Abstract
Sigma1 receptors (Sigma1R) are intracellular chaperone proteins that bind psychotropic drugs and also clinically used drugs such as ketamine and haloperidol. Co-expression of the Sigma1R has been reported to enhance the sensitivity of several voltage-gated ion channels to Sigma1R ligands. Kv1.3 is the predominant voltage-gated potassium channel expressed in T lymphocytes with a documented role in immune activation. To gain a better understanding of Sigma1R modulation of Kv ion channels, we investigated the effects of Sigma1R co-expression on Kv1.3 physiology and pharmacology in ion channels expressed in Xenopus oocytes. We also explored the protein domains of Kv1.3 necessary for protein:protein interaction between Kv1.3 and Sigma1R through co-immunoprecipitation studies. Slowly inactivating outward-going currents consistent with Kv1.3 expression were elicited on step depolarizations. The current characterized by Erev, V1/2, and slope factor remained unchanged when co-expressed with Sigma1R. Analysis of inactivation time constant revealed a faster Kv1.3 current decay when co-expressed with Sigma1R. However the sensitivity to Sigma1R ligands remained unaltered when co-expressed with the Sigma1R in contrast to the previously reported modulation of ligand sensitivity in closely related Kv1.4 and Kv1.5 voltage gated potassium channels. Co-immunoprecipitation assays of various Kv1.3 truncation constructs indicated that the transmembrane domain of the Kv1.3 protein was responsible for the protein:protein interaction with the Sigma1R. Sigma1R likely interacts with different domains of Kv ion channel family proteins resulting in distinct modulation of different channels.
Keywords: Ion channel modulation, Sigma 1 receptor, Kv1.3, Protein interaction
1. Introduction
Sigma1 receptors (Sigma1R) are mostly endoplasmic reticulum-resident two-transmembrane chaperone proteins that bind psychotropic drugs and also drugs such as keta-mine, haloperidol, and intravenous anesthetics relevant to clinical medicine (Cobos et al., 2008; Su et al., 2010; Yamada et al., 2006). Co-expression of the Sigma1R has been reported to enhance the sensitivity of several voltage-gated ion channels including calcium, sodium, and potassium channels to Sigma1R ligands (Zhang and Cuevas, 2002; Johannessen et al., 2009; Wilke et al., 1999). A mechanistic study using the Xenopus oocyte model suggests that the Sigma1R also present in the plasma membrane serve a novel role as a ligand-dependent auxiliary channel subunit protein through direct association with the Kv1.4 and Kv1.5 voltage-gated potassium channel proteins, affecting the ion channel physiology only in the presence of a Sigma1R ligand (Aydar et al., 2002).
In addition to these effects of Sigma1R on ion channels physiology, Sigma1R ligands have been reported to show immuno-modulatory effects. Suppression of splenocyte proliferation and NK cell activity (Liu et al., 1995; Carr et al., 1992), inhibition of mitogen induced lymphocyte proliferative response (Casellas et al., 1994), IL-10 mediated suppression of antitumor immunity (Zhu et al., 2003), and enhancement of monocyte transmigration (Yao et al., 2010) have been ascribed to various Sigma 1R ligands including haloperidol and ketamine (Leykin et al., 1997; Rofael et al., 2003; Ohta et al., 2009). The precise mechanisms of these immuno-modulatory effects remain largely unresolved.
Kv1.3 is the predominant voltage-gated potassium channel expressed in T lymphocytes and this ion channel plays a key role in T cell activation. The functional significance of Kv1.3 in T lymphocyte activation derives from the fact that this potassium channel in the plasma membrane maintains cell membrane at a hyperpolarized negative potential. The negative membrane potential maintains the driving force for Ca2+ allowing sustained influx of extracellular Ca2+ during antigen presentation at the T cell immunological synapse. The sustained influx of extracellular Ca2+ in coordination with Ca2+ released from the intracellular stores triggers Ca2+-calmodulin-dependent phosphatase calcineurin. The consequent activation of the transcription factor NFAT is thought to be the critical signaling responsible for T cell activation (Panyi et al., 2004). As such, blocking plasma membrane Kv1.3 by extracellularly applied toxins has been shown to inhibit T cell activation in an experimental autoimmune encephalomyelitis model of multiple sclerosis, rheumatoid arthritis and type-1 diabetes (Beeton et al., 2001; 2006). Development of a selective extracellular blocker of plasma membrane Kv1.3 as a novel immunosuppressant is an active area of basic and translational research.
Sigma1R are also present in T cells (Ganapathy et al., 1999) but whether this receptor affects Kv1.3 physiology is not known. To gain a better understanding of the potential immunomodulatory role of Sigma1R ligands acting through Kv1.3, we investigated the effects of Sigma1R co-expression on Kv1.3 physiology and pharmacology in Xenopus oocytes. Given the earlier report that Sigma1R alters the ligand inhibition of Kv1.4 and Kv1.5 voltage gated potassium channels functioning as a ligand-dependent auxiliary protein to the channels, we hypothesized that the Sigma1R will modulate the ligand sensitivity of the closely related Kv1.3 channel.
2. Results
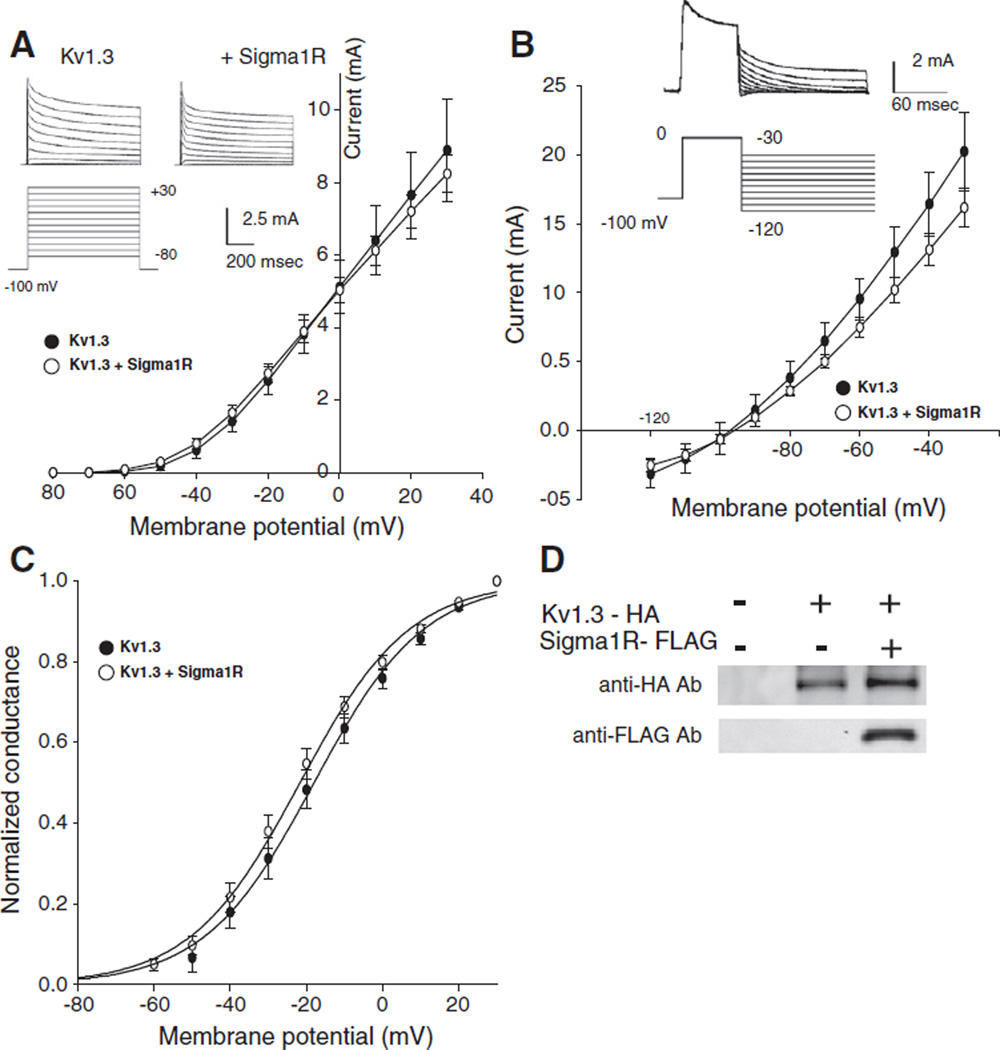
A two-electrode voltage clamp of the injected oocytes demonstrated voltage-activated slowly inactivating outward-going currents consistent with currents flowing through the Kv1.3 channels (Spencer et al., 1997; Kuppar, 1998; DeCoursey et al., 1985). No qualitative difference in the current morphology was seen in oocytes injected with cRNA for HA-tagged Kv1.3 alone or in combination with the FLAG-tagged Sigma1R (Fig. 1A). Reversal potentials were determined by a tail protocol (Fig. 1 B) and normalized conductance–voltage plots (Fig. 1C) were calculated from the current–voltage plot (Fig. 1A) and the reversal potentials. The Kv1.3 current was characterized by: Erev=−99± 4 mV, V1/2=−18±3 mV and slope factor=13.6±0.5 (n=7). The current when co-expressed with the Sigma1R was described by: Erev=−95±2 mV, V1/2=−22±2 mV and slope factor=13.8± 0.4 (n=9)with no significant difference from the parameters derived from Kv1.3 expressed alone. The large size of oocytes allowed a direct mechanical dissection of the plasma membrane and the proper expression of both Kv1.3-HA and Sigma1R-FLAG proteins in the plasma membrane of injected oocytes was demonstrated by a Western blot (Fig. 1D).
Fig. 1.
Sigma1 receptors (Sigma1R) co-expression has no effect on the voltage-dependent activation and Erev of Kv1.3. A. Currents through Kv1.3 or Kv1.3 co-expressed with Sigma1R were elicited by 800 ms depolarizing voltage steps from −80 to +30mV in 10mV increments, applied from a holding potential of −100 mV (inset). IV plots were constructed from peak currents evoked by voltage steps. B. Tail currents elicited by voltage steps from −120 to −30 mV after 60 ms depolarizing prepulse to 0 mV, applied from a holding potential −100 mV. The potential at which no current is observed is the reversal potential (Erev). C. Peak currents at respective voltages normalizing to the maximum current divided by the driving force yielded the gV plot that demonstrates the voltage dependence of channel activation. The smooth curve is the best fitting Boltzmann function. For Kv1.3 alone, n=7; for Kv1.3+Sigma1R, n=9. D. Lysates were prepared from mechanically isolated Xenopus oocytes membranes non-injected or injected with Kv1.3-HA alone, or with Sigma1R-FLAG cRNA. Approximately 3 µg protein was loaded in all lanes, resolved with SDS-PAGE, and replicate membranes were immunoblotted with anti-HA or anti-FLAG antibodies.
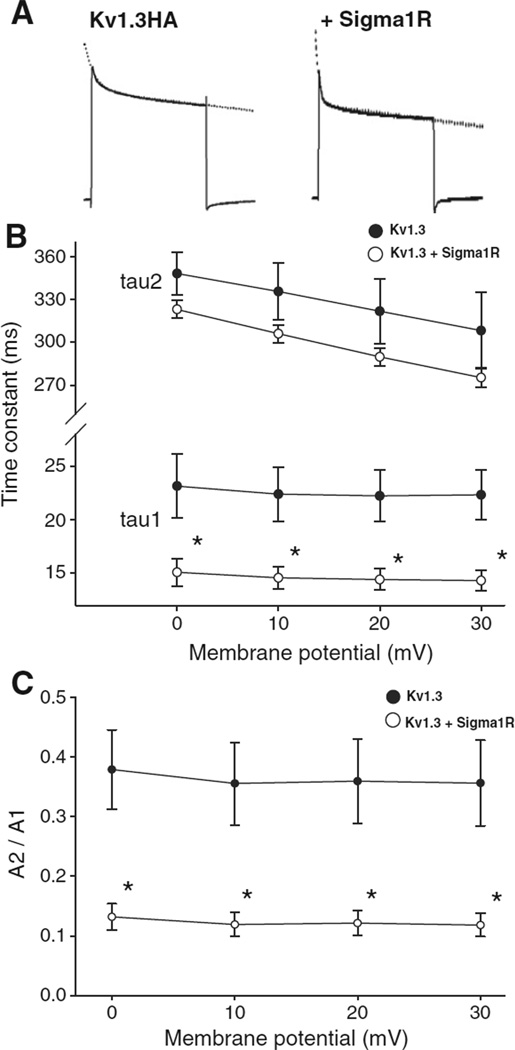
The decay phase of the currents was analyzed by fitting a double exponential function (Fig. 2A). The Kv1.3 current inactivation has been reported to be well described by a monoexponential fit (Spencer et al., 1997). We confirmed that, in contrast to a C-terminal EGFP tag having no effect on inactivation (Kuppar, 1998), an epitope tag with 3 × HA introduced a fast decay phase to the inactivation (data not shown). The current showed a voltage-dependent inactivation with the current decaying faster at a more depolarized potential as reported by others (DeCoursey et al., 1985). In oocytes co-expressing the Sigma1R, a significantly faster current inactivation especially for the fast component of time constants from double exponential fitting was observed at all voltages (Fig. 2B) and with a reduction in the ratio of slow to fast (i.e. A2/A1 ratio) inactivating components (Fig. 2C).
Fig. 2.
Sigma1R co-expression accelerates Kv1.3 inactivation kinetics. A. Current decay of Kv1.3 alone and co-expressed with Sigma1R were fitted with a double exponential function (dashed lines). B. A double exponential fit to the current decay showed a voltage-independent fast (A1*exp(−t/tau1)) and a voltage-dependent slow components (A2*exp(−t/tau2)) of inactivation time constants. Both time constants were smaller in the co-injected oocytes (open circles) compared to those only injected with Kv1.3 cRNA (closed circles). The fast component demonstrated significant difference at all voltages (*p < 0.05). C. The ratio of the amplitudes of slow to fast decaying components (A2/A1 ratio) decreased with Sigma1R co-expression but remained independent of voltage for both groups.
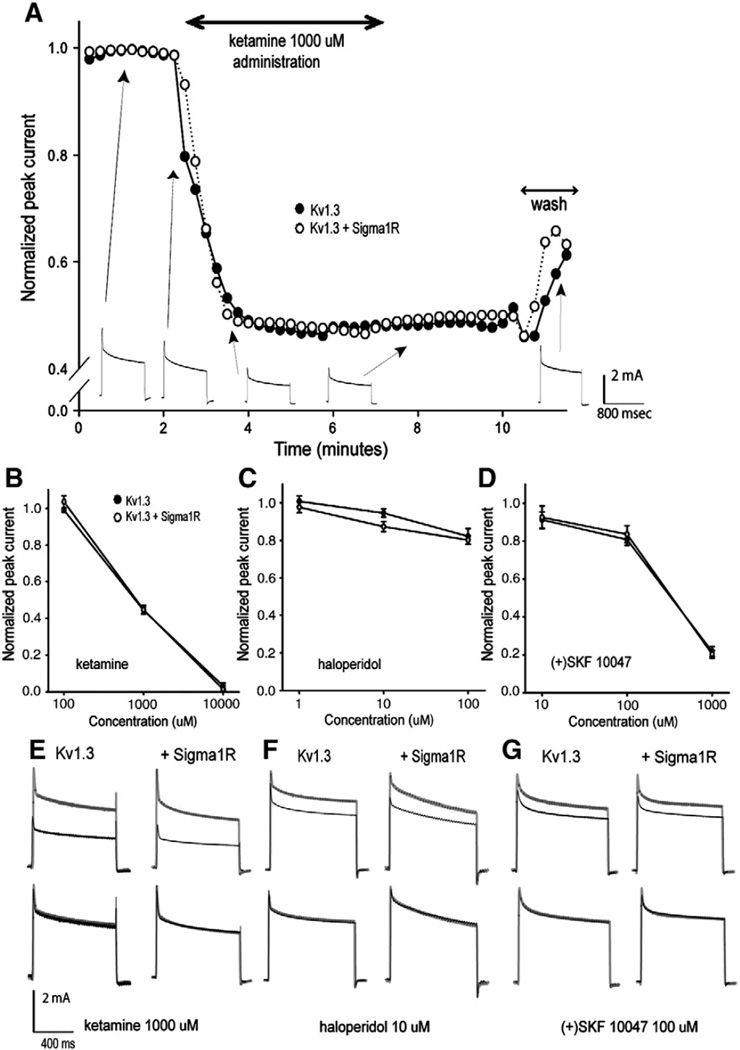
In an earlier study, a dramatic increase in the sensitivity to the blocking effect of Sigma1R ligand was reported when the Sigma1R was co-expressed with Kv1.4 (Aydar et al., 2002). We studied the effects of 3 Sigma1R ligands, ketamine, haloperidol and (+)SKF 10047 on Kv1.3 expressed by itself or in combination with the Sigma1R. These drugs were chosen for the study because ketamine and haloperidol are used clinically and because (+)SKF 10047 is a potent Sigma1R ligand previously reported to demonstrate a profound effect on Kv1.4 and Kv1.5. Currents were evoked by repetitive depolarizing steps and the respective ligands administered. All Sigma1R ligands reversibly inhibited the peak Kv1.3 current (Fig. 3A) in a dose-dependent manner but with no difference in the ligand sensitivity regardless of whether Sigma1R was co-expressed (Fig. 3B–D). Kinetic analysis of currents partially inhibited by the ligands demonstrated no effect on the decay time constants, with or without Sigma1R co-expression, as can be seen from the overlapped scaled current traces (Fig. 3E–G).
Fig. 3.
Sigma1R co-expression had no effect on the sensitivity of Kv1.3 inhibition by Sigma1R ligands. A. Time course of ketamine inhibition of peak currents in oocytes expressing Kv1.3 alone (closed circles) or co-expressing Sigma1R (open circles). Currents were evoked by repetitive 800 ms voltage steps from −80 to 0 mV every 15 seconds to circumvent accumulation of cumulative slow inactivation. Ketamine (1 mM) was administrated at the rate of 1 ml/min by gravity. The current waveforms recorded at different time points during the experiment (Kv1.3 only) are shown below. When administrated in the same way, ketamine (B), haloperidol (C) and (+)SKF 10047 (D), all showed a dose-dependent current inhibition of the Kv1.3 peak current. n=3 for each ligand. E. The upper traces are from before and during drug application. The same traces were scaled and overlapped demonstrating no change in current kinetics induced by ketamine, haloperidol (F) and (+)SKF 10047 (G).
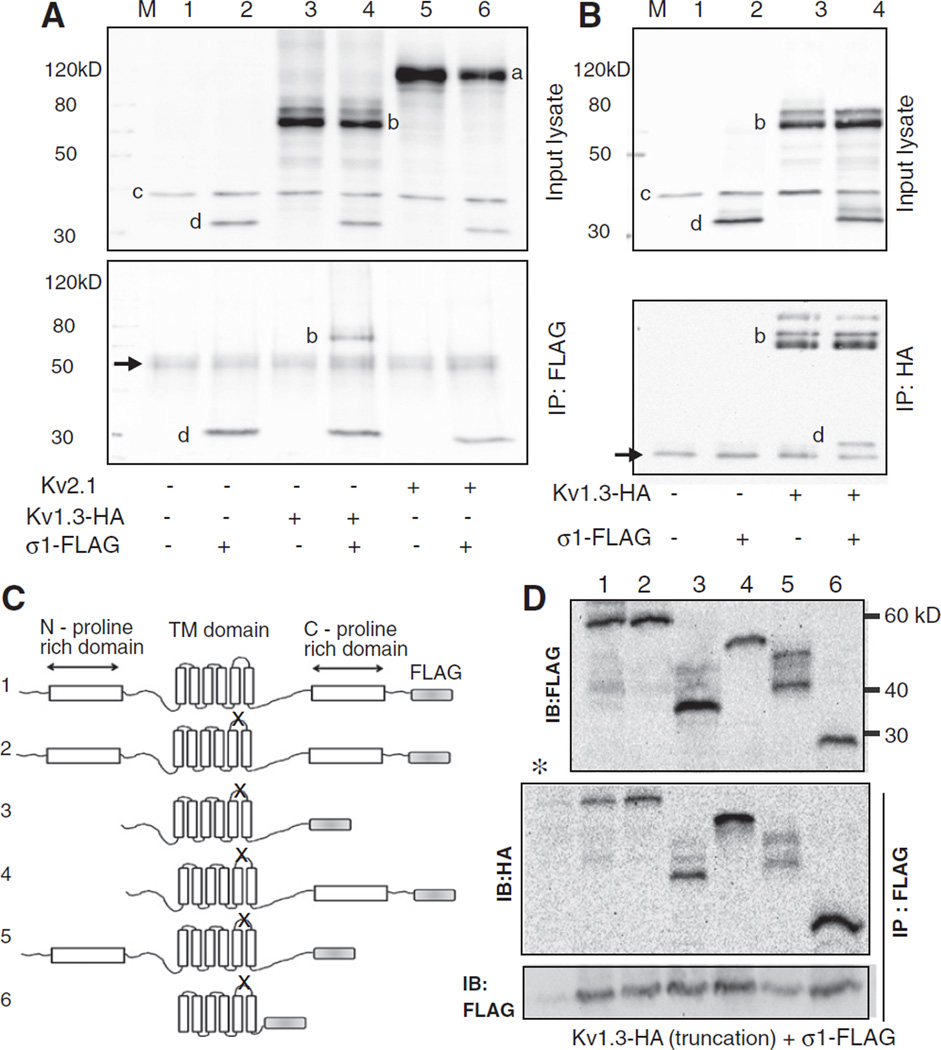
We sought evidence for protein–protein interaction between Kv1.3with Sigma 1 receptor by co-immunoprecipitation studies. These studies were conducted in transfected HEK293 cells since abundant proteins could be extracted. Lysates from cells co-transfected with Kv1.3-HA and Sigma1R-FLAG immunoprecipitated with the anti-FLAG antibody and probed with anti-HA antibody gave a signal consistent with co-immunoprecipitation of the two proteins (Fig. 4A, lanes 3, 4). This protein–protein interaction was confirmed by a reverse immunoprecipitation with the anti-HA antibody and probing with anti-FLAG antibody (Fig. 4B). A similar experiment with another voltage-gated potassium channel Kv2.1 from a different family demonstrated that this channel did not co-immunoprecipitate with the Sigma1R (Fig. 4A, lanes 5, 6) indicating that the Sigma 1R did not interact with all potassium channels. To narrow down the potential domains of the Kv1.3 channel protein interacting with the Sigma 1R, we created a series of Kv1.3 truncation mutants (Fig. 4C). These channels also contained a mutation in the ion permeation domain to demonstrate that the ion flux through the channel was not required for its interaction with the Sigma 1R. The mutant Kv1.3s expressed well and appeared as several mobility shifted species probably reflecting different degrees of protein degradation or post-translational modifications (Fig. 4D, top). Co-immunoprecipitation experiments of truncated ion permeation-deficient Kv1.3s and the Sigma1R suggested that even the channel core domain by itself interacted with the Sigma 1 receptor (Fig. 4C, middle). Probing the immunoprecipitated lanes with anti-FLAG antibody (Fig. 4D, bottom) confirmed the successful pull-down in all lanes except for the control lane where an unrelated rabbit IgG replaced the anti-FLAG antibody.
Fig. 4.
Co-immunoprecipitation study of protein–protein interaction between Kv1.3 and Sigma1R. A. Lysates from HEK 293 cells transfected with HA-tagged Kv1.3 and FLAG-tagged Sigma1R and that immunoprecipitated with anti-FLAG antibody were blotted with a cocktail of mouse anti-Kv2.1 (1:1000), -HA (1:1000, for Kv1.3), -GAPDH (1:100,000), and -FLAG (1:2000, for Sigma 1R) antibodies. The top panel is the input lysate and the bottom after immunoprecipitation. Note the presumed Kv2.1 (a), Kv1.3-HA(b), GAPDH(c), and Sigma 1R-FLAG(d) immunoreactive bands (top) and the IgG heavy chain band (arrow, bottom). Both panels are blots of the entire membrane. B. A reverse co-IP experiment where the input lysates (top) were immunoprecipitated with the anti-HA antibody and probed with a mixture of antibodies as above (bottom).Arrow (bottom panel) is the IgG light chain band. C. Adiagram of various truncated non-conducting (denoted by X in the P-loop) Kv1.3-HA and wild type full length Sigma1R-FLAG. Characteristic structural motifs shown include the N- and C-proline rich domains and the six transmembrane domains. The channel constructs were: 1.wild type full length Kv1.3, 2. non-conducting (P loop GYG to AYA, n1560–1568) full length, mutant, 3.N- and C-proline rich domain deleted non-conducting mutant (ATG at n633–635 and TGA at n1845–1847), 4. N-proline rich domain deleted non-conducting mutant (ATG at n633–635), 5. C-proline rich domain deleted non-conducting mutant (TGA at n1845–1847), and 6. core domain non-conducting mutant (ATG at n906–908 and TGA at n1671–1673). All nucleotide numbers are as denoted on NCBI accession number NM_002232. The expected molecular mass of the constructs are 55.5, 55.5, 45.8, 49.5, 48, and 27.7 kDa for constructs 1–6, respectively. D. Anti-FLAG or anti-HA immunoblots of lysates from cells co-transfected with the various truncated Kv1.3-HAconstructs and Sigma1R-FLAG (top). Kv1.3 proteins that co-immunoprecipitated with Sigma1R are denoted in the IP: Flag IB:HAblot (middle). (*) denotes a control lane identical to lane 1 lysate but immunoprecipitated with an unrelated antibody. Control (IP: FLAG, IB: FLAG) (bottom) confirmed successful immunoprecipitation in all lanes except the negative control * lane. The images are over-exposed to allow detection of the faint protein degradation or post-translationally modified mobility-shifted species. All immunoblots were repeated a minimum of 3 times with consistent results.
3. Discussion
The Sigma1R when expressed in Xenopus oocyte was present in the plasma membrane in the correct cellular compartment for potential interaction with Kv1.3 as reported previously (Aydar et al., 2002) and confirmed here by a Western blot of the isolated oocyte plasma membrane. Sigma1R co-expression with Kv1.3 accelerated the voltage-dependent inactivation of this ion channel at all voltages examined identical to a previously reported ligand-independent effect of Sigma1R on Kv1.4. In contrast, co-expression of Sigma1R did not enhance the sensitivity of Kv1.3 to inhibition by Sigma1R ligands ketamine, haloperidol and (+)SKF 10047. This finding is in contrast to the dramatic sensitization of the closely related Kv1.4 and 1.5 by Sigma1R ligands.
Aydar et al. studied Sigma1R and ion channels expressed in the Xenopus oocyte system, therefore, a difference in the expression system cannot explain the lack of ligand-dependent auxiliary channel protein effect of Sigma1R on Kv1.3. At 100 µM (+)SKF 10047, Kv1.4 was inhibited by ~10% when expressed by itself and the inhibition increased to ~70% when co-expressed with the Sigma1R (Fig. 2 in Aydar et al.). Our results showed a Kv1.3 inhibition of ~15% by the same drug and concentration regardless of whether Sigma1R was co-expressed (Fig. 3D) leading us to the conclusion that the ligand sensitivity of Kv1.3 was not affected by the Sigma1R although Kv1.3 and Kv1.4 demonstrated similar sensitivity to (+)SKF 10047 peak current inhibition.
In Xenopus oocytes and HEK293 cells, the Sigma1R is clearly present in the plasma membrane in the correct cellular compartment for modulating plasma membrane-resident functional ion channels. However, little else is known about how Sigma1R modulates ion channel function. Even the basic topology of the Sigma-1R remains controversial. Aydar et al. suggested intracellular N- and C-termini with the loop between the two hydrophobic domains facing the extracellular side based on differential antibody staining depending on whether the plasma membrane was permeabilized or not. Others suggest a cytosolic localization of the loop between the two hydrophobic domains that allows interaction between ER-resident Sigma1R and the mitochondria at mitochondria-associated-membrane consistent with the earlier report by Hayashi and Su (2007). A recent review attempted to reconcile this controversy suggesting that the Sigma-1R may re-orient as the protein translocates from the ER to plasma membrane with no mechanistic suggestion on how this highly atypical process may occur (Su et al., 2010). At this time, how the Sigma1R modulates the various ion channels and even the Sigma-1R orientation in the plasma membrane is not known.
The dissociation of the Sigma1R ligand-independent effect on the ion channel kinetics from the ligand-dependent sensitization of the ion channel to the ligands suggests the presence of two different mechanisms of ion channel modulation by Sigma1R. Amino acid sequence comparison between Kv1.3 and Kv1.4 reveals significant divergence in the N-terminal cytosolic domain proximal to the first transmembrane domain. It is tempting to speculate that the ligand-dependent effect of Sigma1R on the potassium channels, namely the enhanced sensitivity to Sigma1R ligands, may be mediated by this divergent protein domain while the ligand-independent effect on the channel kinetics, i.e. accelerated inactivation current decay, may be subserved by other domains conserved between the two closely related channels. An insight can be gained from the observation that Kv1.3 inactivation is mostly P/C type in contrast to the predominant N-type inactivation in Kv1.4 (Panyi et al., 1995). Our speculation seemed supported by the fact that the P/C type inactivation is related to the selectivity filter domain, glycine-tyrosine-glycine motif, which is well-conserved among Kv channels with this type of inactivation (Kiss et al., 1999). Since P/C type inactivation occurs by a time-dependent conformational change in the voltage sensing transmembrane domain of ion channels (Loots and Isacoff, 2000) it is reasonable that Sigma 1R acts on the transmembrane domain to modulate inactivation. Our co-immunoprecipitation result confirming Sigma1R interaction with the hydrophobic transmembrane core domain of Kv1.3 is consistent with this idea. Further co-immunoprecipitation experiments with various truncation mutants of Kv1.3, Kv1.4, and Sigma1R looking for potential differences in the protein:protein interaction domains will be necessary to determine whether the two mechanistically different manifestations of ion channel modulation in two related-Kvs are indeed mediated by different protein domain interactions. Conversely, various truncations of the Sigma1R can be studied to define how it interacts with the mostly α-helical transmembrane domains of the ion channel. The only structure-activity study of Sigma1R has been on its interaction with the inositol 1,4,5-trisphosphate (IP3) receptor where it has been demonstrated that the C-terminal of the Sigma1R was critical in the enhancement of IP3-mediated release of calcium (Wu and Bowen, 2008).
Our observation that the acceleration of Kv1.3 inactivation is ligand independent makes it unlikely that the immunomodulatory property of Sigma1R ligands could be mediated by the Sigma1R-Kv1.3 interaction. However, Sigma1R has been shown to play a critical role in intracellular Ca2+ homeostasis through direct inhibition of the L-type voltage gated Ca2+ channels (Church and Fletcher, 1995; Zhang and Cuevas, 2002; Tchedre et al., 2008) and regulation of IP3 receptor-dependent intracellular Ca2+ release (Hayashi and Su, 2007; Wu and Bowen, 2008); both processes likely to play a key role in T lymphocyte activation. Since Ca2+ regulation by Sigma1R is modulated by its ligand, the immuno-modulatory action of Sigma1R ligands could be mediated through modulation of intracellular Ca2+ independent of the receptor’s interaction with Kv1.3. In this study we confirmed that the Sigma1R-Kv1.3 interaction leads to a significant acceleration of current inactivation which likely plays a biological role in the modulation of excitability in cells that express these proteins as in the hippocampus (McCann et al., 1994; Veh et al., 1995).
4. Experimental procedures
4.1. Molecular biology
Full-length cDNA for human Sigma1R variant 1 and human Kv1.3 were obtained from OriGene (Rockville, MD). An epitope tag (3×HA or 3×FLAG) was placed on the C-terminal of the cDNA through a polymerase chain reaction (PCR)-engineered Mlu I restriction enzyme site that replaced the native stop codon. The PCR amplified segments of the cDNA were sequenced to confirm its fidelity. The resulting epitope-tagged proteins are identical to the original sequence except for a threonine–arginine dipeptide linker corresponding to the engineered Mlu I restriction enzyme recognition sequence. Both cDNA were subcloned into pBSMXT (gift of Dr. A. Wei, Washington Univ., St Louis, MO), a modified pBlueScript (Promega, Madison, WI) designed for oocyte expression, where the multiple cloning site is flanked by the Xenopus globin 5′ and 3′ untranslated sequences. Epitope-tagged Sigma1R-3XFLAG and Kv1.3-3XHA cDNA in pBSMXT vector were linearized and cRNA transcribed using mMESSAGE MACHINE T3 (Ambion, Austin, TX). The final cDNAs were also subcloned into the CMV promoter-driven eukaryotic expression vector pCI/neo (Promega, Madison, WI) for expression in human embryonic kidney (HEK) 293 cells (ATCC CRL-1573). Approximately 5×105 cells were plated onto each gelatin-coated well of a 6-well tissue culture plate and 24 hrs after plating 2 µg total of plasmid DNA was transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer’s recommended protocol.
Several Kv1.3 conduction deficient (GYG to AYA in the P-loop) and truncation mutants were created by PCR. The N- and C-proline rich domains thought to be important for protein:protein interactions (Cook and Fadool, 2002) and the amino acids between the proline rich domain and the transmembrane domain were targeted for deletion (see Fig. 4B). For N-deletions where an ectopic start-methionine was inserted, AGCGCC artificial Kozak sequence preceded the ATG codon to enhance translation and expression. As above, all PCR derived constructs were sequenced to confirm fidelity and the final C-terminal 3XFLAG-tagged constructs subcloned into the pCI/neo expression vector.
4.2. Oocyte expression and electrophysiology
Xenopus oocytes were kind gifts from Dr. Baron Chanda (Department of Physiology, UW Madison, WI) harvested under an Institutional Animal Care and Use Committee approved animal protocol. The follicular membranes were removed by digestion in 1.0mg/ml collagenase (type IA; Sigma Aldrich, St. Louis, MO) for 1 hr at room temperature. Oocytes were injected with approximately 80 ng (total) of cRNA and incubated in ND96 (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 5 mM HEPES and 10 µg / ml gentamycin adjusted to pH 7.4 by NaOH) for 24 to 48 hrs at 18 °C.
Electrophysiological recordings were obtained by a conventional two-electrode voltage clamp technique under the control of pClamp 9.2 software (Axon Instruments, Foster City, CA). Electrodes were pulled from borosilicate glass pipettes and filled with 2 M KCl. Electrode resistances were 0.2–1.0 MΩ. Transmembrane potential was controlled with a voltage clamp amplifier (Gene Clamp 500; Axon Instruments) and currents recorded at room temperature with oocytes bathing in a solution consisting of: 93 mM NaCl, 5 mM KCl, 1 mM MgCl2, 0.3 mM CaCl2, 5 mM HEPES (pH 7.4). The current activation protocol consisted of 800 ms voltage steps from −80 to +30 mV in 10 mV increments from a holding potential of −100 mV. The current reversal potential (Erev) was determined by a tail-protocol (activation step to 0 mV followed by a step to −120 to −30 mV) and conductance obtained by dividing the measured peak current by the driving force (V−Erev). Normalized conductance–voltage (gV) curve fitted by a Boltzmann equation yielded the half-activation voltage V1/2 and slope factor. A double exponential function was fitted to the decay phase of the current to define the inactivation time constant. Ketamine and haloperidol were obtained from Sigma Aldrich, and (+)SKF 10047 from TOCRIS bioscience (Ellisville, MO) and compounds dissolved in the bathing solution at the desired concentrations. Currents were elicited by repeated voltage steps from −100 to 0 mV every 15 seconds and after stable baseline currents were obtained, the extracellular control solution was switched to that containing the drug administrated into the recording chamber at a rate of 1 ml/min by gravity. Current recordings were continued until steady state responses were obtained and thereafter returned to the control drug-free solution to demonstrate reversibility. pClamp 9.2 and OriginPro 6.1 (OriginLab, Northampton, MA) and Sigma plot 9.01 (Systat Software Inc., San Jose, CA) were used for data analysis and generating the figures. The significance of the fit parameters (mean± S.E.M.) was tested using a two-tailed t-test. p <0.05 was considered statistically significant.
4.3. Immunoblotting
Cell lysates from mechanically dissected Xenopus oocyte membrane were obtained with a lysis buffer consisting of: 1% Triton X 100, 100mM NaCl, 10mM Tris, 5 mM EDTA supplemented with a protease inhibitor (pH 7.4). The lysate protein concentration was quantified (BCA Protein Assay, Thermo Scientific, Rockford, IL), and an equal amount was loaded in each lane and separated by sodium dodecyl-sulfate-polyacrylamide gel electrophoresis (10% gel). After protein transfer to a nitrocellulose membrane, immunoblot was accomplished by blocking in 1% milk in Tris-buffered saline supplemented with 0.2% Triton X 100 (TBST) for 1 hr at room temperature, primary antibody (mouse anti-HA antibody (1:2500), Covance, Princeton, NJ or mouse anti-FLAG antibody (1:2500), Sigma Aldrich) exposure in 1% milk in TBST for 2 hrs at room temperature or overnight at 4 °C followed by 3 washes, horseradish peroxidase conjugated secondary antibody (goat anti-mouse IgG (1:5000), Bio-Rad Laboratories, Hercules, CA) incubation for 1 hr at room temperature, and visualized using enhanced chemiluminescence reagent (Western Lightning, Perkin Elmer, Waltham, MA).
Cell lysates from HEK cells were obtained with a modified RIPA buffer (1% Nonidet P-40, 10 mM Tris pH7.5, 50 mM NaCl, 30 mM NaPPi, 50 mM NaF, 1% Triton X 100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl-sulfate, 1 mM phenylmethylsulphonyl fluoride, 0.1 mM iodoacetamide, 1 mM Na3VO4, 50 µM N-ethyl maleimide) supplemented with protease inhibitor. For immunoprecipitation, the lysate was processed using Dynabeads (Invitrogen, Carlsbad, CA). Protein 100 µg from lysate was incubated with 2 µg of rabbit anti-FLAG antibody (Sigma Aldrich) or rabbit anti-HA antibody (Thermo scientific, Rockford, IL) bound with 50 µl of Dynabeads protein A for 1 hr at room temperature, followed by washing three times with PBS and eluted in 50 µl PDR by heating at 70 °C for 10 min. The immunoprecipitated proteins were separated, blotted, and detected as described above. For Kv2.1 detection, the mouse anti-Kv2.1 (1:1000, UC Davis/NIH NeuroMab Facility, Davis, CA) was used and probing with mouse anti-GAPDH antibody (1:100,000, Advanced Immunochemical, Long Beach, CA) served as a protein loading control for the input lysate blots.
Acknowledgments
We thank Dr. Arnold Ruoho (Department of Pharmacology, UW Madison, WI) for introducing the senior author to the Sigma1R area of research and for constructive discussions. This research was partly supported by NIH RO1GM086401 (JY) and funds from the University of Wisconsin Department of Anesthesiology.
Contributor Information
Maho Kinoshita, Email: mkinoshita@wisc.edu.
Yoshikazu Matsuoka, Email: matsuoka2@wisc.edu.
Takeshi Suzuki, Email: tsuzuki5@wisc.edu.
Jennifer Mirrielees, Email: mirrielees@wisc.edu.
Jay Yang, Email: Jyang75@wisc.edu.
REFERENCES
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Beeton C, Wulff H, Barbaria J, Clot-Faybesse O, Pennington M, Bernard D, Cahalan MD, Chandy KG, Béraud E. Selective blockade of T lymphocyte K(+) channels ameliorates experimental autoimmune encephalomyelitis: a model for multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13942–13947. doi: 10.1073/pnas.241497298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, Kolski-Andreaco A, Wei E, Grino A, Counts DR, Wang PH, Lee CJ, Healey S, Andrews B, Sankaranarayanan A, Homerick D, Roeck WW, Tehranzadeh J, Stanhope KL, Zimin P, Havel PJ, Griffey S, Knaus HG, Nepom GT, Gutman GA, Calabresi PA, Chandy KG. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17414–17419. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DJ, Mayo S, Woolley TW, DeCosta BR. Immunoregulatory properties of (+)-pentazocine and sigma ligands. Immunology. 1992;77:527–531. [PMC free article] [PubMed] [Google Scholar]
- Casellas P, Bourrie B, Canat Z, Carayon P, Buisson I, Paul R, Brelier JC, Le Fur G. Immunopharmacological profile of SR31747: in vitro and in vivo studies on humoral and cellular responses. J. Immunolol. 1994;52:193–203. doi: 10.1016/0165-5728(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Church J, Fletcher EJ. Blockade by sigma site ligands of high voltage-activated Ca2+ channels in rat and mouse cultured hippocampal pyramidal neurons. Br. J. Pharmacol. 1995;116:2801–2810. doi: 10.1111/j.1476-5381.1995.tb15929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos EJ, Entrena JM, Nieto FR, Cendán CM, Del Pozo E. Pharmacology and therapeutic potential of sigma(1) receptor ligands. Curr. Neuropharmacol. 2008;9:344–366. doi: 10.2174/157015908787386113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook KK, Fadool DA. Two adaptor proteins differentially modulate the phosphorylation and biophysics of Kv1.3 ion channel by Src kinase. J. Biol. Chem. 2002;277:13268–13280. doi: 10.1074/jbc.M108898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-dependent ion channels in T-lymphocytes. J. Neuroimmunol. 1985;10:71–95. doi: 10.1016/0165-5728(85)90035-9. [DOI] [PubMed] [Google Scholar]
- Ganapathy ME, Prasad PD, Huang W, Seth P, Leibach FH, Ganapathy V. Molecular and ligand-binding characterization of the sigma-receptor in the Jurkat human T lymphocyte cell line. J. Pharm. Exp. Ther. 1999;289:251–260. [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Johannessen M, Ramachandran S, Riemer L, Ramos-Serrano A, Ruoho AE, Jackson MB. Voltage-gated sodium channel modulation by sigma-receptors in cardiac myocytes and heterologous systems. Am. J. Physiol. Cell Physiol. 2009;296:C1049–C1057. doi: 10.1152/ajpcell.00431.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss L, Lo Turco J, Korn SJ. Contribution of the selectivity filter to inactivation in potassium channels. Biophy. J. 1999;76:253–263. doi: 10.1016/S0006-3495(99)77194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppar J. Functional expression of GFP-tagged Kv1.3 and Kv1.4 channels in HEK 293 cells. Eur. J. Neurosci. 1998;10(390):8–12. doi: 10.1046/j.1460-9568.1998.00441.x. [DOI] [PubMed] [Google Scholar]
- Leykin I, Mayer R, Shinitzky M. Short and long-term immunosuppressive effects of clozapine and haloperidol. Immunopharmacol. 1997;37:75–86. doi: 10.1016/s0162-3109(97)00037-4. [DOI] [PubMed] [Google Scholar]
- Liu Y, Whitlock BB, Pultz JA, Wolfe SA., Jr Sigma-1 receptors modulate functional activity of rat splenocytes. J. Neuroimmunol. 1995;59:143–154. doi: 10.1016/0165-5728(95)00032-w. [DOI] [PubMed] [Google Scholar]
- Loots E, Isacoff EY. Molecular coupling of S4 to a K(+) channel's slow inactivation gate. J. Gen. Physiol. 2000;116:623–636. doi: 10.1085/jgp.116.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann DJ, Weissman AD, Su TP. Sigma-1 and sigma-2 sites in rat brain: comparison of regional, ontogenetic, and subcellular patterns. Synapse. 1994;17:182–189. doi: 10.1002/syn.890170307. [DOI] [PubMed] [Google Scholar]
- Ohta N, Ohashi Y, Fujino Y. Ketamine inhibits maturation of bone marrow-derived dendritic cells and priming of the Th1-type immune response. Anesth. Analg. 2009;109:793–800. doi: 10.1213/ane.0b013e3181adc384. [DOI] [PubMed] [Google Scholar]
- Panyi G, Sheng Z, Tu L, Deutsch C. C-type Inactivation of a voltage-gated K+ channel occurs by a cooperative mechanism. Biophys. J. 1995;69:896–903. doi: 10.1016/S0006-3495(95)79963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyi G, Varga Z, Gáspár R. Ion channels and lymphocyte activation. Immunol. Lett. 2004;92:55–66. doi: 10.1016/j.imlet.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Rofael HZ, Turkall RM, Abdel-Rahman MS. Immunomodulation by cocaine and ketamine in postnatal rats. Toxicology. 2003;188:101–114. doi: 10.1016/s0300-483x(03)00081-7. [DOI] [PubMed] [Google Scholar]
- Spencer RH, Sokolov Y, Li H, Takenaka B, Milici AJ, Aiyar J, Nguyen A, Park H, Jap BK, Hall JE, Gutman GA, Chandy KG. Purification, visualization, and biophysical characterization of Kv1.3 tetramers. J. Biol. Chem. 1997;272:2389–2395. doi: 10.1074/jbc.272.4.2389. [DOI] [PubMed] [Google Scholar]
- Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol. Sci. 2010;31:557–566. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchedre KT, Huang RQ, Dibas A, Krishnomoorthy RR, Dillon GH, Yorio T. Sigma-1 receptor regulation of voltage-gated calcium channels involves a direct interaction. Invest. Ophthalmol. Vis. Sci. 2008;49:4993–5002. doi: 10.1167/iovs.08-1867. [DOI] [PubMed] [Google Scholar]
- Veh RW, Lichtinghagen R, Sewing S, Wunder F, Grumbach IM, Pongs O. Immunohistochemical localization of five members of the Kv1 channel subunits: contrasting subcellular locations and neuron-specific co-localizations in rat brain. Eur. J. Neurosci. 1995;7:2189–2205. doi: 10.1111/j.1460-9568.1995.tb00641.x. [DOI] [PubMed] [Google Scholar]
- Wilke RA, Lupardus PJ, Grandy DK, Rubinstein M, Low MJ, Jackson MB. K+ channel modulation in rodent neurohypophysial nerve terminals by sigma receptors and not by dopamine receptors. J. Physiol. 1999;517:391–406. doi: 10.1111/j.1469-7793.1999.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Bowen WD. Role of Sigma-1 receptor C-terminal segment in inositol 1,4,5-trisphosphate receptor activation. J. Biol. Chem. 2008;283:28198–28215. doi: 10.1074/jbc.M802099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Nakao S, Sakamoto S, Takamori Y, Tamura Y, Mochizuki-Oda N, Kataoka Y, Yamada H, Shingu K. Propofol acts at the sigma-1 receptor and inhibits pentazocine-induced c-Fos expression in the mouse posterior cingulate and retrosplenial cortices. Acta Anaesthesiol. Scand. 2006;50:875–881. doi: 10.1111/j.1399-6576.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- Yao H, Yang Y, Kim KJ, Bethel-Brown C, Gong N, Funa K, Gendelman HE, Su TP, Wang JQ, Buch S. Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration. Blood. 2010;115:4951–4962. doi: 10.1182/blood-2010-01-266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cuevas J. Sigma receptors inhibit high-voltage-activated calcium channels in rat sympathetic and parasympathetic neurons. J. Neurophysiol. 2002;87:2867–2879. doi: 10.1152/jn.2002.87.6.2867. [DOI] [PubMed] [Google Scholar]
- Zhu LX, Sharma S, Gardner B, Escuadro B, Atianzar K, Tashkin DP, Duninett SM. IL-10 mediates sigma1 receptor-dependent suppression of antitumor immunity. J. Immunol. 2003;170:3585–3591. doi: 10.4049/jimmunol.170.7.3585. [DOI] [PubMed] [Google Scholar]