycaemic control, HbA1c testing, pharmacokinetics of antidiabetic medication and metabolism, often leading to unpredictable blood glucose levels. Various effects of ESRD can make blood glucose levels fluctuate widely, placing these patients at risk of hypoglycaemia and often becoming a challenge for the testing physicians. Shorter life-span of red blood cells, iron deficiency, recent transfusion, use of erythropoietin-stimulating agents and several factors including uraemic toxins, may increase insulin resistance, leading to a blunted ability to suppress hepatic gluconeogenesis and regulate peripheral glucose utilisation.4
For T1DM patients receiving exogenous insulin, renal metabolism plays significant role since there is no first-pass metabolism in the liver. As renal function starts declining, insulin clearance does not change appreciably, due to compensatory peritubular insulin uptake. Thus, despite the increase in insulin resistance caused by renal failure, the net effect is a reduced requirement for exogenous insulin in ESRD.5 Hence in patients with ESRD with T1DM, insulin therapy is started at 0.5 IU/kg, which is half the calculated dose in patients without renal failure and most oral antidiabetes drugs are contraindicated in ESRD as main adverse effect of these agents is oedema, especially when they are combined with insulin therapy.6
ADMSC have been a driving force to initiate studies testing their therapeutic effectiveness for many diseases and their immunomodulatory properties have been equally promising. This is the first case report to our knowledge where co-infusion of in vitro generated HSC, ADMSC combined with insulin-making cells was carried out successfully along with LDRT to control the blood glucose level as a treatment of T1DM–ESRD patient.
This report shows that the patient has been able to maintain steady renal allograft function without rejection and deterioration in diabetes status in spite of taking calcineurin inhibitors and steroids. However, it suggests that dose requirement of insulin-making cells for such patients needs to be worked out and long-term follow-up of sustainability of such therapy needs to be studied. This approach will open up new avenues of SC therapy for patients with T1DM with ESRD along with RT and they need not wait for availability of deceased donor organs. We conclude that co-infusion of insulin-making cells, HSC, ADMSC and LDRT is a safe and effective novel therapy and will open up avenues for patients with T1DM with ESRD.
Learning points.
In type 1 diabetes mellitus (T1DM) patients who have developed end-stage renal disease (ESRD), uraemia and many other factors complicate glycaemic control.
In T1DM–ESRD patient, insulin therapy is started at 0.5 IU/kg, which is half the calculated dose in patients without renal failure.
Most oral hypoglycaemic agents are ineffective in T1DM–ESRD patients
Co-infusion of in vitro generated insulin-making cells and HSC with LDRT is a safe and effective therapy to control blood glucose level as a treatment of T1DM in a RT patient with DM associated ESRD. This approach will open up new avenues for patients with T1DM with ESRD.
Footnotes
Contributors: HLT was the principal investigator and helped in writing and reviewing manuscript SDD and AVV carried out stem cell generation and other laboratory work and carried out manuscript writing
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Vanikar AV, Dave SD, Thakkar UG, et al. Co-transplantation of adipose tissue-derived insulin-secreting mesenchymal stem cells and hematopoietic stem cells: A novel therapy for insulin-dependent diabetes mellitus. Stem Cells Int 2010; Article ID 582382: 5 pages [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oluwole OO, Depaz HA, Gopinathan R, et al. Indirect allorecognition in acquired thymic tolerance induction of donor-specific permanent acceptance of rat islets by adoptive transfer of allopeptide-pulse host myeloid and thymic dendritic cells. Diabetes 2001;2013:1546. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo R. Diabetic nephropathy: etiologic and therapeutic considerations. Diabetes Rev 1995;2013:510.2013 [Google Scholar]
- 4.Joy MS, Cefali WT, Hogan SL, et al. Long-term glycemic control measurements in diabetic patients receiving hemodialysis. Am J Kidney Dis 2002;2013:297–307 [DOI] [PubMed] [Google Scholar]
- 5.Biesenbach G, Raml A, Schmekal B, et al. Decreased insulin requirement in relation to GFR in nephropathic type 1 and insulin-treated type 2 diabetic patients. Diabet Med 2003;2013:642–5 [DOI] [PubMed] [Google Scholar]
- 6.Kumarpal S, Peter H, Franklin M. Managing diabetes in hemodialysis patients: observations and recommendations. Cleve Clin J Med 2009;2013:649–55 [DOI] [PubMed] [Google Scholar]


