Abstract
PURPOSE
To investigate the association of endothelial-related markers with organ dysfunction and in-hospital mortality to validate our earlier findings in a multicenter study. We hypothesize that: 1) endothelial biomarkers will be associated with organ dysfunction and mortality in sepsis; and, that sFlt-1, holds promise as novel prognostic markers in sepsis.
METHODS
A prospective, multicenter, observational study of a convenience sample of Emergency Department (ED) patients with a suspected infection presenting to one of four urban, academic medical center EDs between January 2009 and January 2010. We collected plasma while the patients were in the ED, and subsequently assayed endothelial-related biomarkers, namely sFlt-1, sE-Selectin, sICAM-1, sVCAM-1, and PAI-1. Outcomes were organ dysfunction and in-hospital mortality.
RESULTS
We enrolled at a total of 166 patients: 63 with sepsis (38%), 61 with severe sepsis (37%) and 42 with septic shock (25%). All endothelial biomarkers were significantly associated with sepsis severity, P < 0.002. We found a significant inter-correlation between all biomarkers, strongest between sFlt1 and PAI-1 (r=0.61, P < 0.001) and PAI-1 and sE-selectin and sICAM-1 (r=0.49, P < 0.001). Among the endothelial biomarkers, sFlt-1 had the strongest association with SOFA score (r=0.58, P < 0.001). sFlt-1 and PAI-1 had the highest area under the operating receiver characteristic curve for mortality of 0.87.
CONCLUSIONS
This multi-center validation study confirms that markers of endothelial activation are associated with sepsis severity, organ dysfunction and mortality in sepsis. This supports the hypothesis that the endothelium plays a central role in the pathophysiology of sepsis and may serve as a more accurate prediction tool and a target for therapies aimed at ameliorating endothelial cell dysfunction. Additionally, sFLT-1 holds promise as a novel sepsis severity biomarker.
Keywords: Sepsis, endothelial, biomarker, inflammation, shock, infection
INTRODUCTION
Sepsis is a heterogeneous syndrome with variable physiological manifestations in patients. One of the biggest challenges in sepsis is to develop a more detailed understanding of the underlying pathophysiological mechanism. Numerous studies have found an association between endothelial cell activation and sepsis [1-5] and it is becoming evident that the endothelium plays a key role in sepsis. The endothelial activation in sepsis is associated with changes in hemostatic balance, leukocyte trafficking, vascular permeability, inflammation and microcirculatory flow. Although these changes is a part of the adaptive host response to infection, they may become excessive resulting in the dysfunctional phenotype that a hallmark of sepsis.
In a previous single-center study, we found compelling evidence that sepsis in humans is associated with activation of the endothelium as evidenced by increased levels of circulating endothelial biomarkers [6]. We also found an association between these markers and severity of illness and organ dysfunction in sepsis. Such findings may not only improve our understanding of a component of the pathophysiology in sepsis but may also suggest targets for diagnostic platforms and specific therapeutic interventions. Given the central role of the endothelium in sepsis pathophysiology there is a need for linking a proposed biomarker to a mechanism of endothelial activation and introducing endothelial assay in the clinic.
In the current study we sought to perform a multicenter validation in a new cohort of adult ED patients. We will validate our previous findings by testing the hypothesis that markers of endothelial cell activation soluble vascular cell adhesion molecule (VCAM-1), soluble intercellular adhesion molecule (ICAM-1), sE-selectin, plasminogen activator inhibitors (PAI-1), and soluble fms-like tyrosine kinase-1 (sFlt-1) are associated with sepsis severity, organ dysfunction and in-hospital mortality in patients with varying degrees of sepsis acuity.
MATERIALS AND METHODS
Study design and population
This was a prospective, multicenter observational cohort study of a convenience sample of adult patients (age 18 years or older) with suspected infection presenting to four urban academic emergency departments. The patients had to meet the American College of Chest Physicians/Society of Critical Care Medicine criteria for sepsis, specifically 1) suspected infection and 2.) fulfillment of two or more of the criteria for systemic inflammatory response syndrome (SIRS) (temperature > 100.4°F or < 96.8°F, heart rate > 90 beats/minute, respiratory rate > 20 breaths/minute or partial pressure of carbon dioxide < 32 mmHg, white blood cell count > 12,000/μL or < 4,000/μL or > 10% bands)7. Suspected infection was defined as a clinical suspicion of an infectious etiology as assessed by the treating physician, and determined by interviewing the treating physician to determine if infection was suspected based on the ED work-up.
Exclusion criteria included any of the following: age < 18 years, pregnancy, established “Do Not Resuscitate” orders prior to enrollment, acute traumatic or burn injury (primary diagnosis), acute cerebrovascular event (primary diagnosis), acute coronary syndrome (primary diagnosis), acute pulmonary edema (primary diagnosis), cardiac dysrhythmia (primary diagnosis), acute and active gastrointestinal bleeding (primary diagnosis), acute drug overdose (primary diagnosis), requirement for immediate surgery and inability to obtain written informed consent. Clinical management at each institution is in agreement with the Surviving Sepsis Campaign guidelines.
The study period was between January 2009 and January 2010. The setting was Beth Israel Deaconess Medical Center (BIDMC), Boston, Carolinas Medical Center, Charlotte, Cooper University Hospital, Camden and Detroit Medical Center, Detroit. The ethics boards in all four hospitals approved this study, and written informed consent was obtained.
Demographics and clinical covariates
We collected demographic variables (age, sex and race), co-morbid disease (cerebrovascular disease, chemotherapy, congestive heart failure, chronic obstructive pulmonary disease, dementia, diabetes, HIV or AIDS, Hodgkin’s disease, intravenous drug use, leukemia, liver disease, myocardial infarction, non-Hodgkin’s lymphoma, peripheral vascular disease, renal disease, splenectomy, steroid use, ulcer disease, transplant, presence of any malignancy and residence in a nursing home), vital sign information (temperature, blood pressure, heart rate, respiratory rate and oxygen saturation) and the result of laboratory testing (serum lactate, complete blood count, chemistry panels, markers of coagulation and liver function tests)
Biomarker Analysis
All subjects received a blood draw while in the Emergency Department. Samples were drawn in EDTA tubes, centrifuged at 2,500 × g at 4°C and frozen at -80°C within one hour of collection. Plasma was assayed for sE-selectin, sICAM-1, VCAM-1 and PAI-1 as a multiplex panel using the human cardiovascular-1 panel (Millipore, Billerica, MA, USA) and Interleukin-6 (IL-6) using the human cardiovascular-3 panel (Millipore, Billerica, MA, USA) on the Luminex 200 instrument (Millipore). The sFlt-1 assays were performed using the Quantikine ELISA kits (R&D systems, Minneapolis, MN, USA). All assays were performed in duplicate and the average levels were used for analysis.
Outcomes
Sepsis severity was characterized according to a modified version of the ACCP/SCCM sepsis syndrome [7]. Patients were characterized into one of the following groups: sepsis, severe sepsis and septic shock. For assessment of organ dysfunction, we used the SOFA-score, and for additional assessment of the severity of illness, we used the Acute Physiologic and Chronic Health Evaluation (APACHE)-II score based on the worst values over the first 24 hours8,9. Serum lactate levels were used as another severity of illness marker.
Sepsis severity classification
Sepsis was comprised ED patients with suspected infection with a least 2 SIRS criteria (as described above). Severe sepsis was defined as sepsis with concomitant organ dysfunction defined by meeting one or more of the following organ dysfunction definitions; central nervous system: new altered mental state and/or new onset of GCS < 15; respiratory: any mechanical ventilation, supplemental oxygen required to maintain oxygen saturation > 95%, and/or respiratory rate > 24 beats per minute; cardiovascular: any vasopressor use, SBP < 90 mmHg after 20 mL/kg bolus; renal: urine output < 0.5 mL/kg/hr, or creatinine > 50% of baseline or > 2. mg/dl if baseline is unknown; hepatic: AST/ALT > 80 (new); hematopoietic: platelet count < 100,000 and/or PT/PTT > 50% of normal; or metabolic: lactate > 2.5 mmol/l. Septic Shock was defined as sepsis plus hypotension (SBP < 90 mmHg after 20 to 30 cc/kg fluid challenge). Suspected infection was defined as a clinical suspicion of an infectious etiology as assessed by the treating physician, and determined by interviewing the treating physician to determine if infection was suspected based on the ED work-up. The sepsis severity was assessed on presentation and daily for the first 72 hours or until hospital discharge, assigning a patient to the worst syndrome achieved.
Organ dysfunction
The sequential organ failure assessment (SOFA) score was used to assess organ failures[8]. The SOFA score is designed to identify morbidity and individualizes the dysfunction or failure of each organ system. It has been established as a valid predictor for both initial and serial assessments [10,11]. The SOFA score was assessed on presentation and at 24 hours after enrollment.
Other inflammatory response and illness severity markers
IL-6 level was used as a prototype marker of inflammatory response. APACHE-II score was used as a secondary assessment of severity based on worst vital signs, as originally described [9]. This score has been validated as an assessment tool for risk-stratification, and was utilized to characterize disease severity. While some of the baseline variables make it a score that is not necessarily responsive to acute disease state, its prognostic ability has been well established. Mortality was defined by hospital discharge disposition.
Data Analysis
Means with standard deviations, medians with inter-quartile ranges, and proportions were used for descriptive statistics, as appropriate. To analyze the association between the endothelial biomarkers and sepsis severity, we used generalized linear modeling. Next, we calculated Spearman rank correlation coefficients to assess the bivariable association among the biomarkers. We report the calculated Spearman correlation coefficient (r-value) along with the associated p-value. We performed a similar analysis between the target biomarkers and organ dysfunction (SOFA score) and, the inflammatory response marker IL-6, and APACHE-II score. Due to a non-normal distribution, SOFA score was log transformed throughout the analysis. As a comparator, we also examined the correlation of IL-6 with SOFA score.
To compare the strength of association between each of the biomarkers and organ dysfunction, we standardized each biomarker using the following formula: ((Biomarker – Biomarker mean)/biomarker SD). We then used a linear regression model adjusted for age, gender and co-morbid illness burden (any type of diabetes, congestive heart failure, cancer, chronic obstructive lung disease). We report the beta coefficient with standard error as well as the adjusted r2 value for each biomarker model. We also tested multi-marker models to determine the value of combinations of biomarkers. To assess the clinical predictive ability of the biomarkers, we calculated the area under the receiver operating characteristic curve (AUC) with 95% confidence interval for each biomarker to predict the outcome in-hospital mortality. The AUCs were compared using a nonparametric approach [12].
RESULTS
Patient characteristics
We enrolled a total of 166 patients in the study with a mean age of 60 (SD +/- 17) years; 59% were male, 67% Caucasian (Table 1). On admission, 42 (25%) had septic shock, 61 (37%) had severe sepsis, and 63 (38%) had sepsis. The mortality rates were 45% for the shock cohort, 6% for the severe sepsis cohort, and 2% for the sepsis cohort.
Table 1.
Patient Characteristics
| N=166 | |
|---|---|
| Demographics | |
| Age median, mean (SD) | 59, 59 (17) |
| Race: White n (%) | 108 (66%) |
| African-american | 53 (32%) |
| Other | 3 (2%) |
| Female gender n (%) | 61 (37%) |
| Comorbidities n (%) | |
| COPD | 20 (12%) |
| Chronic Heart Failure | 23 (14%) |
| Diabetes | 54 (31%) |
| Cancer | 17 (10%) |
| Sepsis Syndrome n (%) | |
| Sepsis | 63 (38%) |
| Severe Sepsis | 61 (37%) |
| Septic Shock | 42 (25%) |
| Severity of Disease, median, mean (SD) | |
| SOFA score | 1, 3 (3) |
| APACHE score | 14, 16 (11) |
| Lactate (mmol/L) | 2.4, 2.4 (0.8) |
| Marker levels on admission, median, mean (SD) | |
| sE-selectin (ng/mL) | 67.6, 104.2 (92.9) |
| sVCAM-1 (ng/mL) | 1,215, 1,638 (1,386) |
| ICAM-1 (ng/mL) | 202, 229 (118) |
| PAI-1 (ng/mL) | 78, 120 (167) |
| sFlt-1 (pg/mL) | 168, 333 (482) |
| IL-6 (ng/mL) | 64, 1,076 (2.684) |
Endothelial cell activation and sepsis severity
We found an association between biomarker levels and sepsis severity (worst sepsis syndrome within 72 hours) for sFlt-1 (p<0.0001 for trend across groups), sE-selectin (p<0.001), sVCAM-1 (p<0.002), PAI-1 (p<0.001) and sICAM-1 (p<0.001).
Evidence of endothelial cell activation
In order to assess whether there was evidence of net endothelial cell activation in response to infection, we correlated the selected biomarkers, which individually represent various components of the endothelial cell-signaling pathway. Using a Spearman rank correlation coefficient, we found a significant correlation between all biomarkers (sE-selectin, sFlt-1, sVCAM-1, PAI-1 and sICAM-1) (Figure 1). The strongest correlations were found between sFlt-1 and PAI-1 (r=0.61, p < 0.001) and PAI-1 and sE-selectin and sICAM-1 (r=0.49, p < 0.001)
Figure 1. Spearman rank correlation of biomarkers of endothelial cell activation with each other.
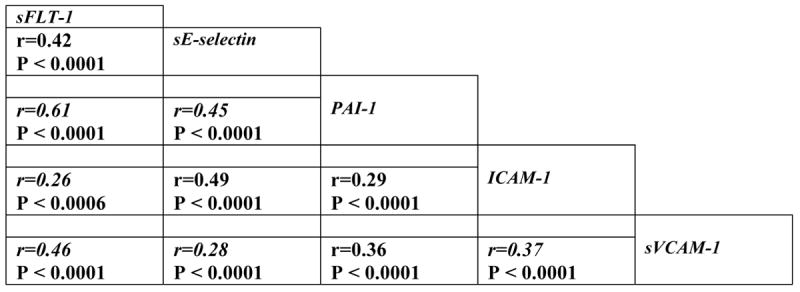
Spearman rank correlation coefficients (r value) are shown with statistical significance of the correlation.
Endothelial cell activation biomarkers and organ dysfunction
To assess the association between endothelial cell biomarkers with organ dysfunction, we analyzed the correlation between the endothelial cell related biomarkers and IL-6 with SOFA score at enrollment. All biomarkers were significantly correlated with SOFA score at enrollment (Figure 2 shows Spearman correlations for different endothelial biomarkers and SOFA at enrollment). In particular sFlt-1 (r=0.58, p < 0.0001) and PAI-1 (r=0.41, p < 0.0001) were correlated with SOFA score at enrollment. For comparison, the correlation between IL-6 and SOFA score at enrollment was r= 0.47, p < 0.0001 and for lactate and SOFA score r=0.26, p<0.01). Furthermore, all endothelial biomarker levels at enrollment correlated with SOFA score at 24 hours: sE-selectin (r=0.27), sFlt-1 (r=0.60), sVCAM-1 (r=0.35), and PAI-1 (r=0.45), p< 0.0001 for all comparisons except sICAM-1 (r=0.15), p=0.03. For comparison, the correlation between IL-6 and SOFA score at 24 hours was r=0.47, p < 0.0001.
Figure 2. Correlation of biomarkers of endothelial cell activation with SOFA-score and IL-6.
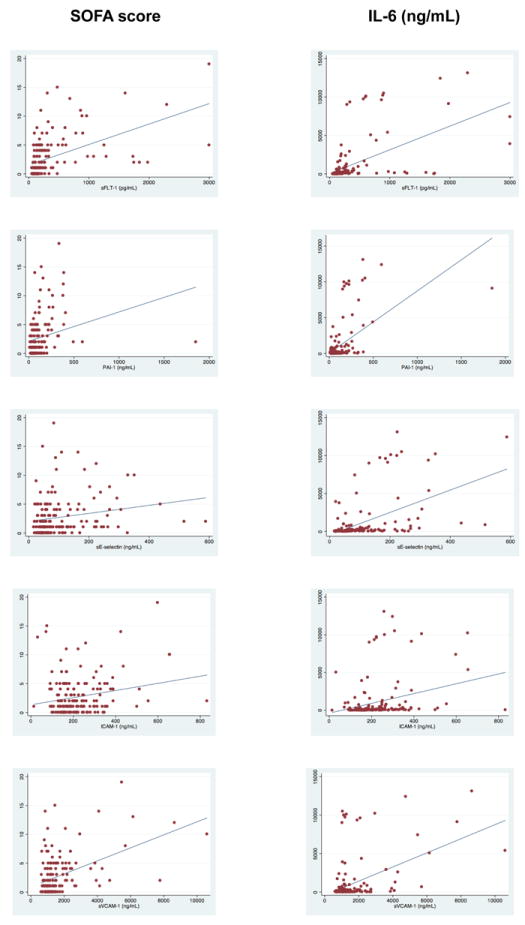
Endothelial cell activation biomarkers and inflammation
We used circulating IL-6 concentrations as a marker of pro-inflammatory response. We found an association between all biomarker levels of endothelial activation and IL-6 (Figure 2) showing Spearman correlations for different endothelial biomarkers and IL-6). sFlt-1 had a particularly strong correlation with IL-6 (r=0.63, p< 0.0001).
Endothelial cell activation biomarkers and other severity of illness markers
Endothelial cell activation markers correlated with two independent markers of disease severity, lactate and APACHE-II scores. There was a significant correlation using spearman rank between the target biomarkers and APACHE-II score: sFlt-1 (r=0.64, P < 0.0001), PAI-1 (r=0.58, p < 0.0001), sE-selectin (r=0.31, p < 0.0001), sICAM-1 (r=0.17, p < 0.05), and sVCAM-1 (r=0.38, p < 0.0001). These results compare favorably to the r-value for the correlation between classic biomarkers such as lactate with APACHE-II (r=0.30) and IL-6 with APACHE-II (r=0.54). Furthermore, there was a significant association between some of the endothelial cell biomarkers and lactate level: sFlt-1 (r=0.31, p < 0.0001), and PAI1 (r=0.38, p < 0.0001). The rest did not meet significance: sE-selectin (r=0.09, p =0.25), sICAM-1 (r=0.09, p =0.24), and sVCAM-1 (r=0.04, p = 0.65). As a comparator, the IL-6 correlation coefficient with lactate was (r=0.19, p<0.05)
Endothelial cell activation biomarkers and organ dysfunction adjusted for age, gender and co-morbid illness burden
We analyzed the association of the biomarkers with organ dysfunction with linear models adjusted for age, gender, diabetes and history of cancer (Table 2) using standardized beta coefficients to allow for equal comparisons. We report the models testing one biomarker at a time (Table 2). The R2 value in the adjusted model for sFlt-1 alone was 0.36. There was no other combination of two or more biomarkers that exceeds the R2 of the model with sFlt-1 alone. Thus sFlt-1 appears to have the strongest association with organ dysfunction, and in this instance, a multi-marker approach was not as beneficial.
Table 2. Association of individual biomarkers with organ dysfunction, adjusted for age, gender and comorbid burden.
This table shows the results from each individual biomarker incorporated into a linear regression model (one marker per model) with outcome SOFA, adjusted for age (years), gender and comorbid burden. The biomarkers are standardized [biomarker – biomarker mean)/SD] so beta estimates are comparable. Each biomarker showed a statistically significant association with SOFA score.
| Organ Dysfunction | ||||
|---|---|---|---|---|
| Biomarker | Std. beta | SE | P-value | Model adjusted r2 |
| sFlt | 2.55 | 0.31 | <0.001 | 0.36 |
| PAI-1 | 1.27 | 0.32 | <0.001 | 0.15 |
| sVCAM-1 | 1.88 | 0.31 | <0.001 | 0.24 |
| ICAM | 0.88 | 0.32 | <0.008 | 0.10 |
| sE-selectin | 1.44 | 0.30 | <0.001 | 0.18 |
| IL-6 | 2.24 | 0.27 | <0.001 | 0.34 |
Biomarkers as predictors of mortality
In order to further assess the accuracy of the different markers, we report the area under the receiver operating characteristic curve for the ability of the biomarker drawn at enrollment in the ED to in-hospital mortality (Table 3). sFlt-1 and PAI-1 performed with the highest accuracy, both with an AUC of 0.85 (95% CI: 0.75 - 0.94 and 0.76 - 0.93), and was higher (P<0.05) than the AUC for all the other endothelial markers. For comparison IL-6 had an AUC of 0.78 (0.67 - 0.89), however not statistically different, p=0.14 and lactate measured at enrollment had an AUC of 0.80 (0.69 - 0.92), p=0.54. We then investigated whether the model would be improved by any combination of multiple markers. The AUC values of sFlt-1 and PAI-1 were alone 0.85, and adding any second marker did not improve the model fit above this level. When adjusting for age, diabetes, COPD and CHF, the AUCs of the models with sFlt-1 and PAI-1 were both 0.87 (0.79 - 0.95 and 0.78 - 0.95).
Table 3.
Area under the curve for each biomarker as a predictor of in-hospital mortality, adjusted for comorbid disease.
| Biomarker | AUC | 95% CI |
|---|---|---|
| sFLT-1 | 0.87 | 0.79-0.95 |
| PAI-1 | 0.87 | 0.78-0.95 |
| sE-selectin | 0.77 | 0.69-0.85 |
| ICAM | 0.71 | 0.60-0.81 |
| sVCAM-1 | 0.78 | 0.68-0.89 |
| IL-6 | 0.80 | 0.69-0.91 |
DISCUSSION
In one of the largest ED-based multicenter endothelial activation in sepsis studies to date, we assembled a group of moderately ill ED patients with varying sepsis syndrome severity (overall mortality rate= 14%) with the overall goal of investigating the association of endothelial activation with sepsis severity, organ dysfunction and mortality. We found that increased levels of the endothelial biomarkers sFlt-1, PAI-1, sICAM-1 and sVCAM-1 were associated with sepsis severity, organ dysfunction and mortality in this patient group, which is consistent with our earlier findings that the endothelium is activated in sepsis6. Furthermore, sFlt-1 remains promising as a novel prognostic marker for morbidity and mortality in sepsis, and is in this study comparable to IL-6 in predicting inflammation, organ dysfunction and mortality. The associations of these biomarkers with sepsis are biologically plausible as they incorporate key components of the endothelial cell response in sepsis, including leukocyte trafficking (sICAM-1 and sVCAM-1), coagulation activity (PAI-1), and signaling (sFlt-1).
Prior work supports sFlt-1 as an important prognostic marker in sepsis. We have previously showed that sFlt-1 is significantly associated with sepsis severity, organ dysfunction and is highly predictive of mortality13,14. We have in this study provided additional biologic evidence supporting this novel finding. Preclinical studies have shown that VEGF exacerbates sepsis and mediates morbidity and mortality, The circulating decoy molecule sFlt-1 binds to VEGF and acts as a competitive inhibitor of VEGF signaling in endothelial cells and thus neutralizes the pro-inflammatory effects of VEGF. This is indeed supported by the observation that supratherapeutic levels of sFlt-1 in animal sepsis models deplete free VEGF and protect against morbidity and mortality15,16. Increased levels of sFlt-1 may represent a critical component of the host anti-inflammatory response. Indeed it seems to be a promising marker of sepsis compared with the other markers tested and is equivalent to IL-6, a prototype marker of inflammation. We found that levels of circulating PAI-1 was associated with sepsis severity, consistent with previous reports [17-22]. The expression of PAI-1 is largely restricted to the endothelial cells, which adds further evidence that the endothelium is activated during sepsis.
Our findings add to the existing literature in several ways. This is one of the largest cohorts of sepsis patients analyzed to date for soluble markers of endothelial activation. We have included markers that are indicative of both leukocyte adhesion and coagulation in the same cohort. Furthermore, we have validated the novel discovery that sFlt-1 levels correlate more closely with severity of illness, organ dysfunction and mortality compared with the other markers tested and lactate. In this study we found sFlt-1 to perform in a similar fashion as IL-6. We have also validated earlier results that adding multiple markers fail to provide additional information over a single marker.
Limitations
This study has a number of limitations. First, we did not use a consecutive patient population so the study may be subject to selection bias. Second, we only analyzed blood from the initial draw and we did not follow the biomarker dynamics over time. Third, There may have been unmeasured confounders or confounding that was not accounted for in the analysis. Fourth, we use circulating levels of endothelial markers as a surrogate for endothelial activation and may not accurately reflect the degree, nature and site of activation of the endothelium (though this is also a strength as it is what clinicians have access to). Fifth, we did not include an uninfected control group, making us unable to answer whether the observed endothelial activation is specific to sepsis. Sixth, some patients enrolled with sepsis may have been misclassified and ultimately had other etiologies of disease, but included in our cohorts. Lastly, even though this is one of the largest studies looking at endothelial activation in sepsis, our sample size is not large. A larger study may have afforded the opportunity for more complete subset analysis.
CONCLUSION
We have addressed the challenges of sepsis heterogeneity by prospectively validating the existence of an endothelial activation during sepsis that is correlated with infection, organ dysfunction and in-hospital mortality. In this study we found s-Flt-1 to perform very favorably and in a similar fashion to IL-6 and lactate. This further strengthens the hypothesis that the endothelium is activated as evidence by increased levels of circulating endothelial biomarkers.
Acknowledgments
Funding Sources:
This study was in part funded by an investigator-initiated grant from Hutchinson Technology (Hutchinson, MN, USA). Dr. Skibsted is supported by a research grant from Aarhus University, Denmark. Dr. Shapiro is supported by National Institutes of Health grants HL091757, GM076659 and 5R01HL093234-02. Dr. jones is supported by NIH grant GM076652. Dr. Trzeciak is supported by NIH grant GM083211. Dr. Aird is supported by NIH grants HL091757 and GM088184.
Abbreviations
- APACHE II score
acute physiologic and chronic health evaluation II score
- AUC
area under the curve
- ED
emergency department
- sICAM-1
soluble intercellular adhesion molecule
- IL-6
interleukin-6
- PAI-1
plasminogen activator inhibitors -1
- sFlt-1
soluble fms-like tyrosine kinase-1
- SIRS
systemic inflammatory response syndrome
- SOFA score
sequential organ failure assessment score
- sVCAM-1
soluble vascular cell adhesion molecule
Footnotes
Competing Interests:
Dr. Shapiro has received research grants from Hutchinson Technologies, Eli Lilly and Inverness Medical and has received speaker’s honorarium from Inverness Medical. Dr. Schuetz receives speaker’s honoraria from BRAHMS’s Diagnostics and Biomerieux Inc. Dr. Trzeciak receives research support from Ikaria and serves as a consultant to Spectral Diagnostics, but does not receive any personal remuneration from any commercial interest.
References
- 1.Hotchkiss RS, Karl IE. Endothelial cell apoptosis in sepsis: a case of habeas corpus? Crit Care Med. 2004;32:901–2. doi: 10.1097/01.ccm.0000115264.93926.ec. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Tinsley KW, Swanson PE, Karl IE. Endothelial cell apoptosis in sepsis. Crit Care Med. 2002;30:S225–8. doi: 10.1097/00003246-200205001-00009. [DOI] [PubMed] [Google Scholar]
- 3.Hemmer CJ, Vogt A, Unverricht M, Krause R, Lademann M, Reisinger EC. Malaria and bacterial sepsis: similar mechanisms of endothelial apoptosis and its prevention in vitro. Crit Care Med. 2008;36:2562–8. doi: 10.1097/CCM.0b013e31818441ee. [DOI] [PubMed] [Google Scholar]
- 4.Hack CE, Zeerleder S. The endothelium in sepsis: source of and a target for inflammation. Crit Care Med. 2001;29:S21–7. doi: 10.1097/00003246-200107001-00011. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda N, Teramae H, Yamamoto S, Takano K, Takano Y, Hattori Y. Increased death receptor pathway of apoptotic signaling in septic mouse aorta: effect of systemic delivery of FADD siRNA. American journal of physiology Heart and circulatory physiology. 2010;298:H92–101. doi: 10.1152/ajpheart.00069.2009. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro NI, Schuetz P, Yano K, et al. The association of endothelial cell signaling, severity of illness, and organ dysfunction in sepsis. Critical Care. 2010;14:R182. doi: 10.1186/cc9290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 8.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Medicine. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 9.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical care medicine. 1985;13:818–29. [PubMed] [Google Scholar]
- 10.Moreno R, Vincent JL, Matos R, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study Working Group on Sepsis related Problems of the ESICM. Intensive Care Medicine. 1999;25:686–96. doi: 10.1007/s001340050931. [DOI] [PubMed] [Google Scholar]
- 11.Balci C, Sungurtekin H, Gurses E, Sungurtekin U. APACHE II, APACHE III, SOFA scoring systems, platelet counts and mortality in septic and nonseptic patients. Ulus Travma Acil Cerrahi Derg. 2005;11:29–34. [PubMed] [Google Scholar]
- 12.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 13.Schuetz P, Jones AE, Aird WC, Shapiro NI. Endothelial cell activation in emergency department patients with sepsis-related and non-sepsis-related hypotension. Shock. 2011;36:104–8. doi: 10.1097/SHK.0b013e31821e4e04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro NI, Schuetz P, Yano K, et al. The association of endothelial cell signaling, severity of illness, and organ dysfunction in sepsis. Critical care. 2010;14:R182. doi: 10.1186/cc9290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yano K, Liaw PC, Mullington JM, et al. Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. The Journal of experimental medicine. 2006;203:1447–58. doi: 10.1084/jem.20060375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsao PN, Chan FT, Wei SC, et al. Soluble vascular endothelial growth factor receptor-1 protects mice in sepsis. Critical care medicine. 2007;35:1955–60. doi: 10.1097/01.CCM.0000275273.56547.B8. [DOI] [PubMed] [Google Scholar]
- 17.Kinasewitz GT, Yan SB, Basson B, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism [ISRCTN74215569] Critical Care. 2004;8:R82–90. doi: 10.1186/cc2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorente JA, Garcia-Frade LJ, Landin L, et al. Time course of hemostatic abnormalities in sepsis and its relation to outcome. Chest. 1993;103:1536–42. doi: 10.1378/chest.103.5.1536. [DOI] [PubMed] [Google Scholar]
- 19.Kidokoro A, Iba T, Fukunaga M, Yagi Y. Alterations in coagulation and fibrinolysis during sepsis. Shock. 1996;5:223–8. doi: 10.1097/00024382-199603000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Martinez MA, Pena JM, Fernandez A, et al. Time course and prognostic significance of hemostatic changes in sepsis: relation to tumor necrosis factor-alpha. Critical care medicine. 1999;27:1303–8. doi: 10.1097/00003246-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Savioli M, Cugno M, Polli F, et al. Tight glycemic control may favor fibrinolysis in patients with sepsis. Critical care medicine. 2009;37:424–31. doi: 10.1097/CCM.0b013e31819542da. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Segarra G, Espinosa G, Tassies D, et al. Increased mortality in septic shock with the 4G/4G genotype of plasminogen activator inhibitor 1 in patients of white descent. Intensive Care Medicine. 2007;33:1354–62. doi: 10.1007/s00134-007-0695-y. [DOI] [PubMed] [Google Scholar]


