Abstract
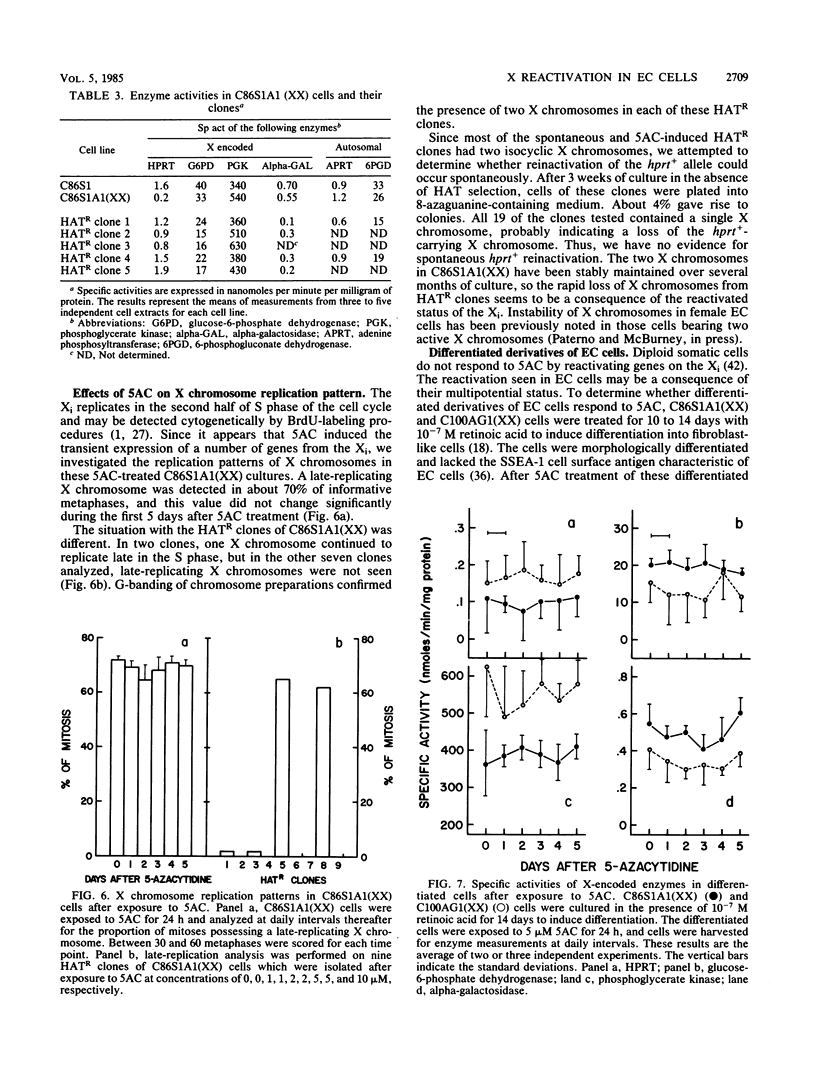
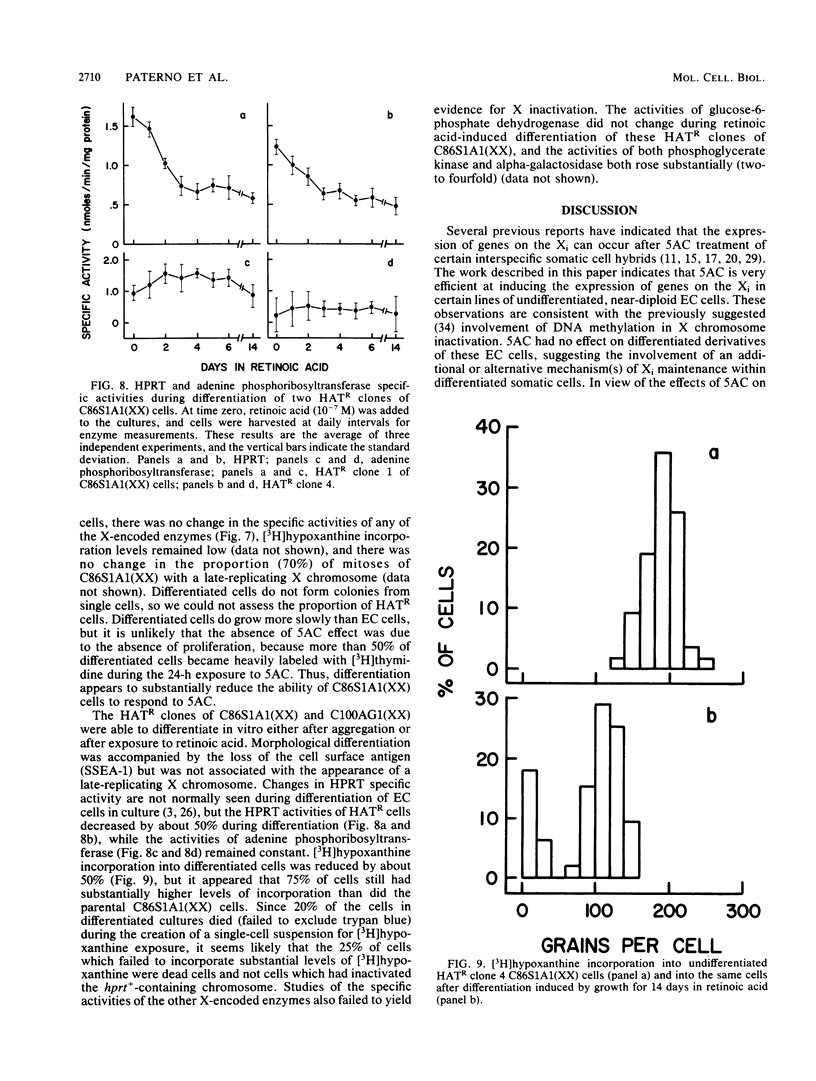
The embryonal carcinoma cell line, C86S1, carries two X chromosomes, one of which replicates late during S phase of the cell cycle and appears to be genetically inactive. C86S1A1 is a mutant which lacks activity of the X-encoded enzyme, hypoxanthine phosphoribosyltransferase (HPRT). Treatment of C86S1A1 cells with DNA-demethylating agents, such as 5-azacytidine (5AC), resulted in (i) the transient expression in almost all cells of elevated levels of HPRT and three other enzymes encoded by X-linked genes and (ii) the stable expression of HPRT in up to 5 to 20% of surviving cells. Most cells which stably expressed HPRT had two X chromosomes which replicated in early S phase. C86S1A1 cells which had lost the inactive X chromosome did not respond to 5AC. These results suggest that DNA demethylation results in the reactivation of genes on the inactive X chromosome and perhaps in the reactivation of the entire X chromosome. No such reactivation occurred in C86S1A1 cells when the cells were differentiated before exposure to 5AC. Thus, the process of X chromosome inactivation may be a sequential one involving, as a first step, methylation of certain DNA sequences and, as a second step, some other mechanism(s) of transcriptional repression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alves P., Jonasson J. New staining method for the detection of sister-chromatid exchanges in BrdU-labelled chromosomes. J Cell Sci. 1978 Aug;32:185–195. doi: 10.1242/jcs.32.1.185. [DOI] [PubMed] [Google Scholar]
- Busslinger M., Hurst J., Flavell R. A. DNA methylation and the regulation of globin gene expression. Cell. 1983 Aug;34(1):197–206. doi: 10.1016/0092-8674(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Campione-Piccardo J., Sun J. J., Craig J., McBurney M. W. Cell-cell interaction can influence drug-induced differentiation of murine embryonal carcinoma cells. Dev Biol. 1985 May;109(1):25–31. doi: 10.1016/0012-1606(85)90342-2. [DOI] [PubMed] [Google Scholar]
- Chapman V. M., Kratzer P. G., Siracusa L. D., Quarantillo B. A., Evans R., Liskay R. M. Evidence for DNA modification in the maintenance of X-chromosome inactivation of adult mouse tissues. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5357–5361. doi: 10.1073/pnas.79.17.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Malcolm L. A., Yoshida A., Giblett E. R. Phosphoglycerate kinase: an X-linked polymorphism in man. Am J Hum Genet. 1971 Jan;23(1):87–91. [PMC free article] [PubMed] [Google Scholar]
- Creusot F., Acs G., Christman J. K. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2'-deoxycytidine. J Biol Chem. 1982 Feb 25;257(4):2041–2048. [PubMed] [Google Scholar]
- Epstein C. J., Smith S., Travis B., Tucker G. Both X chromosomes function before visible X-chromosome inactivation in female mouse embryos. Nature. 1978 Aug 3;274(5670):500–503. doi: 10.1038/274500a0. [DOI] [PubMed] [Google Scholar]
- Fenwick R. G., Jr, Fuscoe J. C., Caskey C. T. Amplification versus mutation as a mechanism for reversion of an HGPRT mutation. Somat Cell Mol Genet. 1984 Jan;10(1):71–84. doi: 10.1007/BF01534474. [DOI] [PubMed] [Google Scholar]
- Gartler S. M., Riggs A. D. Mammalian X-chromosome inactivation. Annu Rev Genet. 1983;17:155–190. doi: 10.1146/annurev.ge.17.120183.001103. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Wilson M. C. Delayed de novo methylation in teratocarcinoma suggests additional tissue-specific mechanisms for controlling gene expression. Nature. 1983 Jan 6;301(5895):32–37. doi: 10.1038/301032a0. [DOI] [PubMed] [Google Scholar]
- Graves J. A. 5-azacytidine-induced re-expression of alleles on the inactive X chromosome in a hybrid mouse cell line. Exp Cell Res. 1982 Sep;141(1):99–105. doi: 10.1016/0014-4827(82)90072-6. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hoffman R. M. Altered methionine metabolism, DNA methylation and oncogene expression in carcinogenesis. A review and synthesis. Biochim Biophys Acta. 1984;738(1-2):49–87. doi: 10.1016/0304-419x(84)90019-2. [DOI] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Hors-Cayla M. C., Heuertz S., Frezal J. Coreactivation of four inactive X genes in a hamster x human hybrid and persistence of late replication of reactivated X chromosome. Somatic Cell Genet. 1983 Nov;9(6):645–657. doi: 10.1007/BF01539470. [DOI] [PubMed] [Google Scholar]
- Jones-Villeneuve E. M., McBurney M. W., Rogers K. A., Kalnins V. I. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol. 1982 Aug;94(2):253–262. doi: 10.1083/jcb.94.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Villeneuve E. M., Rudnicki M. A., Harris J. F., McBurney M. W. Retinoic acid-induced neural differentiation of embryonal carcinoma cells. Mol Cell Biol. 1983 Dec;3(12):2271–2279. doi: 10.1128/mcb.3.12.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Lester S. C., Korn N. J., DeMars R. Derepression of genes on the human inactive X chromosome: evidence for differences in locus-specific rates of derepression and rates of transfer of active and inactive genes after DNA-mediated transformation. Somatic Cell Genet. 1982 Mar;8(2):265–284. doi: 10.1007/BF01538681. [DOI] [PubMed] [Google Scholar]
- Ley T. J., Chiang Y. L., Haidaris D., Anagnou N. P., Wilson V. L., Anderson W. F. DNA methylation and regulation of the human beta-globin-like genes in mouse erythroleukemia cells containing human chromosome 11. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6618–6622. doi: 10.1073/pnas.81.21.6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liskay R. M., Evans R. J. Inactive X chromosome DNA does not function in DNA-mediated cell transformation for the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4895–4898. doi: 10.1073/pnas.77.8.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R., Epstein C. J., Travis B., Tucker G., Yatziv S., Martin D. W., Jr, Clift S., Cohen S. X-chromosome inactivation during differentiation of female teratocarcinoma stem cells in vitro. Nature. 1978 Jan 26;271(5643):329–333. doi: 10.1038/271329a0. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Adamson E. D. Studies on the activity of the X chromosomes in female teratocarcinoma cells in culture. Cell. 1976 Sep;9(1):57–70. doi: 10.1016/0092-8674(76)90052-0. [DOI] [PubMed] [Google Scholar]
- McBurney M. W. Clonal lines of teratocarcinoma cells in vitro: differentiation and cytogenetic characteristics. J Cell Physiol. 1976 Nov;89(3):441–455. doi: 10.1002/jcp.1040890310. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Strutt B. J. Genetic activity of X chromosomes in pluripotent female teratocarcinoma cells and their differentiated progeny. Cell. 1980 Sep;21(2):357–364. doi: 10.1016/0092-8674(80)90472-9. [DOI] [PubMed] [Google Scholar]
- Miller D. A., Okamoto E., Erlanger B. F., Miller O. J. Is DNA methylation responsible for mammalian X chromosome inactivation? Cytogenet Cell Genet. 1982;33(4):345–349. doi: 10.1159/000131782. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Monk M., Harper M. I. Sequential X chromosome inactivation coupled with cellular differentiation in early mouse embryos. Nature. 1979 Sep 27;281(5729):311–313. doi: 10.1038/281311a0. [DOI] [PubMed] [Google Scholar]
- Niwa O., Yokota Y., Ishida H., Sugahara T. Independent mechanisms involved in suppression of the Moloney leukemia virus genome during differentiation of murine teratocarcinoma cells. Cell. 1983 Apr;32(4):1105–1113. doi: 10.1016/0092-8674(83)90294-5. [DOI] [PubMed] [Google Scholar]
- Oshima R. Stimulation of the clonal growth and differentiation of feeder layer dependent mouse embryonal carcinoma cells by beta-mercaptoethanol. Differentiation. 1978;11(3):149–155. doi: 10.1111/j.1432-0436.1978.tb00978.x. [DOI] [PubMed] [Google Scholar]
- Riggs A. D. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14(1):9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- Rosenstraus M., Chasin L. A. Isolation of mammalian cell mutants deficient in glucose-6-phosphate dehydrogenase activity: linkage to hypoxanthine phosphoribosyl transferase. Proc Natl Acad Sci U S A. 1975 Feb;72(2):493–497. doi: 10.1073/pnas.72.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A. 1978 Nov;75(11):5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. L., Stuhlmann H., Jähner D., Jaenisch R. De novo methylation, expression, and infectivity of retroviral genomes introduced into embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4098–4102. doi: 10.1073/pnas.79.13.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi N., Martin G. R. Studies of the temporal relationship between the cytogenetic and biochemical manifestations of X-chromosome inactivation during the differentiation of LT-1 teratocarcinoma stem cells. Dev Biol. 1984 Jun;103(2):425–433. doi: 10.1016/0012-1606(84)90330-0. [DOI] [PubMed] [Google Scholar]
- Toniolo D., D'Urso M., Martini G., Persico M., Tufano V., Battistuzzi G., Luzzatto L. Specific methylation pattern at the 3' end of the human housekeeping gene for glucose 6-phosphate dehydrogenase. EMBO J. 1984 Sep;3(9):1987–1995. doi: 10.1002/j.1460-2075.1984.tb02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venolia L., Gartler S. M., Wassman E. R., Yen P., Mohandas T., Shapiro L. J. Transformation with DNA from 5-azacytidine-reactivated X chromosomes. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2352–2354. doi: 10.1073/pnas.79.7.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Jolly D. J., Lunnen K. D., Friedmann T., Migeon B. R. Methylation of the hypoxanthine phosphoribosyltransferase locus on the human X chromosome: implications for X-chromosome inactivation. Proc Natl Acad Sci U S A. 1984 May;81(9):2806–2810. doi: 10.1073/pnas.81.9.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Migeon B. R. Studies of X chromosome DNA methylation in normal human cells. Nature. 1982 Feb 25;295(5851):667–671. doi: 10.1038/295667a0. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Patel P., Chinault A. C., Mohandas T., Shapiro L. J. Differential methylation of hypoxanthine phosphoribosyltransferase genes on active and inactive human X chromosomes. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1759–1763. doi: 10.1073/pnas.81.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]