Abstract
Wilson disease is a rare heredodegenerative inborn error of copper metabolism with varied neuropsychiatric, hepatic and other manifestations. Here we report a case of Wilson disease with neurological manifestations in a 7-year-old girl with concurrent asymptomatic liver involvement and characteristic radiological findings of signal intensity alterations in bilateral striata and thalami along with changes in central pons too like central pontine myelinolysis (CPM), which is of rare occurrence.
Background
Wilson disease, also known as hepatolenticular degeneration, is an autosomal recessive inborn error of copper metabolism caused by mutation of the gene encoding for a copper transporting P-type ATPase (ATP7B) on chromosome 13q14.3.1 This leads to impaired copper transport and copper toxicity with its deposition in liver, brain, eye, kidney, bones and blood tissues.2 It commonly presents with neurological (40%), hepatic (40%) and psychiatric (15%), while other (5%) manifestations include ocular, renal, skeletal and haematological. Patients of Wilson disease with neurological manifestations usually present in the second or third decade of life with a few of these patients having prior or concurrent hepatic disease. Imaging of brain usually reveals changes in basal ganglia, substantia nigra pars compacta, periaqueductal grey matter and pontine tegmentum. MRI finding like that of central pontine myelinolysis (CPM) has been infrequently reported in Wilson disease. Here we describe a case of Wilson disease with neurological manifestations and concurrent hepatic involvement in a 7-year-old girl with MRI findings like that of CPM.
Case presentation
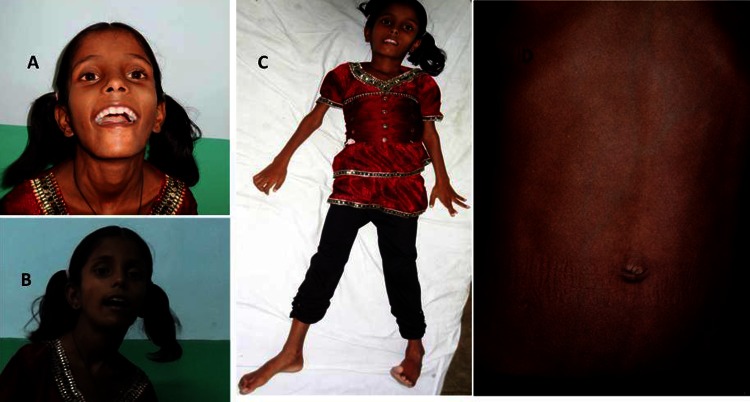
A 7-year-old girl born from a non-consanguineous marriage with full-term normal vaginal delivery with no perinatal or natal complications and normal developmental history presented to us with a 6-month history of difficulty in swallowing and speaking, and drooling of saliva. She also had difficulty in walking and performing activities using upper limbs along with abnormal posturing. According to her family members, she had been apparently well 6 months ago when she initially developed difficulty in swallowing for both solids and liquids without nasal regurgitation, with change in speech in the form of strained hypophonic speech with reduced speech output. They also noticed her mouth used to be open most times of the day with excessive drooling of saliva. After 1 month of her illness, she also developed difficulty holding footwear with her feet and also occasional tilting of neck during walking. Objects occasionally used to fall from her hands and she used to find it difficult to do activities using her hands like eating, dressing and undressing herself. There was no history of tremulousness of limbs, behavioural abnormalities, delusions or hallucinations, seizures, drug intake or any history suggestive of cognitive decline. No history of jaundice or any history suggestive of liver disease, febrile encephalopathy or psychiatry illness could be elicited. Family history was not contributory. On examination, her blood pressure was 96/60 mm Hg, pulse was 78/min and she was afebrile. She had reduced facial expressions, facial and mandibular dystonia leading to dropped jaw (sustained opened mouth) and excessive grinning even to minimal stimuli, what is commonly known as ‘vacuous smile’ (figure 1A,B). She was conscious and attentive but not cooperative for other detailed higher function examinations. Dystonia of face, neck, fingers and toes (striatal toe) was present (figure 1C). Her motor system examination revealed increased tone (rigidity) in all four limbs with normal power, deep tendon reflexes were 2+ and planters were bilateral downgoing. Her motor, sensory and cerebellar system was normal. Abdominal system examination revealed dilated vein over abdomen (figure 1D) and splenomegaly. Slit-lamp examination of eyes revealed bilateral presence of Kayser-Fleischer ring (KF ring) (figure 2) and fundus examination was normal. Her cardiovascular and respiratory system examination was within normal limits.
Figure 1.
(A, B) Patient photograph showing vacuous smile and open mouth appearance, (C) presence of abnormal postures and (D) abdominal examination demonstrated dilated veins.
Figure 2.

Kayser-Fleischer ring seen on slit-lamp examination.
Investigations
Her routine biochemical parameters were normal. Complete haemogram, renal function, thyroid function, coagulation parameters were within normal limits. Serum sodium level was 137 meq/l and serum potassium level was 4.1 meq/l. General blood picture did not reveal acanthocytes or any other abnormalities. Liver function test revealed slightly elevated serum aspartate aminotransferase 70.0 IU/l (normal range 7–56 IU/l) with normal serum alanine transaminase 40 IU/l (normal range 5–47 IU/l) and alkaline phosphatase and reduced total protein and albumin. The serum cereluplasmin level was 0.08 g/l (normal range 0.25–0.46). The 24 h urinary copper excretion level was found to be 473.20 µg (normal range <60 µg/24 h). MRI of cranium revealed T2 hyperintense signals involving bilateral putamen, caudate and bilateral thalamus. Similar signal changes were also observed in the central pons with peripheral sparing (figure 3). The abdominal ultrasound depicted coarse echotexture of liver with normal size, splenomegaly and dilation of splenic and portal veins.
Figure 3.
MRI of cranium revealed T2 hyperintense signals involving bilateral putamen, caudate and thalamus. The pontine area revealed central signal alterations with peripheral sparing.
The clinical description, presence of KF ring, low serum ceruloplamin levels and MRI findings favoured the diagnosis of Wilson disease.
Differential diagnosis
Treatment
The patient was treated with chelating agent d-pencillamine 250 mg twice a day and 50 mg of elemental zinc thrice a day with careful monitoring of haematological and renal functions. Dietary advice to avoid copper-rich foods was given. Trihexyphenidyl was prescribed for dystonia.
Outcome and follow-up
During initial follow-up after starting the treatment, there was gradual improvement. At 3 months of follow-up, her bulbar symptoms, drooling and facial dystonia markedly diminished. However, generalised dystonia was persisting.
Discussion
CPM was first described by Adams, Victor and Mancall in 1959 and it is characterised by symmetric destruction of myelin in the basis pontis. Clinical manifestations vary from asymptomatic to comatose, findings include dysarthria, mutism, behavioural abnormalities, pseudobulbar palsy, quadriparesis, hyperreflexia, seizures and coma. It usually occurs in the setting of chronic alcoholism, malnutrition and electrolyte imbalance (most commonly due to rapid correction of hyponatraemia). Wilson disease is an autosomal recessive disorder of copper metabolism characterised by copper toxicity and its accumulation primarily in liver and brain, other sites less commonly involved. There are varied presentations of Wilson disease depending on the site of involvement like neurological (40%), hepatic (40%), psychiatric (15%) and others (ocular, renal, skeletal and haematological). A wide spectrum of neurological manifestations occurs in Wilson disease, and it should be considered as a possible cause of movement disorder in any patient under the age of 50 years.3 Of special mention is the fact that it is one of the very few curable movement disorders at present time. Common neurological manifestations of Wilson disease are tremors, dysarthria, dystonia, choreoathetosis, drooling of saliva, clumsiness or gait disturbances, parkinsonian syndrome and pseudosclerotic syndrome of postural and intentional tremor.3 Patients with neurological manifestations usually present in their second or third decade (after the age of 12 years).4 5 However, the age of presentation in cases with hepatic manifestations is earlier.6 According to Menkes and Wilcox7 neurological manifestations in children (<12 years) primarily consist of bulbar symptoms (usually the first symptom) likes indistinct speech and difficulty in swallowing, generalised or focal dystonia, gait disturbances and drooling. Noureen et al8 in their study on neurological Wilson disease in children (4–16 years) found dystonia, dysarthria and cognitive decline as a common clinical presentation. Many patients of Wilson disease with neurological manifestations have concurrent subclinical liver disease or at least a prior history of it.9 However, the exact incidence of associated symptomatic liver disease or portal hypertension (like in our case) is not known. CT or MRI of the brain usually show changes in basal ganglia (mainly putamen and caudate nucleus), thalamus and brain stem (substantia nigra, periaqueductal grey matter and pontine tegmentum) which generally reverse on treatment.10 However, signal intensity alterations or changes in the central pons like CPM have been rarely described in literature.11 12 Sinha et al in their study described three distinct patterns of CPM like changes in patients with Wilson disease (age 14.2±4.6 years): (1) classical central hyperintensity with clear periphery, (2) bisected central pontine signal by a horizontal line, (3) ‘Mercedes Benz’ or ‘Trident’ sign.13 To the best of our knowledge, there is no literature so far that reported CPM like changes in children with Wilson disease (age <12 year). They also reported that bulbar symptoms and drooling were more common in patients’ with CPM like changes as compared with those without it. Here we described a case of Wilson disease with neurological manifestations like dysarthria, dysphagia, dystonia and characteristic facial expression with ‘vacuous smile’ in a 7-year-old girl with concurrent hepatic involvement in the form of liver cirrhosis and portal hypertension. Her MRI brain revealed the classical sign of CPM that is, central hyperintensity with spared periphery.
Learning points.
The central pontine lesions mimicking central pontine myelinolysis can be a manifestation of Wilson disease.
There are three distinct pattern of central pontine myelinosis like change in the Wilson disease: (1)) classical central hyperintensity with clear periphery, (2) bisected central pontine signal by a horizontal line, (3) ‘Mercedes Benz’ or ‘Trident’ sign.
They usually presents with bulbar symptoms (dysphagia and dysarthria) and drooling.
Footnotes
Contributors: RV observed the patient and DR helped in preparing the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bull PC, Thomas GR, Rommens JM, et al. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet 1993;2013:327–37 [DOI] [PubMed] [Google Scholar]
- 2.Schilsky M,, Tavill AS. Wilson disease. In: Schiff ER, Forbes JR, Cox DW. eds. Disease of the liver, 9th edn., Philadelphia: Lippincott Williams & Wilkins, 2003:1169–86 [Google Scholar]
- 3.Le Witt P, Pfeiffe R. Neurologic aspects of Wilson's disease: clinical manifestations and treatment considerations. In: Jankovic JJ, Tolosa E, eds. Parkinson's disease and movement disorders. 4th edn. Philadelphia: Lippincott Williams & Wilkins, 2002:240–55 [Google Scholar]
- 4.Brewer GJ. Recognition, diagnosis, and management of Wilson's disease. Soc Exp Biol Med, 2000;2013:39–46 [DOI] [PubMed] [Google Scholar]
- 5.Lorincz MT. Neurologic Wilson's disease. Ann N Y Acad Sci 2010;2013:173–87 [DOI] [PubMed] [Google Scholar]
- 6.Merle U, Schaefer M, Ferenci P, et al. Clinical presentation, diagnosis and long-term outcome of Wilson's disease: a cohort study. Gut 2007;2013:115–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menkes JH, Wilcox WR. Inherited metabolic diseases of the nervous system. In: Menkes JH, Sarnat HB, Maria BL, eds. Child Neurology. 7th edn. Lippincott Williams & Wilkins, 2006:109–15. [Google Scholar]
- 8.Noureen N, Rana MT. Neurological Wilson disease in children: a three years experience from Multan. J Pak Med Assoc 2011;2013:743–8 [PubMed] [Google Scholar]
- 9.Scheinberg IH, Sternlieb I. Wilson's disease. Philadelphia, PA: W B Saunders, 1984 [Google Scholar]
- 10.Saatci I, Topcu M, Baltaoglu FF, et al. MR findings in WD. Acta Radiol 1997;2013:250–8 [DOI] [PubMed] [Google Scholar]
- 11.Sinha S, Taly AB, Ravishankar S, et al. Wilson's disease: cranial MRI observations and clinical correlation. Neuroradiology 2006;2013:613–21 [DOI] [PubMed] [Google Scholar]
- 12.Kizkin S, Sarac K, Ozisik HI, et al. Central pontine myelinolysis in Wilson's disease: MR spectroscopy findings. Magn Reson Imaging 2004;2013:117–21 [DOI] [PubMed] [Google Scholar]
- 13.Sinha S, Taly AB, Ravishankar S, et al. Central pontine signal changes in Wilson's disease: distinct MRI morphology and sequential changes with de-coppering therapy. J Neuroimaging 2007;2013:286–91 [DOI] [PubMed] [Google Scholar]