Abstract
Several peptides are produced and released from endocrine cells scattered within the gastric oxyntic and the small intestinal mucosa. These peptide hormones are crucially involved in the regulation of gastrointestinal functions and food intake by conveying their information to central regulatory sites located in the brainstem as well as in the forebrain, such as hypothalamic nuclei. So far, ghrelin is the only known hormone that is peripherally produced in gastric X/A-like cells and centrally acting to stimulate food intake, whereas the suppression of feeding seems to be much more redundantly controlled by a number of gut peptides. Cholecystokinin produced in the duodenum is a well established anorexigenic hormone that interacts with ghrelin to modulate food intake indicating a regulatory network located at the first site of contact with nutrients in the stomach and upper small intestine. In addition, a number of peptides including leptin, urocortin 2, amylin and glucagon-like peptide 1 interact synergistically with CCK to potentiate its satiety signaling effect. New developments have led to the identification of additional peptides in X/A-like cells either derived from the pro-ghrelin gene by alternative splicing and posttranslational processing (obestatin) or a distinct gene (nucleobindin2/nesfatin-1) which have been investigated for their influence on food intake.
Keywords: Brain-gut, duodenum, food intake, gut peptides, stomach, vagus
INTRODUCTION – PERIPHERAL REGULATION OF FOOD INTAKE
After rapid passage through the oral cavity and the esophagus, ingested food has contact with the stomach first and consecutively with aboral parts of the small intestine. Along this way, specialized endocrine cells respond with the release of gut peptide hormones, many of which play a prime role in modulating nutrient intake. Most of them induce satiation (meal termination) and/or satiety (postponing next meal initiation) while ghrelin is the only known gut orexigenic hormone. As gut peptides located in endocrine cells of the mucosa are not released in an isolated but often in an orchestrated manner in response to change in autonomic and metabolic conditions, their interactions need to be taken into consideration to understand mechanisms at play connecting circuitries regulating food intake.
In the present review, we will highlight ghrelin derived from the stomach and cholecystokinin (CCK) released from the small intestine as established physiologically relevant peptides regulating food intake. We will also highlight the state-of-knowledge on newly identified peptides in gastric X/A-like cells, namely obestatin and nucleobindin2 (NUCB2)/ nesfatin-1. Other gastrointestinal peptides including urocortin 2, amylin and glucagon-like peptide 1 (GLP-1) or the adipocyte-derived protein, leptin will only be discussed with respect to their interactions with gastric (ghrelin) and duodenal (CCK) food intake regulatory hormones. Regulation of food intake by distal gut hormones has been extensively reviewed elsewhere [1, 2].
GHRELIN
Expression in the Stomach, Release and Receptor Interaction
Ghrelin was discovered a decade ago as the endogenous ligand of the orphan growth hormone secretagogue receptor 1a (GHS-R1a) [3]. The X/A-like cells are located in the gastric mucosa and represent the major source of circulating ghrelin [4] as indicated by the 75% decrease of plasma ghrelin occurring immediately after gastrectomy [5]. Additionally, although in much smaller quantities, ghrelin is synthesized in the small and large intestine [6], pancreas [7] and other peripheral organs including liver, heart, testis, kidney, skin and adipose tissue [8, 9].
Ghrelin consists of 28 amino acids and has a characteristic acyl group at the serine in position three, which is necessary for its binding to the GHS-R1a receptor [10]. The octanoyl chain was initially identified as the acyl group in rodent and human ghrelin [11] and recently another acyl-form, decanoyl ghrelin (D-ghrelin) has been found in the mouse stomach and plasma contributing to about half of the circulating acyl ghrelin [12]. Whether D-ghrelin is also a prominent circulating form in humans is still to be established. The enzyme that acylates ghrelin has been recently identified in mice and humans by two independent groups as the fourth member of the membrane-bound O-acyltransferases (MBOAT4) and renamed ghrelin-O-acyltransferase (GOAT) [13, 14]. Mouse GOAT is co-localized with ghrelin in gastric X/A-like cells as detected by in situ hybridization [15] and immunohistochemistry [16]. In contrast, in rats, only half of the GOAT immunoreactive gastric endocrine cells co-localize with ghrelin, whereas the other half co-expresses histidine decarboxylase, a marker for enterochromaffin-like cells [16] indicating a species difference in the distribution of GOAT. This also points towards additional functions of GOAT in other gastric non-ghrelin synthesizing endocrine cells.
Ghrelin is considered a classical orexigenic peptide hormone since its plasma levels rise before and decrease after ingestion of food in animals and humans [17]. A recent study indicates the presence of the T1R3 chemoreceptor on closed-type ghrelin cells of the mouse stomach which do not have contact to the lumen but are located in close proximity to blood vessels as well as on duodenal open-type ghrelin cells in the duodenal mucosa [18]. The distribution of T1R3 receptors may qualify the ghrelin-containing cells to detect sugars and amino acids arising from the blood stream and/or luminal contents to operate as sensor as well as effector cells adapting ghrelin release accordingly [18]. Indeed, proteins and carbohydrates are more effective than lipids to suppress circulating postprandial levels of ghrelin [19]. Of further relevance is the recent demonstration that GOAT immunore-activity in the plasma was increased in response to a 24-h fast in rats and more prominently in mice [16]. This finding suggests that the octanoylation of ghrelin can also take place in the circulation. The mechanisms regulating GOAT expression under different metabolic conditions are still to be determined. Besides the short term fluctuations, circulating ghrelin levels negatively correlate with the body mass index, with increased levels in underweight conditions such as anorexia and cachexia and reduced levels in overweight and obese subjects [20, 21]. One exception is the Prader-Willi syndrome where obese patients display elevated ghrelin plasma levels associated with voracious appetite [22].
The ghrelin receptor, GHS-R1a is widely expressed at the mRNA level in various peripheral tissues (pituitary, stomach, intestine, pancreas, adipose tissue, adrenal gland, thyroid gland, myocardium, spleen, immune cells and on vagal afferents) and the brain [8, 23-27] indicative of broader functions besides the regulation of food intake. In the brain, GHS-R1a mRNA is localized in the arcuate nucleus (Arc), hypothalamic ventromedial nucleus, hippocampus (CA2 and CA3 areas and dentate gyrus), ventral tegmental area, substantia nigra as well as raphe magnus nucleus [23-25, 28] and receptor expression has been confirmed at the protein level in the hypothalamus [29, 30]. The N-terminal five amino acids including the acyl group represent the shortest fragment that interacts with and activates the GHS-R1a depicting the active binding site of ghrelin [10]. However, even in the absence of ghrelin, the GHS-R1a shows a high constitutive activity [31]. This activity is modulated by homo- and heterodimerization, the first contributing to stimulation of growth hormone release, whereas heterodimerization of GHS-R1a and GHS-R1b reduces the responsiveness to ghrelin [32] suggesting a regulatory role of the functionally inactive variant, GHS-R1b.
Effect on Food Intake
Convergent experimental and clinical studies established ghrelin as a hormone physiologically stimulating food ingestion in various animals [20, 33] and humans [34]. The recently described D-ghrelin increases food intake in mice as well [12] indicating biological activity. The orexigenic effect of ghrelin is mediated by the GHS-R1a as indicated by the lack of increased food intake following ghrelin injection in GHS-R1a knockout mice [35, 36] and blockade of the orexigenic response by pre-treatment with GHS-R1a antagonists, JMV 3002 and JMV 2959 in rodents [37]. Circulating ghrelin can act on hypothalamic nuclei involved in the regulation of food intake after crossing the blood-brain barrier by a saturable transporter [38, 39] or directly at hypothalamic sites with incomplete blood-brain barrier. In addition, the ghrelin action can also be initiated on vagal afferents as supported by the presence of the GHS-R1a in the rat nodose ganglion [40, 41] and the finding that intravenous injection of ghrelin fails to stimulate food intake after vagotomy [40]. Further supporting a vagally-mediated action, ghrelin injected intravenously in rats increases the neuronal activation in the nucleus of the solitary tract (NTS) as assessed by Fos immunohistochemistry [42]. Moreover, ghrelin microinjected into the dorsal vagal complex stimulated food intake with a similar sensitivity to that observed after intra-arcuate nucleus injection [43] indicating also a brainstem site of action for ghrelin. After intravenous injection, ghrelin increases the noradrenergic signaling from the NTS to the Arc resulting in the activation of neuropeptide Y (NPY) positive neurons [44]. Conversely, bilateral transections rostral to the NTS abolished the ghrelin-induced feeding [44] highlighting the signaling of ghrelin via the vagus – brainstem – hypothalamus pathway. Taken together, systemic administration of ghrelin reproducing its circulating delivery from gastric endocrine cells can act on brain food intake regulatory centers by both, passage of the blood-brain barrier and direct influence on vagal signaling. However, one report demonstrated retained orexigenic signaling following intraperitoneal ghrelin administration in rats that underwent selective subdiaphragmatic vagal de-afferentiation [45] suggesting that intravenous versus intraperitoneal injection of ghrelin may have different sites of action to induce orexigenic behavior.
The direct central action of ghrelin is also supported by the detection of the peptide in neurons of the Arc [46], a nucleus predominantly implicated in the regulation of ingestive behavior [47], and in vicinity to the third brain ventricle [48]. Ghrelin positive neurons in the Arc make synaptic contacts with NPY and agouti-related peptide (AgRP) positive neurons [48, 49], two main orexigenic neuropeptides [50] suggesting the involvement of NPY and AgRP in the effects of ghrelin injected either centrally or peripherally. Ghrelin delivered into the lateral brain ventricle increases the expression of NPY and AgRP mRNA and activates NPY/AgRP-expressing neurons in the Arc [51]. Other studies showed that the orexigenic effect of centrally injected ghrelin is abolished by co-treatment with anti-NPY or anti-AgRP antibodies into the lateral brain ventricle [25] consistent with NPY and AgRP being downstream peptides mediating ghrelin's food intake stimulatory effect. Likewise, ghrelin injected intraperitoneally in mice selectively activates NPY positive neurons in the Arc as assessed by Fos immunohistochemistry [52]. Conversely, intraperitoneal injection of ghrelin fails to stimulate food intake in mice deficient in NPY and AgRP, whereas in mice bearing single deletion of NPY or AgRP, ghrelin's orexigenic action was retained pointing towards compensation by the other neurotransmitter [53].
Besides its acute effect on food intake, ghrelin is also involved in long term energy homeostasis as reflected by increased energy expenditure and reduced body weight observed in mice lacking both ghrelin and the GHS-R1a [54]. In addition, ghrelin knockout mice fed a high fat diet display a reduced respiratory quotient indicating increased fat consumption [55]. Moreover, the resistance of GHS-R1a deficient mice to diet-induced obesity [36] is indicative of an important role of ghrelin in adipogenesis.
Desacyl Ghrelin: Release and Effects on Food Intake
Desacyl ghrelin was initially reported to represent the major form of circulating ghrelin with an acyl/total ghrelin ratio ranging from 1:15 to 1:55 [56, 57]. However, recent optimization of blood processing improves the recovery of the labile acyl ghrelin resulting in an acyl/total ghrelin ratio of 1:5 [58]. This ratio could become higher when the recently identified D-ghrelin form is added as well. However, this will require the availability of a specific radioimmunoassay developed to quantify this acyl form [12]. Until now it is not clear whether desacyl ghrelin merely represents a degradation product of acyl ghrelin or could also be the circulating precursor of acyl ghrelin, especially in light of the recent finding that the ghrelin acylating enzyme, GOAT, is present in the blood [16]. Since desacyl ghrelin does not bind to the GHS-R1a, the peptide was initially considered biologically inactive [3]. However, several cellular actions of desacyl ghrelin have been reported. The peptide increases insulin release from INS-1E cells [59], decreases glucose secretion from hepatocytes [60], inhibits apoptosis of cardiomyocytes and endothelial cells [61], inhibits lipolysis in epididymal adipocytes [62], reduces synthesis of inflammatory cytokines in activated microglia cells [63], stimulates proliferation of C2C12 skeletal myoblasts [64] and inhibits cancer cell proliferation in vitro [65]. Since the GHS-R1a is not expressed in these cells and/or GHS-R1a antagonists have no effect on these desacyl ghrelin actions, the existence of a yet to be characterized, specific desacyl ghrelin receptor has been postulated.
In contrast to ghrelin's well established role as a hormone stimulating food intake, the physiological role of desacyl ghrelin is much less well understood. Few studies indicated an anorexigenic effect of desacyl ghrelin after intraperitoneal administration of 16 μg/animal in ad libitum fed rats during the dark phase or during the light phase after an overnight fast [66]. Likewise, desacyl ghrelin reduced food intake in mice following central (intracerebroventricular, 3 μg/mouse) or peripheral (intraperitoneal, 10 μg/mouse) injection [67]. However, one study reported an increase of food intake following intracerebroventricular injection of 0.6 μg desacyl ghrelin [68], which may be explained by a possible acylation of this low dose of exogenously administered peptide, since also low doses (<1 μg/rat, intracerebroventricularly) are able to stimulate food intake [68]. In addition, another study injecting 24 μg/mouse intraperitoneally was unable to show an inhibitory effect on food intake in mice [69]. However, we recently reported that desacyl ghrelin injected intraperitoneally blocked the ghrelin (13 μg/kg body weight)-induced food intake in rats when administered simultaneously at 5- or 10-times higher doses, whereas the peptide alone had no effect on food ingestion (Table 1) [70]. These data give rise to a potential acyl ghrelin/desacyl ghrelin interaction that may have relevance in the regulation of food intake and deserves further investigation. Modulation of GOAT activity resulting in an altered acyl/desacyl ghrelin ratio could help to investigate this potential interaction. Recent studies expanded these finding to the pancreas where desacyl ghrelin was found to be a powerful inhibitor of the acylated ghrelin-induced secretion of pancreatic polypeptide from mouse islets [71], providing additional support for the relevance of the acyl ghrelin/desacyl ghrelin interaction.
Table 1.
Synergistic or Antagonistic Interaction between Ghrelin Orexigenic/CCK Anorexigenic Effects and Other Food Intake Regulatory Hormones
| Interaction | Peptide(s) | Effect on | Reference |
|---|---|---|---|
| Synergistic | anandamide | Ghrelin plasma levels | [159] |
| CCK + leptin | Food intake, neuronal activation | [160] | |
| CCK + urocortin 2 | Food intake, gastric emptying, neuronal activation | [161] | |
| CCK + GLP-1 | Food intake | [162] | |
| CCK + amylin | Food intake | [163] | |
| Antagonistic | Insulin | Ghrelin plasma levels | [153] |
| Somatostatin | Ghrelin plasma levels | [154, 155] | |
| GLP-1 | Ghrelin plasma levels | [156-158] | |
| Bombesin | Ghrelin plasma levels | [155] | |
| Ghrelin + desacyl ghrelin | Food intake, neuronal activation | [70] | |
| Ghrelin + CCK | Food intake, neuronal activation, ghrelin plasma levels, CART mRNA expression | [147, 150, 151, 166] |
OTHER PRODUCTS OF THE X/A-LIKE CELL
Besides ghrelin and desacyl ghrelin, other peptides originating from the X/A-like cells have been recently identified and investigated with regards to their implication in the regulation of food intake. These peptides encompass products originating from the same gene as ghrelin (obestatin) [72] or a different gene (nucleobindin2, NUCB2/nesfatin-1) [73].
Obestatin: Release and Effects on Food Intake
The 23 amino acid peptide obestatin was described to be derived from the pro-ghrelin gene by alternative splicing and posttranslational modification at a computer-based predicted cleavage site [72]. In line with this finding, obestatin immunosignals co-localized with ghrelin in human gastric oxyntic endocrine cells [74, 75]. However, in rats only 60% of obestatin immunoreactive cells co-labeled with ghrelin [76] possibly indicating a different distribution between species. Along with its putative identification five years ago, obestatin was reported to be the endogenous ligand of the orphan seven transmembrane domain G protein coupled receptor, GPR39 [72]. However, subsequent reports by various independent groups [77-80] and also the initial investigators [81] failed to reproduce this finding and showed the lack of binding to and activation of the GPR39 by obestatin added to native tissue or transfected cells bearing the receptor. Based on these converging negative data, obestatin is no longer considered as the endogenous ligand for the GPR39 and the receptor through which obestatin acts is still to be identified.
In contrast to the observed variations of ghrelin levels both in the stomach and the systemic circulation induced by different metabolic conditions, gastric expression of obestatin did not vary with different feeding conditions including short or long term food reduction and did not differ between lean and obese rats as assessed by immunohistochemistry [76, 82]. In addition, most reports showed that blood obestatin levels were below threshold [83, 84] and not altered by the metabolic status [82] which does not support the initial assumption of obestatin being a physiological anorexigenic hormone [72]. Moreover, by far, most of the subsequent studies by independent groups were unable to demonstrate an anorexigenic effect of obestatin [78, 79, 84-98] although a few reports were able to only partially reproduce the initially reported food intake reducing effects [89, 99-101]. In summary, it is still a matter of debate whether obestatin is a relevant peptide in food intake regulation. However, two recent reports describe a decrease in the acyl ghrelin/obestatin ratio in the blood of patients with restrictive type of anorexia nervosa, whereas it is not changed in constitutionally thin subjects [102, 103]. This change was caused by an increase in plasma acyl ghrelin levels and an even more pronounced elevation of circulating obestatin levels [102]. On the other hand, the possible alteration of the ghrelin/obestatin ratio under conditions of obesity is still unknown [104]. Whether the ghrelin/obestatin ratio plays a regulatory role in the control of food intake and/or body weight is still an object of debate.
Nesfatin-1: Release and Effect on Food Intake
Nesfatin-1 is an 82 amino acid polypeptide that was initially identified in the cerebrospinal fluid of rats as the cleavage product of NUCB2 which is a 396 amino acid protein prominently expressed in brain feeding regulatory centers [105]. Consistent reports indicate that nesfatin-1 injected into the brain at picomolar levels induces a sustained reduction of dark phase food intake. Nesfatin-1's effect is mediated by several hypothalamic anorexigenic pathways including melanocortin, corticotropin-releasing factor receptor 2 (CRF2) and oxytocin as well as medullary pro-opiomelanocortin signaling [105-107].
In addition to brain NUCB2/nesfatin-1 expression and action, we recently reported that NUCB2 mRNA has a 20- and 12-times higher expression in the stomach than the brain and other peripheral viscera such as the heart respectively [73]. Enrichment of gastric small endocrine cells indicated prominent expression of NUCB2 mRNA in these cells and down-regulation of expression during fasting [73]. These changes translated into reduced nesfatin-1/NUCB2 plasma levels after fasting [106], supporting the suggested physiological role as an anorexigenic modulator of food intake. In humans, nesfatin-1/NUCB2 plasma levels are negatively correlated with body mass index [108]. Interestingly, in the rat stomach, nesfatin-1 immunoreactivity was co-localized with ghrelin in the same cell but in a different pool of vesicles [73] giving rise to differential regulation and release of nesfatin-1 and ghrelin from gastric X/A-like cells. In addition, nesfatin-1 immunoreactivity has been detected in the pancreas, testis and pituitary gland [73, 109, 110]. However, still little is known on the biological actions of peripherally administered nesfatin-1. So far, one group of investigators reported a reduction of dark phase food intake induced by intraperitoneal injection of nesfatin-1 at a dose of 70 μg/mouse and the nesfatin-1 24-53 amino acid fragment, whereas a lower dose of 14 μg/mouse was without effect [111]. This contrasts with the low dose (1 μg/mouse) at which nesfatin-1 injected into the third ventricle of chronically cannulated mice reduced dark phase food intake [107]. The effect of the intraperitoneally injected nesfatin-1 fragment was no longer observed in mice with chemical deafferentiation of C-fibers by capsaicin suggesting a vagally mediated action [112]. It is still to be established, whether the cleavage of nesfatin-1 leads to this 30 amino acid fragment in vivo. The receptor involved in nesfatin-1's anorexigenic action is unknown and its identification and localization will be a key step to enhance the understanding of (sub) cellular events involved in nesfatin-1's effects.
CHOLECYSTOKININ
Main Production in the Duodenum and Release
CCK is mainly produced in small intestinal I cells, most prominently in the duodenum but also in the jejunum and brain structures including the cortex, hippocampus, amygdala, hypothalamus (ventromedial nucleus, Arc, dorsomedial nucleus, supraoptic nucleus and paraventricular nucleus) and hindbrain [113]. CCK is released postprandially with a rapid onset as shown by a rise of plasma levels within 15 minutes post meal initiation in humans [114]. Proteins and fat are potent stimulants of CCK release, whereas glucose caused a significant but small CCK increase in the blood stream [114]. Recently, CCK expression has been described at the gene and peptide levels in taste receptor cells of the oral cavity along with the CCK1 receptor suggesting a potential autocrine action of CCK [115]. In addition, a major portion of those cells expresses alpha-gustducin involved in bitter sensing and, to a lesser extent, T1R2 implicated in the sensations of sweetness [116]. The importance of CCK signaling in the sensation of nutrients is further highlighted by the overconsumption of sweets [117] and oils [118] in CCK1 receptor deficient Otsuka Long-Evans Tokushima fatty (OLETF) rats, most likely resulting from differential sensing of these nutrients. In addition to sensing in the oral cavity, CCK is also involved in the sensation of nutrients in the stomach and small intestine as indicated by an increased Fos expression in the nucleus of the solitary tract following intragastric administration of bitter taste TR2 agonists and the suppression of this Fos response by vagotomy or the CCK1 receptor antagonist, devazepide [119]. These data point towards a direct role for CCK in the mediation of chemosensory signals in the oral cavity and further aboral in the stomach and small intestine.
Molecular Circulating Forms and Importance of Proper Blood Sampling
Several molecular forms of CCK have been reported in the systemic circulation, namely CCK-8 [120, 121], CCK-22 [122], CCK-33/39 [123] and CCK-58 [124, 125]. The most studied form of CCK is CCK-8, based on its predominant detection after standard blood processing. However, after specific blood processing to reduce peptide break down and to increase peptide recovery, only the larger form, CCK-58 is detectable [124] indicating ex vivo degradation of CCK. This is particularly important in light of the different in vivo actions of CCK-8 and CCK-58 on e.g. myenteric neuron [126] and vagal afferent [127] activation, food intake [128] and pancreatic water and chloride secretion [125, 129], whereas similar effects were observed in vitro on acinar cell function [130].
Receptor Interaction and Effect on Food Intake
CCK exerts a variety of actions to orchestrate digestive functions including gall bladder contraction, stimulation of pancreatic secretion as well as induction of gastric accommodation [114, 131, 132]. It was the first gut peptide established to have physiological relevance to inhibit food intake in rats [133] and its role as a short term meal-reducing signal has been extensively documented in mammalian species including humans [134]. CCK's effects are mediated via two receptors, the CCK1 and CCK2 receptor. Intravenous infusion of CCK reduces food intake [135] through binding to the CCK1 receptor based on studies using selective CCK1 receptor agonists [136]. CCK decreases meal size and increases inter-meal interval [137] indicating an effect on satiation as well as satiety. Since the CCK1 receptor has a low and a high affinity state, selective agonists for the high affinity state such as JMV-180 [138] are useful tools to characterize the mediation of this action [139]. In addition, CCK-8S injected intraperitoneally reduces food intake in CCK2 receptor but not in CCK1 receptor knockout mice [140]. CCK1 null mice have a markedly altered feeding pattern comprising of longer and bigger meals compared to their wild type littermates [141]. However, meal frequency was reduced resulting in a similar cumulative food intake [141]. With regard to CCK knockout mice, they did not present alterations in overall food intake but gained significantly less body weight compared to their wild type littermates due to impaired absorption of fat and increased energy expenditure reflecting preferential use of carbohydrates [142]. These findings support a role of CCK not only as a short term meal-reducing signal but also as a regulator of fat absorption and body weight. In addition, mice lacking the CCK2 receptor, a subtype predominantly expressed in the brain, presented hyperphagia resulting in increased body weight gain compared to their wild type littermates [143] indicating a role for this receptor in the brain regulation of food intake and body weight as well.
CCK's satiety effect is mediated via vagal afferent fibers innervating the gut and relaying the information to the brain. This was demonstrated by the localization of the CCK1 receptor on vagal afferents [144] and the absence of food intake reduction in response to intraperitoneal injection of CCK in rats that underwent bilateral abdominal or gastric vagotomy [145]. Conversely, intravenous infusion of a CCK1 receptor antagonist, loxiglumide, increased caloric intake in healthy volunteers accompanied by an increased sensation of hunger [146] underlining the physiological relevance of CCK as satiety signal.
INTERACTION BETWEEN GASTRIC AND INTESTINAL HORMONES IN THE REGULATION OF FOOD INTAKE
Under different metabolic conditions, gut peptides are not released singly but simultaneously to orchestrate adapted food intake and digestive responses which has led to a number of studies focusing on the interactions between gastrointestinal food intake regulatory peptides.
Interaction between Ghrelin and CCK
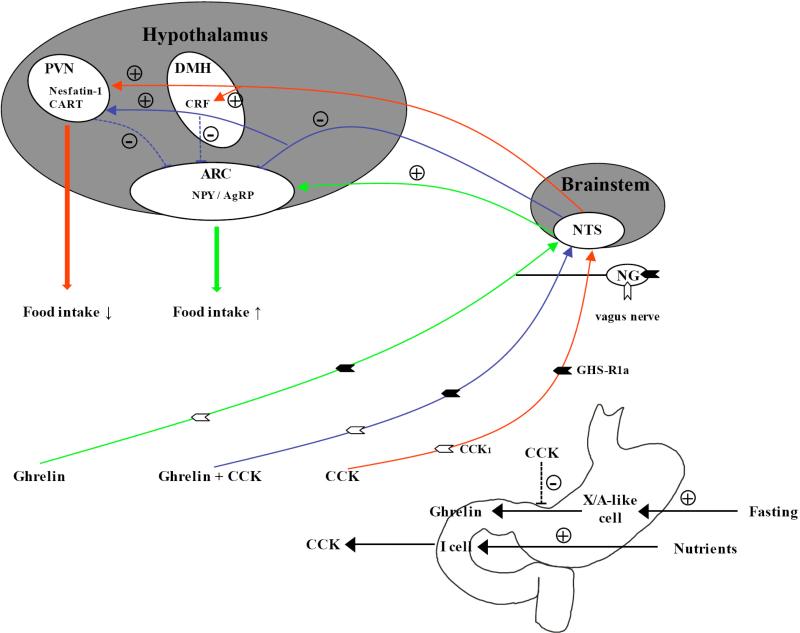
We previously reported that simultaneous intraperitoneal injection of ghrelin and CCK-8S inhibits the ghrelin-induced stimulation of food intake and activation of neurons in the Arc (Table 1) [147] possibly through an intraperitoneal CCK-8S related activation of nesfatin-1 immunoreactive [148] or cocaine- and amphetamine-regulated transcript (CART) positive neurons in the hypothalamic paraventricular nucleus [149] Fig. (1). These findings were confirmed by an independent group and extended by demonstrating the lack of intravenous CCK inhibitory effect on intravenous ghrelin action in CCK1 receptor deficient Otsuka Long-Evans Tokushima fatty (OLETF) rats [150]. Since both GHS-R1a and CCK1 have been detected on vagal afferents by immunohistochemistry [150] and ghrelin injected intravenously suppresses, whereas intravenous CCK stimulates vagal afferent activity [40], the negative interaction between CCK and ghrelin could be initiated directly on the vagus nerve. However, CCK2 receptor knockout mice have an increased hypothalamic expression of GHS-R1a most likely contributing to the increased food intake and body weight observed in those mice [143] indicating an interaction also on the level of the hypothalamus. In addition, CCK-8S injected intravenously reduces circulating ghrelin in healthy volunteers [151] suggesting a regulatory action of CCK on ghrelin release, possibly directly exerted on X/A-like cells or through the release of somatostatin [152] Fig. (1).
Fig. (1).
Interaction between ghrelin and CCK in the regulation of food intake. During fasting ghrelin is released from X/A-like cells of the gastric mucosa stomach and mediates its orexigenic effect via the afferent vagus nerve bearing the GHS-R1a and CCK1 receptors and activation of NPY/AgRP positive neurons in the hypothalamic arcuate nucleus. CCK is released from the upper small intestine after nutrient exposure and reduces food intake by activating central neurons containing anorexigenic mediators such as CRF, CART and nesfatin-1. When injected simultaneously with ghrelin, CCK blocks the ghrelin induced food intake most likely by inhibiting ghrelin stimulated neuronal activation in the arcuate nucleus. CCK also reduces circulating ghrelin levels. + stimulation; - inhibition; ↑ increase; ↓ decrease; dotted line, potential pathways; AgRP, Agouti-related peptide; ARC, arcuate nucleus; CRF, corticotropin-releasing factor; DMH, dorsomedial nucleus of the hypothalamus; NG, nodose ganglion; NPY, neuropeptide Y; NTS, nucleus of the solitary tract; PVN, paraventricular nucleus. References: [40, 147-151, 170, 171]
Modulation of Ghrelin Release by Peripheral Food Intake Regulatory Hormones
Circulating levels of ghrelin are affected by a variety of gastrointestinal hormones and neurotransmitters. A reduction of ghrelin plasma concentrations is induced by CCK [151], insulin [153], somatostatin [154, 155], GLP-1 [156-158] and bombesin [155], whereas increased levels have been reported following treatment with anandamide, a cannabinoid analog [159] (Table 1). Yet to be established is whether these modulations of ghrelin release reflect direct actions on gastric X/A-like cells or are secondary to other transmitter/hormone release.
Interaction between CCK and Other Peripheral Food Intake Regulatory Hormones
The synergistic interaction between CCK and several other anorexigenic peptides/proteins namely leptin [160], urocortin 2 [161], GLP-1 [162] and amylin [163] has been described. The long-term modulator of energy homeostasis, leptin, injected intraperitoneally at a subthreshold dose induced a rapid in onset reduction of food ingestion when simultaneously injected with a subthreshold dose of CCK-8, whereas alone leptin had no effect (Table 1) [160]. This potentiating action was specific for CCK as bombesin injected intraperitoneally at a similar dose had no effect on the leptin induced reduction of food intake [160]. The potentiating effect of CCK on leptin's action was exerted via vagal afferent activation and was associated with increased Fos expression in the hypothalamic paraventricular nucleus [160]. In line with these findings, the majority of cultured nodose ganglion neurons responsive to CCK also responded to leptin [164] likely providing the afferent projections described as type 2 gastric vagal afferents in which pretreatment with CCK increased sensitivity for leptin [165]. In addition, the CCK1 receptor antagonist, devazepide prevented the reduction of food intake occurring 5–7 h after intraperitoneal injection of leptin alone [160] indicating that the synergistic interaction of leptin and CCK occurs physiologically. Similarly, CCK-8 simultaneously administered with urocortin 2 reduced food ingestion and delayed gastric emptying, whereas both peptides alone had no effect (Table 1) [161]. Both CRF2 and CCK1 receptors are expressed in the nodose ganglia supporting a synergistic action occurring on vagal afferents. This was demonstrated in vitro in stomach vagus preparations where intragastric artery injections of subthreshold doses of CCK-8 and urocortin 2 increased gastric vagal afferent activity [161]. Other synergistic peptide interactions include intravenous co-infusion of GLP-1 and CCK-33 which decreased food ingestion more potently than the summation of effects of both peptides injected alone in healthy volunteers [162], and amylin and CCK that when injected intraperitoneally at subthreshold doses synergistically reduce stimulated food intake after fasting in mice (Table 1) [163]. By contrast, ghrelin is able to suppress the CCK-induced increase in mRNA expression of the anorexigenic cocaine- and amphetamine-regulated transcript (CART) in vitro using cultured rat vagal afferent neurons (Table 1) [166].
Interaction between CCK and Gut Peptide Receptors
Recent studies indicate that the metabolic status and related changes in gut peptides also impact on the expression of their G protein-coupled receptors. For instance the melanin-concentrating hormone receptor 1 (MCH1) [167] and the cannabinoid receptor CB1 [168] in the nodose ganglia were increased at the mRNA and protein levels after fasting as assessed by PCR and immunohistochemistry respectively. Conversely, re-feeding decreased the nodose ganglia expression of MCH1 and CB1 which was blocked by the CCK1 antagonist, lorglumide [167, 168] indicating a CCK signaling dependent regulation. In addition, fasting reduces Y2 receptor mRNA expression in vagal afferent neurons which was stimulated by re-feeding and intraperitoneal injection of CCK [169]. These studies indicate that under different metabolic conditions a host of gut peptides are differently regulated at the levels of their expression and release which may impact on cognate receptor expression in the nodose ganglia to orchestrate food intake regulation.
SUMMARY
Peptides produced in specialized endocrine cells in the stomach and small intestine are not only involved in the appropriate regulation of gastrointestinal digestive functions such as motility and secretion but also in the control of food intake and maintenance of energy balance. Whereas most of the gut peptides inhibit food intake to prevent overeating and increase of body weight, one gastric hormone, ghrelin, stimulates food intake. In addition, ghrelin has also been implicated in the promotion of adipogenesis and long-term regulation of food intake. Besides the production of ghrelin, the X/A-like endocrine cell of the stomach also produces desacyl ghrelin, although it remains to be established whether desacyl ghrelin is the ghrelin precursor or its deacylation product, particularly in light of the recent demonstration of GOAT protein expression in the circulation and its regulation by changes in nutritional status. Contrary to the fact that desacyl ghrelin was initially thought to represent an inactive peptide, growing evidence gives rise to biological functions of desacyl ghrelin. Interestingly, desacyl ghrelin seems to antagonize ghrelin's effects on several functions including food intake. The identification of the desacyl ghrelin receptor will be a big step forward to characterize the mediation of desacyl ghrelin's effects. Another product of the gastric X/A-like cell, obestatin, has been controversially discussed during the past 5 years due to the divergent results on food intake obtained. Therefore, it is still a topic of debate whether obestatin has a role in the regulation of food intake and/or body weight or if it will be useful as a biomarker in eating disorders. Interestingly, the novel anorexigenic peptide, nesfatin-1 has been recently identified in the gastric X/A-like cell as well. Nesfatin-1/NUCB2 is located in a different pool of vesicles than ghrelin and differentially regulated by metabolic status. However, the mechanism controlling this differential production and release remains to be investigated along with the role of peripheral nesfatin-1.
The different peptides located in gut endocrine cells are controlled neuronally or by direct contact with nutrients and upon release can synergistically interact to efficiently curtail food intake (CCK and leptin, urocortin 2, GLP-1 or amylin) or can modulate each other in an opposite direction (ghrelin and CCK or ghrelin and desacyl ghrelin). Recent years have witnessed a marked increase in unraveling interactions among these regulatory pathways and potential underlying mechanisms of actions. In addition, still very little is known at the cellular level on how the synergistic or counteracting signaling of gastrointestinal peptides is taking place particularly within nodose ganglion cells harboring many receptors for gut peptides ultimately leading to the effect on food intake.
ACKNOWLEDGEMENTS
We thank Ms. Eugenia Hu for reviewing the manuscript.
Supported by: German Research Foundation Grants STE 1765/1-1 (A.S.), VA Research Career Scientist Award, NIHDK 33061, Center Grant DK-41301 (Animal Core) (Y.T.)
Footnotes
CONFLICT OF INTEREST STATEMENT
A.S. and Y.T. have nothing to disclose. No conflicts of interest exist.
REFERENCES
- 1.Moran TH, Dailey MJ. Minireview: Gut peptides: targets for antiobesity drug development? Endocrinology. 2009;150(6):2526–2530. doi: 10.1210/en.2009-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karra E, Batterham RL. The role of gut hormones in the regulation of body weight and energy homeostasis. Mol. Cell. Endocrinol. 2010;316(2):120–128. doi: 10.1016/j.mce.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–560. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 4.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J. Clin. Endocrinol. Metab. 2001;86(10):4753–4658. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 5.Jeon TY, Lee S, Kim HH, Kim YJ, Son HC, Kim DH, Sim MS. Changes in plasma ghrelin concentration immediately after gastrectomy in patients with early gastric cancer. J. Clin. Endocrinol. Metab. 2004;89(11):5392–5396. doi: 10.1210/jc.2004-0872. [DOI] [PubMed] [Google Scholar]
- 6.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141(11):4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 7.Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, Kojima M, Kangawa K, Arima T, Matsuo H, Yada T, Matsukura S. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes. 2002;51(1):124–129. doi: 10.2337/diabetes.51.1.124. [DOI] [PubMed] [Google Scholar]
- 8.Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 2002;87(6):2988. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 9.Barreiro ML, Gaytan F, Caminos JE, Pinilla L, Casanueva FF, Aguilar E, Dieguez C, Tena-Sempere M. Cellular location and hormonal regulation of ghrelin expression in rat testis. Biol. Reprod. 2002;67(6):1768–1776. doi: 10.1095/biolreprod.102.006965. [DOI] [PubMed] [Google Scholar]
- 10.Bednarek MA, Feighner SD, Pong SS, McKee KK, Hreniuk DL, Silva MV, Warren VA, Howard AD, Van Der Ploeg LH, Heck JV. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J. Med. Chem. 2000;43(23):4370–4376. doi: 10.1021/jm0001727. [DOI] [PubMed] [Google Scholar]
- 11.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol. Rev. 2005;85(2):495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 12.Hiejima H, Nishi Y, Hosoda H, Yoh J, Mifune H, Satou M, Sugimoto H, Chiba S, Kawahara Y, Tanaka E, Yoshimatsu H, Uchimura N, Kangawa K, Kojima M. Regional distribution and the dynamics of n-decanoyl ghrelin, another acyl-form of ghrelin, upon fasting in rodents. Regul. Pept. 2009;156(1-3):47–56. doi: 10.1016/j.regpep.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132(3):387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc. Natl. Acad. Sci. U S A. 2008;105(17):6320–5325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakata I, Yang J, Lee CE, Osborne-Lawrence S, Rovinsky SA, Elmquist JK, Zigman JM. Colocalization of ghrelin O-acyltransferase and ghrelin in gastric mucosal cells. Am. J. Physiol. Endocrinol. Metab. 2009;297(1):E134–E141. doi: 10.1152/ajpendo.90859.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stengel A, Goebel M, Wang L, Taché Y, Sachs G, Lambrecht NW. Differential distribution of ghrelin-O-acyltransferase (GOAT) immunoreactive cells in the mouse and rat gastric oxyntic mucosa. Biochem. Biophys. Res. Commun. 2010;392(1):67–71. doi: 10.1016/j.bbrc.2009.12.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 18.Hass N, Schwarzenbacher K, Breer H. T1R3 is expressed in brush cells and ghrelin-producing cells of murine stomach. Cell Tissue Res. 2010;339(3):493–504. doi: 10.1007/s00441-009-0907-6. [DOI] [PubMed] [Google Scholar]
- 19.Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, Thorner MO, Cummings DE. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J. Clin. Endocrinol. Metab. 2008;93(5):1971–1979. doi: 10.1210/jc.2007-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 21.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J. Med. 2002;346(21):1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 22.Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, Schwartz MW, Basdevant A, Weigle DS. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat. Med. 2002;8(7):643–644. doi: 10.1038/nm0702-643. [DOI] [PubMed] [Google Scholar]
- 23.Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273(5277):974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 24.Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res. Mol. Brain Res. 1997;48(1):23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 25.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 26.Hattori N, Saito T, Yagyu T, Jiang BH, Kitagawa K, Inagaki C. GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J. Clin. Endocrinol. Metab. 2001;86(9):4284–4291. doi: 10.1210/jcem.86.9.7866. [DOI] [PubMed] [Google Scholar]
- 27.Schellekens H, Dinan TG, Cryan JF. Lean Mean Fat Reducing “Ghrelin” Machine: Hypothalamic Ghrelin and Ghrelin Receptors as Therapeutic Targets in Obesity. Neuropharmacology. 2010;58(1):2–16. doi: 10.1016/j.neuropharm.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 28.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J. Comp. Neurol. 2006;494(3):528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shuto Y, Shibasaki T, Wada K, Parhar I, Kamegai J, Sugihara H, Oikawa S, Wakabayashi I. Generation of polyclonal antiserum against the growth hormone secretagogue receptor (GHS-R): evidence that the GHS-R exists in the hypothalamus, pituitary and stomach of rats. Life Sci. 2001;68(9):991–996. doi: 10.1016/s0024-3205(00)01001-8. [DOI] [PubMed] [Google Scholar]
- 30.Mondal MS, Date Y, Yamaguchi H, Toshinai K, Tsuruta T, Kangawa K, Nakazato M. Identification of ghrelin and its receptor in neurons of the rat arcuate nucleus. Regul. Pept. 2005;126(1-2):55–59. doi: 10.1016/j.regpep.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 31.Holst B, Schwartz TW. Constitutive ghrelin receptor activity as a signaling set-point in appetite regulation. Trends Pharmacol. Sci. 2004;25(3):113–117. doi: 10.1016/j.tips.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Schellekens H, Dinan TG, Cryan JF. Lean Mean Fat Reducing “Ghrelin” Machine: Hypothalamic Ghrelin and Ghrelin Receptors as Therapeutic Targets in Obesity. Neuropharmacology. 2010;58(1):2–16. doi: 10.1016/j.neuropharm.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 33.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141(11):4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 34.Druce MR, Wren AM, Park AJ, Milton JE, Patterson M, Frost G, Ghatei MA, Small C, Bloom SR. Ghrelin increases food intake in obese as well as lean subjects. Int. J. Obes. (Lond) 2005;29(9):1130–1136. doi: 10.1038/sj.ijo.0803001. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc. Natl. Acad. Sci. USA. 2004;101(13):4679–84. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J. Clin. Invest. 2005;115(12):3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salome N, Haage D, Perrissoud D, Moulin A, Demange L, Egecioglu E, Fehrentz JA, Martinez J, Dickson SL. Anorexigenic and electrophysiological actions of novel ghrelin receptor (GHS-R1A) antagonists in rats. Eur. J. Pharmacol. 2009;612(1-3):167–173. doi: 10.1016/j.ejphar.2009.03.066. [DOI] [PubMed] [Google Scholar]
- 38.Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J. Pharmacol. Exp. Ther. 2002;302(2):822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 39.Pan W, Tu H, Kastin AJ. Differential BBB interactions of three ingestive peptides: obestatin, ghrelin, and adiponectin. Peptides. 2006;27(4):911–916. doi: 10.1016/j.peptides.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123(4):1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 41.Sakata I, Yamazaki M, Inoue K, Hayashi Y, Kangawa K, Sakai T. Growth hormone secretagogue receptor expression in the cells of the stomach-projected afferent nerve in the rat nodose ganglion. Neurosci. Lett. 2003;342(3):183–186. doi: 10.1016/s0304-3940(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 42.Takayama K, Johno Y, Hayashi K, Yakabi K, Tanaka T, Ro S. Expression of c-Fos protein in the brain after intravenous injection of ghrelin in rats. Neurosci. Lett. 2007;417(3):292–296. doi: 10.1016/j.neulet.2007.02.089. [DOI] [PubMed] [Google Scholar]
- 43.Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52(9):2260–2265. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- 44.Date Y, Shimbara T, Koda S, Toshinai K, Ida T, Murakami N, Miyazato M, Kokame K, Ishizuka Y, Ishida Y, Kageyama H, Shioda S, Kangawa K, Nakazato M. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell. Metab. 2006;4(4):323–331. doi: 10.1016/j.cmet.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J. Neurosci. 2006;26(43):11052–11060. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu S, Guan JL, Wang QP, Uehara K, Yamada S, Goto N, Date Y, Nakazato M, Kojima M, Kangawa K, Shioda S. Immunocytochemical observation of ghrelin-containing neurons in the rat arcuate nucleus. Neurosci. Lett. 2002;321(3):157–160. doi: 10.1016/s0304-3940(01)02544-7. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 48.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 49.Guan JL, Wang QP, Kageyama H, Takenoya F, Kita T, Matsuoka T, Funahashi H, Shioda S. Synaptic interactions between ghrelin- and neuropeptide Y-containing neurons in the rat arcuate nucleus. Peptides. 2003;24(12):1921–1928. doi: 10.1016/j.peptides.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Abizaid A, Horvath TL. Brain circuits regulating energy homeostasis. Regul. Pept. 2008;149(1-3):3–10. doi: 10.1016/j.regpep.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50(11):2438–2443. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- 52.Wang L, Saint-Pierre DH, Taché Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y - synthesizing neurons in mouse hypothalamic arcuate nucleusNeurosci. Lett. 2002;325(1):47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]
- 53.Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, Ye Z, Nargund RP, Smith RG, Van der Ploeg LH, Howard AD, MacNeil DJ, Qian S. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145(6):2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 54.Pfluger PT, Kirchner H, Gunnel S, Schrott B, Perez-Tilve D, Fu S, Benoit SC, Horvath T, Joost HG, Wortley KE, Sleeman MW, Tschop MH. Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditure. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294(3):G610–G618. doi: 10.1152/ajpgi.00321.2007. [DOI] [PubMed] [Google Scholar]
- 55.Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, Moncrieffe M, Thabet K, Cox HJ, Yancopoulos GD, Wiegand SJ, Sleeman MW. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc. Natl. Acad. Sci. USA. 2004;101(21):8227–32. doi: 10.1073/pnas.0402763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raff H. Total and active ghrelin in developing rats during hypoxia. Endocrine. 2003;21(2):159–561. doi: 10.1385/ENDO:21:2:159. [DOI] [PubMed] [Google Scholar]
- 57.Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem. Biophys. Res. Commun. 2000;279(3):909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- 58.Stengel A, Keire D, Goebel M, Evilevitch L, Wiggins B, Taché Y, Reeve JR., Jr. The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology. 2009;150(11):5113–5118. doi: 10.1210/en.2009-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gauna C, Delhanty PJ, van Aken MO, Janssen JA, Themmen AP, Hofland LJ, Culler M, Broglio F, Ghigo E, van der Lely AJ. Unacylated ghrelin is active on the INS-1E rat insulinoma cell line independently of the growth hormone secretagogue receptor type 1a and the corticotropin releasing factor 2 receptor. Mol. Cell. Endocrinol. 2006;251(1-2):103–111. doi: 10.1016/j.mce.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 60.Gauna C, Delhanty PJ, Hofland LJ, Janssen JA, Broglio F, Ross RJ, Ghigo E, van der Lely AJ. Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. J. Clin. Endocrinol. Metab. 2005;90(2):1055–1060. doi: 10.1210/jc.2004-1069. [DOI] [PubMed] [Google Scholar]
- 61.Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F, Papotti M, Surico N, Bussolino F, Isgaard J, Deghenghi R, Sinigaglia F, Prat M, Muccioli G, Ghigo E, Graziani A. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J. Cell Biol. 2002;159(6):1029–1037. doi: 10.1083/jcb.200207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muccioli G, Pons N, Ghe C, Catapano F, Granata R, Ghigo E. Ghrelin and des-acyl ghrelin both inhibit isoproterenol-induced lipolysis in rat adipocytes via a non-type 1a growth hormone secretagogue receptor. Eur. J. Pharmacol. 2004;498(1-3):27–35. doi: 10.1016/j.ejphar.2004.07.066. [DOI] [PubMed] [Google Scholar]
- 63.Bulgarelli I, Tamiazzo L, Bresciani E, Rapetti D, Caporali S, Lattuada D, Locatelli V, Torsello A. Desacyl-ghrelin and synthetic GH-secretagogues modulate the production of inflammatory cytokines in mouse microglia cells stimulated by beta-amyloid fibrils. J. Neurosci. Res. 2009;87(12):2718–2727. doi: 10.1002/jnr.22088. [DOI] [PubMed] [Google Scholar]
- 64.Filigheddu N, Gnocchi VF, Coscia M, Cappelli M, Porporato PE, Taulli R, Traini S, Baldanzi G, Chianale F, Cutrupi S, Arnoletti E, Ghe C, Fubini A, Surico N, Sinigaglia F, Ponzetto C, Muccioli G, Crepaldi T, Graziani A. Ghrelin and des-acyl ghrelin promote differentiation and fusion of C2C12 skeletal muscle cells. Mol. Biol. Cell. 2007;18(3):986–994. doi: 10.1091/mbc.E06-05-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cassoni P, Papotti M, Ghe C, Catapano F, Sapino A, Graziani A, Deghenghi R, Reissmann T, Ghigo E, Muccioli G. Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J. Clin. Endocrinol. Metab. 2001;86(4):1738–1745. doi: 10.1210/jcem.86.4.7402. [DOI] [PubMed] [Google Scholar]
- 66.Chen CY, Inui A, Asakawa A, Fujino K, Kato I, Chen CC, Ueno N, Fujimiya M. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology. 2005;129(1):8–25. doi: 10.1053/j.gastro.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 67.Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, Meguid MM, Kasuga M. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut. 2005;54(1):18–24. doi: 10.1136/gut.2004.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Toshinai K, Yamaguchi H, Sun Y, Smith RG, Yamanaka A, Sakurai T, Date Y, Mondal MS, Shimbara T, Kawagoe T, Murakami N, Miyazato M, Kangawa K, Nakazato M. Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology. 2006;147(5):2306–2314. doi: 10.1210/en.2005-1357. [DOI] [PubMed] [Google Scholar]
- 69.Neary NM, Druce MR, Small CJ, Bloom SR. Acylated ghrelin stimulates food intake in the fed and fasted states but desacylated ghrelin has no effect. Gut. 2006;55(1):135. [PMC free article] [PubMed] [Google Scholar]
- 70.Inhoff T, Mönnikes H, Noetzel S, Stengel A, Goebel M, Dinh QT, Riedl A, Bannert N, Wisser AS, Wiedenmann B, Klapp BF, Taché Y, Kobelt P. Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides. 2008;29:2159–2168. doi: 10.1016/j.peptides.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumar R, Salehi A, Rehfeld JF, Hoglund P, Lindstrom E, Hakanson R. Proghrelin peptides: Desacyl ghrelin is a powerful inhibitor of acylated ghrelin, likely to impair physiological effects of acyl ghrelin but not of obestatin A study of pancreatic polypeptide secretion from mouse islets. Regul. Pept. 2010 doi: 10.1016/j.regpep.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 72.Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ. Obestatin, a peptide encoded by the ghre lin gene, opposes ghrelin's effects on food intake. Science. 2005;310(5750):996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- 73.Stengel A, Goebel M, Yakubov I, Wang L, Witcher D, Coskun T, Taché Y, Sachs G, Lambrecht NW. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology. 2009;150(1):232–238. doi: 10.1210/en.2008-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gronberg M, Tsolakis AV, Magnusson L, Janson ET, Saras J. Distribution of Obestatin and Ghrelin in Human Tissues: Immunoreactive Cells in the Gastrointestinal Tract, Pancreas, and Mammary Glands. J. Histochem. Cytochem. 2008;56(9):793–801. doi: 10.1369/jhc.2008.951145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsolakis AV, Grimelius L, Stridsberg M, Falkmer SE, Waldum HL, Saras J, Janson ET. Obestatin/ghrelin cells in normal mucosa and endocrine tumours of the stomach. Eur. J. Endocrinol. 2009;160(6):941–949. doi: 10.1530/EJE-09-0001. [DOI] [PubMed] [Google Scholar]
- 76.Zhao CM, Furnes MW, Stenstrom B, Kulseng B, Chen D. Characterization of obestatin- and ghrelin-producing cells in the gastrointestinal tract and pancreas of rats: an immunohistochemical and electron-microscopic study. Cell Tissue Res. 2008;331(3):575–587. doi: 10.1007/s00441-007-0514-3. [DOI] [PubMed] [Google Scholar]
- 77.Lauwers E, Landuyt B, Arckens L, Schoofs L, Luyten W. Obestatin does not activate orphan G protein-coupled receptor GPR39. Biochem. Biophys. Res. Commun. 2006;351(1):21–25. doi: 10.1016/j.bbrc.2006.09.141. [DOI] [PubMed] [Google Scholar]
- 78.Tremblay F, Perreault M, Klaman LD, Tobin JF, Smith E, Gimeno RE. Normal food intake and body weight in mice lacking the G protein-coupled receptor GPR39. Endocrinology. 2007;148(2):501–506. doi: 10.1210/en.2006-1275. [DOI] [PubMed] [Google Scholar]
- 79.Holst B, Egerod KL, Schild E, Vickers SP, Cheetham S, Gerlach LO, Storjohann L, Stidsen CE, Jones R, Beck-Sickinger AG, Schwartz TW. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology. 2007;148(1):13–20. doi: 10.1210/en.2006-0933. [DOI] [PubMed] [Google Scholar]
- 80.Chartrel N, Alvear-Perez R, Leprince J, Iturrioz X, Reaux-Le Goazigo A, Audinot V, Chomarat P, Coge F, Nosjean O, Rodriguez M, Galizzi JP, Boutin JA, Vaudry H, Llorens-Cortes C. Comment on “Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake”. Science. 2007;315(5813):766. doi: 10.1126/science.1135047. author reply 766. [DOI] [PubMed] [Google Scholar]
- 81.Zhang JV, Klein C, Ren P-G, Kass S, Donck LV, Moechars D, Hsueh AJW. Response to Comment on “Obestatin, a Peptide Encoded by the Ghrelin Gene, Opposes Ghrelin's Effects on Food Intake”. Science. 2007;315:766d. doi: 10.1126/science.1135047. [DOI] [PubMed] [Google Scholar]
- 82.Beck B, Bossenmeyer-Pourie C, Pourie G. Association of neuropeptide W, but not obestatin, with energy intake and endocrine status in Zucker rats. A new player in long-term stress-feeding interactions. Appetite. 2010 doi: 10.1016/j.appet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 83.Bang AS, Soule SG, Yandle TG, Richards AM, Pemberton CJ. Characterisation of proghrelin peptides in mammalian tissue and plasma. J. Endocrinol. 2007;192(2):313–323. doi: 10.1677/JOE-06-0021. [DOI] [PubMed] [Google Scholar]
- 84.Mondal M, Toshinai K, Ueno H, Koshinaka K, Nakazato M. Characterization of obestatin in rat and human stomach and plasma, and its lack of acute effect on feeding behavior in rodents. J. Endocrinol. 2008;198(2):339–346. doi: 10.1677/JOE-08-0082. [DOI] [PubMed] [Google Scholar]
- 85.Seoane LM, Al-Massadi O, Pazos Y, Pagotto U, Casanueva FF. Central obestatin administration does not modify either spontaneous or ghrelin-induced food intake in rats. J. Endocrinol. Invest. 2006;29(8):RC13–RC15. doi: 10.1007/BF03344174. [DOI] [PubMed] [Google Scholar]
- 86.Moechars D, Depoortere I, Moreaux B, de Smet B, Goris I, Hoskens L, Daneels G, Kass S, Ver Donck L, Peeters T, Coulie B. Altered gastrointestinal and metabolic function in the GPR39-obestatin receptor-knockout mouse. Gastroenterology. 2006;131(4):1131–1141. doi: 10.1053/j.gastro.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 87.Gourcerol G, Million M, Adelson DW, Wang Y, Wang L, Rivier J, St-Pierre DH, Taché Y. Lack of interaction between peripheral injection of CCK and obestatin in the regulation of gastric satiety signaling in rodents. Peptides. 2006;27(11):2811–2819. doi: 10.1016/j.peptides.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 88.Samson WK, White MM, Price C, Ferguson AV. Obestatin acts in brain to inhibit thirst. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292(1):R637–R643. doi: 10.1152/ajpregu.00395.2006. [DOI] [PubMed] [Google Scholar]
- 89.Lagaud GJ, Young A, Acena A, Morton MF, Barrett TD, Shankley NP. Obestatin reduces food intake and suppresses body weight gain in rodents. Biochem. Biophys. Res. Commun. 2007;357(1):264–269. doi: 10.1016/j.bbrc.2007.03.138. [DOI] [PubMed] [Google Scholar]
- 90.Yamamoto D, Ikeshita N, Daito R, Herningtyas EH, Toda K, Takahashi K, Iida K, Takahashi Y, Kaji H, Chihara K, Okimura Y. Neither intravenous nor intracerebroventricular ad ministration of obestatin affects the secretion of GH, PRL, TSH and ACTH in rats. Regul. Pept. 2007;138(2-3):141–144. doi: 10.1016/j.regpep.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 91.Zizzari P, Longchamps R, Epelbaum J, Bluet-Pajot MT. Obestatin partially affects ghrelin stimulation of food intake and growth hormone secretion in rodents. Endocrinology. 2007;148(4):1648–1653. doi: 10.1210/en.2006-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nogueiras R, Pfluger P, Tovar S, Arnold M, Mitchell S, Morris A, Perez-Tilve D, Vazquez MJ, Wiedmer P, Castaneda TR, DiMarchi R, Tschop M, Schurmann A, Joost HG, Williams LM, Langhans W, Dieguez C. Effects of obestatin on energy balance and growth hormone secretion in rodents. Endocrinology. 2007;148(1):21–26. doi: 10.1210/en.2006-0915. [DOI] [PubMed] [Google Scholar]
- 93.Kobelt P, Wisser AS, Stengel A, Goebel M, Bannert N, Gourcerol G, Inhoff T, Noetzel S, Wiedenmann B, Klapp BF, Taché Y, Mönnikes H. Peripheral obestatin has no effect on feeding behavior and brain Fos expression in rodents. Peptides. 2008;29(6):1018–1027. doi: 10.1016/j.peptides.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Depoortere I, Thijs T, Moechars D, De Smet B, Ver Donck L, Peeters TL. Effect of peripheral obestatin on food intake and gastric emptying in ghrelin-knockout mice. Br. J. Pharmacol. 2008;153(7):1550–1557. doi: 10.1038/sj.bjp.0707683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Annemie VD, Debby VD, Valentijn V, Bart de S, Walter L, Liliane S, Peter Paul de D. Central administration of obestatin fails to show inhibitory effects on food and water intake in mice. Regul. Pept. 2009;156(1-3):77–82. doi: 10.1016/j.regpep.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 96.Brunetti L, Leone S, Orlando G, Recinella L, Ferrante C, Chiavaroli A, Di Nisio C, Di Michele P, Vacca M. Effects of obestatin on feeding and body weight after standard or cafeteria diet in the rat. Peptides. 2009;30(7):1323–1327. doi: 10.1016/j.peptides.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 97.Unniappan S, Speck M, Kieffer TJ. Metabolic effects of chronic obestatin infusion in rats. Peptides. 2008;29(8):1354–1361. doi: 10.1016/j.peptides.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 98.Lagaud GJ, Young A, Acena A, Morton MF, Barrett TD, Shankley NP. Retracted notice to: “Obestatin reduces food intake and suppresses body 4 weight gain in rodents” [Biochem. Biophys. Res. Commun. 357(1) (2007) 5 264–269]. Biochem. Biophys. Res. Commun. 2009;388(3):619. doi: 10.1016/j.bbrc.2007.03.138. [DOI] [PubMed] [Google Scholar]
- 99.Bresciani E, Rapetti D, Dona F, Bulgarelli I, Tamiazzo L, Locatelli V, Torsello A. Obestatin inhibits feeding but does not modulate GH and corticosterone secretion in the rat. J. Endocrinol. Invest. 2006;29(8):RC16–RC18. doi: 10.1007/BF03344175. [DOI] [PubMed] [Google Scholar]
- 100.Carlini VP, Schioth HB, Debarioglio SR. Obestatin improves memory performance and causes anxiolytic effects in rats. Biochem. Biophys. Res. Commun. 2007;352(4):907–912. doi: 10.1016/j.bbrc.2006.11.112. [DOI] [PubMed] [Google Scholar]
- 101.Nagaraj S, Peddha MS, Manjappara UV. Fragments of obestatin as modulators of feed intake, circulating lipids, and stored fat. Biochem. Biophys. Res. Commun. 2008;366(3):731–737. doi: 10.1016/j.bbrc.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 102.Germain N, Galusca B, Grouselle D, Frere D, Tolle V, Zizzari P, Lang F, Epelbaum J, Estour B. Ghrelin/obestatin ratio in two populations with low bodyweight: constitutional thinness and anorexia nervosa. Psychoneuroendocrinology. 2009;34(3):413–419. doi: 10.1016/j.psyneuen.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 103.Germain N, Galusca B, Grouselle D, Frere D, Billard S, Epelbaum J, Estour B. Ghrelin and obestatin circadian levels differentiate bingeing-purging from restrictive anorexia nervosa. J. Clin. Endocrinol. Metab. 2010;95(6):3057–3062. doi: 10.1210/jc.2009-2196. [DOI] [PubMed] [Google Scholar]
- 104.Hassouna R, Zizzari P, Tolle V. The ghrelin/obestatin balance in the physiological and pathological control of growth hormone secretion, body composition and food intake. J. Neuroendocrinol. 2010;22(7):793–804. doi: 10.1111/j.1365-2826.2010.02019.x. [DOI] [PubMed] [Google Scholar]
- 105.Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443(7112):709–712. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 106.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Lambrecht NW, Taché Y. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology. 2009;150(11):4911–4919. doi: 10.1210/en.2009-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, Yoshida N, Koike M, Uchiyama Y, Fujiwara K, Yashiro T, Horvath TL, Dietrich MO, Tanaka S, Dezaki K, Oh IS, Hashimoto K, Shimizu H, Nakata M, Mori M, Yada T. Nesfatin-1-regulated oxytocinergic signaling in the paraventricu lar nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab. 2009;10(5):355–365. doi: 10.1016/j.cmet.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 108.Tsuchiya T, Shimizu H, Yamada M, Osaki A, Oh IS, Ariyama Y, Takahashi H, Okada S, Hashimoto K, Satoh T, Kojima M, Mori M. Fasting Concentrations of Nesfatin-1 Are Negatively Correlated with Body Mass Index in Non-Obese Males. Clin. Endocrinol. (Oxf) 2010 doi: 10.1111/j.1365-2265.2010.03835.x. [DOI] [PubMed] [Google Scholar]
- 109.Gonzalez R, Tiwari A, Unniappan S. Pancreatic beta cells colocalize insulin and pronesfatin immunoreactivity in rodents. Biochem. Biophys. Res. Commun. 2009;381(4):643–648. doi: 10.1016/j.bbrc.2009.02.104. [DOI] [PubMed] [Google Scholar]
- 110.Foo KS, Brauner H, Ostenson CG, Broberger C. Nucleobindin-2/nesfatin in the endocrine pancreas: distribution and relationship to glycaemic state. J. Endocrinol. 2010;204(3):255–263. doi: 10.1677/JOE-09-0254. [DOI] [PubMed] [Google Scholar]
- 111.Shimizu H, Oh-I S, Hashimoto K, Nakata M, Yamamoto S, Yoshida N, Eguchi H, Kato I, Inoue K, Satoh T, Okada S, Yamada M, Yada T, Mori M. Peripheral Administration of Nesfatin-1 Reduces Food Intake in Mice: The leptin-independent mechanism. Endocrinology. 2009;150:662–671. doi: 10.1210/en.2008-0598. [DOI] [PubMed] [Google Scholar]
- 112.Shimizu H, Ohsaki A, Oh IS, Okada S, Mori M. A new anorexigenic protein, nesfatin-1. Peptides. 2009;30(5):995–998. doi: 10.1016/j.peptides.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 113.Beinfeld MC, Korchak DM. The regional distribution and the chemical, chromatographic, and immunologic characterization of motilin brain peptides: the evidence for a difference between brain and intestinal motilin-immunoreactive peptides. J. Neurosci. 1985;5(9):2502–2509. doi: 10.1523/JNEUROSCI.05-09-02502.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J. Clin. Invest. 1985;75(4):1144–1152. doi: 10.1172/JCI111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Herness S, Zhao FL, Lu SG, Kaya N, Shen T. Expression and physiological actions of cholecystokinin in rat taste receptor cells. J. Neurosci. 2002;22(22):10018–10029. doi: 10.1523/JNEUROSCI.22-22-10018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shen T, Kaya N, Zhao FL, Lu SG, Cao Y, Herness S. Co-expression patterns of the neuropeptides vasoactive intestinal peptide and cholecystokinin with the transduction molecules alpha-gustducin and T1R2 in rat taste receptor cells. Neuroscience. 2005;130(1):229–238. doi: 10.1016/j.neuroscience.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 117.Hajnal A, Covasa M, Bello NT. Altered taste sensitivity in obese, prediabetic OLETF rats lacking CCK-1 receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289(6):R1675–R1686. doi: 10.1152/ajpregu.00412.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Swartz TD, Hajnal A, Covasa M. Altered orosensory sensitivity to oils in CCK-1 receptor deficient rats. Physiol. Behav. 2010;99(1):109–117. doi: 10.1016/j.physbeh.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hao S, Sternini C, Raybould HE. Role of CCK1 and Y2 receptors in activation of hindbrain neurons induced by intragastric administration of bitter taste receptor ligands. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294(1):R33–R38. doi: 10.1152/ajpregu.00675.2007. [DOI] [PubMed] [Google Scholar]
- 120.Linden A, Carlquist M, Hansen S, Uvnas-Moberg K. Plasma concentrations of cholecystokinin, CCK-8, and CCK-33, 39 in rats, determined by a method based on enzyme digestion of gastrin before HPLC and RIA detection of CCK. Gut. 1989;30(2):213–222. doi: 10.1136/gut.30.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Folsch UR, Cantor P, Wilms HM, Schafmayer A, Becker HD, Creutzfeldt W. Role of cholecystokinin in the negative feedback control of pancreatic enzyme secretion in conscious rats. Gastroenterology. 1987;92(2):449–458. doi: 10.1016/0016-5085(87)90141-7. [DOI] [PubMed] [Google Scholar]
- 122.Liddle RA, Goldfine ID, Williams JA. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology. 1984;87(3):542–549. [PubMed] [Google Scholar]
- 123.Izzo RS, Brugge WR, Praissman M. Immunoreactive cholecystokinin in human and rat plasma: correlation of pancreatic secretion in response to CCK. Regul. Pept. 1984;9(1-2):21–34. doi: 10.1016/0167-0115(84)90004-1. [DOI] [PubMed] [Google Scholar]
- 124.Reeve JR, Jr., Green GM, Chew P, Eysselein VE, Keire DA. CCK-58 is the only detectable endocrine form of cholecystokinin in rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285(2):G255–G265. doi: 10.1152/ajpgi.00523.2002. [DOI] [PubMed] [Google Scholar]
- 125.Reeve JR, Jr., Wu SV, Keire DA, Faull K, Chew P, Solomon TE, Green GM, Coskun T. Differential bile-pancreatic secretory effects of CCK-58 and CCK-8. Am. J. Physiol. Gastroin-test. Liver Physiol. 2004;286(3):G395–G402. doi: 10.1152/ajpgi.00020.2003. [DOI] [PubMed] [Google Scholar]
- 126.Cooper MS, Reeve JR, Jr., Raboin SJ, Abdalla MO, Green GM, Sayegh AI. Cholecystokinin-58 and cholecystokinin-8 produce similar but not identical activations of myenteric plexus and dorsal vagal complex. Regul. Pept. 2008;148(1-3):88–94. doi: 10.1016/j.regpep.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 127.Kreis ME, Zittel TT, Raybould HE, Reeve JR, Jr., Grundy D. Prolonged intestinal afferent nerve discharge in response to cholecystokinin-58 compared to cholecystokinin-8 in rats. Neurosci. Lett. 1997;230(2):89–92. doi: 10.1016/s0304-3940(97)00483-7. [DOI] [PubMed] [Google Scholar]
- 128.Glatzle J, Raybould HE, Kueper MA, Reeve JR, Jr., Zittel TT. Cholecystokinin-58 is more potent in inhibiting food intake than cholecystokinin-8 in rats. Nutr. Neurosci. 2008;11(2):69–74. doi: 10.1179/147683008X301432. [DOI] [PubMed] [Google Scholar]
- 129.Yamamoto M, Reeve JR, Jr., Keire DA, Green GM. Water and enzyme secretion are tightly coupled in pancreatic secretion stimulated by food or CCK-58 but not by CCK-8. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288(5):G866–G879. doi: 10.1152/ajpgi.00389.2003. [DOI] [PubMed] [Google Scholar]
- 130.Criddle DN, Booth DM, Mukherjee R, McLaughlin E, Green GM, Sutton R, Petersen OH, Reeve JR., Jr. Cholecystokinin-58 and cholecystokinin-8 exhibit similar actions on calcium signaling, zymogen secretion, and cell fate in murine pancreatic acinar cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297(6):G1085–G1092. doi: 10.1152/ajpgi.00119.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liddle RA, Elashoff J, Reeve JR., Jr. Relative bioactivities of cholecystokinins-8 and -33 on rat pancreatic acini. Peptides. 1986;7(5):723–727. doi: 10.1016/0196-9781(86)90085-9. [DOI] [PubMed] [Google Scholar]
- 132.van der Schaar PJ, Bremer Y, Lamers CB, Masclee AA. Role of cholecystokinin in relaxation of the proximal stomach. Scand. J. Gastroenterol. 2001;36(4):361–366. doi: 10.1080/003655201300051117. [DOI] [PubMed] [Google Scholar]
- 133.Gibbs J, Young RC, Smith GP. Cholecystokinin elicits satiety in rats with open gastric fistulas. Nature. 1973;245(5424):323–325. doi: 10.1038/245323a0. [DOI] [PubMed] [Google Scholar]
- 134.Stacher G, Steinringer H, Schmierer G, Schneider C, Winklehner S. Cholecystokinin octapeptide decreases intake of solid food in man. Peptides. 1982;3(2):133–136. doi: 10.1016/0196-9781(82)90041-9. [DOI] [PubMed] [Google Scholar]
- 135.Reidelberger RD, Arnelo U, Granqvist L, Permert J. Comparative effects of amylin and cholecystokinin on food intake and gastric emptying in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280(3):R605–R611. doi: 10.1152/ajpregu.2001.280.3.R605. [DOI] [PubMed] [Google Scholar]
- 136.Asin KE, Gore PA, Jr., Bednarz L, Holladay M, Nadzan AM. Effects of selective CCK receptor agonists on food intake after central or peripheral administration in rats. Brain Res. 1992;571(1):169–174. doi: 10.1016/0006-8993(92)90527-g. [DOI] [PubMed] [Google Scholar]
- 137.Kraly FS, Carty WJ, Resnick S, Smith GP. Effect of cholecystokinin on meal size and intermeal interval in the sham-feeding rat. J. Comp. Physiol. Psychol. 1978;92(4):697–707. doi: 10.1037/h0077501. [DOI] [PubMed] [Google Scholar]
- 138.Stark HA, Sharp CM, Sutliff VE, Martinez J, Jensen RT, Gardner JD. CCK-JMV-180: a peptide that distinguishes high-affinity cholecystokinin receptors from low-affinity cholecystokinin receptors. Biochim. Biophys. Acta. 1989;1010(2):145–150. doi: 10.1016/0167-4889(89)90154-7. [DOI] [PubMed] [Google Scholar]
- 139.Weatherford SC, Laughton WB, Salabarria J, Danho W, Tilley JW, Netterville LA, Schwartz GJ, Moran TH. CCK satiety is differentially mediated by high- and low-affinity CCK receptors in mice and rats. Am. J. Physiol. 1993;264(2 Pt 2):R244–R249. doi: 10.1152/ajpregu.1993.264.2.R244. [DOI] [PubMed] [Google Scholar]
- 140.Sakurai C, Ohta M, Kanai S, Uematsu H, Funakoshi A, Miyasaka K. Lack of ghrelin secretion in response to fasting in cholecystokinin-A (-1), -B (-2) receptor-deficient mice. J. Physiol. Sci. 2006;56(6):441–417. doi: 10.2170/physiolsci.RP003306. [DOI] [PubMed] [Google Scholar]
- 141.Donovan MJ, Paulino G, Raybould HE. CCK(1) receptor is essential for normal meal patterning in mice fed high fat diet. Physiol. Behav. 2007;92(5):969–9674. doi: 10.1016/j.physbeh.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lo CM, King A, Samuelson LC, Kindel TL, Rider T, Jandacek RJ, Raybould HE, Woods SC, Tso P. Cholecystokinin knockout mice are resistant to high-fat diet-induced obesity. Gastroenterology. 2010;138(5):1997–2005. doi: 10.1053/j.gastro.2010.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Clerc P, Coll Constans MG, Lulka H, Broussaud S, Guigne C, Leung-Theung-Long S, Perrin C, Knauf C, Carpene C, Penicaud L, Seva C, Burcelin R, Valet P, Fourmy D, Dufresne M. Involvement of cholecystokinin 2 receptor in food intake regulation: hyperphagia and increased fat deposition in cholecystokinin 2 receptor-deficient mice. Endocrinology. 2007;148(3):1039–1049. doi: 10.1210/en.2006-1064. [DOI] [PubMed] [Google Scholar]
- 144.Blackshaw LA, Grundy D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J. Auton. Nerv. Syst. 1990;31(3):191–201. doi: 10.1016/0165-1838(90)90185-l. [DOI] [PubMed] [Google Scholar]
- 145.Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science. 1981;213(4511):1036–1037. doi: 10.1126/science.7268408. [DOI] [PubMed] [Google Scholar]
- 146.Beglinger C, Degen L, Matzinger D, D'Amato M, Drewe J. Loxiglumide, a CCK-A receptor antagonist, stimulates calorie intake and hunger feelings in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280(4):R1149–R1154. doi: 10.1152/ajpregu.2001.280.4.R1149. [DOI] [PubMed] [Google Scholar]
- 147.Kobelt P, Tebbe JJ, Tjandra I, Stengel A, Bae HG, Andresen V, van der Voort IR, Veh RW, Werner CR, Klapp BF, Wiedenmann B, Wang L, Taché Y, Mönnikes H. CCK inhibits the orexigenic effect of peripheral ghrelin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288(3):R751–R758. doi: 10.1152/ajpregu.00094.2004. [DOI] [PubMed] [Google Scholar]
- 148.Noetzel S, Stengel A, Inhoff T, Goebel M, Wisser AS, Bannert N, Wiedenmann B, Klapp BF, Taché Y, Mönnikes H, Kobelt P. CCK-8S activates c-Fos in a dose-dependent manner in nesfatin-1 immunoreactive neurons in the paraventricular nucleus of the hypothalamus and in the nucleus of the solitary tract of the brainstem. Regul. Pept. 2009;157(1-3):84–91. doi: 10.1016/j.regpep.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 149.Peter L, Stengel A, Noetzel S, Inhoff T, Goebel M, Taché Y, Veh RW, Bannert N, Grötzinger C, Wiedenmann B, Klapp BF, Mönnikes H, Kobelt P. Peripherally injected CCK-8S activates CART positive Neurons of the Paraventricular Nucleus in Rats. Peptides. 2010;31(6):1118–1123. doi: 10.1016/j.peptides.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Date Y, Toshinai K, Koda S, Miyazato M, Shimbara T, Tsuruta T, Niijima A, Kangawa K, Nakazato M. Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology. 2005;146(8):3518–3525. doi: 10.1210/en.2004-1240. [DOI] [PubMed] [Google Scholar]
- 151.Brennan IM, Otto B, Feltrin KL, Meyer JH, Horowitz M, Feinle-Bisset C. Intravenous CCK-8, but not GLP-1, suppresses ghrelin and stimulates PYY release in healthy men. Peptides. 2007;28(3):607–611. doi: 10.1016/j.peptides.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 152.Eissele R, Koop I, Schaar M, Koop H, Arnold R. Role of cholecystokinin in the control of gastric somatostatin in the rat: in vivo and in vitro studies. Regul. Pept. 1991;32(3):333–340. doi: 10.1016/0167-0115(91)90026-d. [DOI] [PubMed] [Google Scholar]
- 153.Saad MF, Bernaba B, Hwu CM, Jinagouda S, Fahmi S, Kogosov E, Boyadjian R. Insulin regulates plasma ghrelin concentration. J. Clin. Endocrinol. Metab. 2002;87(8):3997–4000. doi: 10.1210/jcem.87.8.8879. [DOI] [PubMed] [Google Scholar]
- 154.Shimada M, Date Y, Mondal MS, Toshinai K, Shimbara T, Fukunaga K, Murakami N, Miyazato M, Kangawa K, Yoshimatsu H, Matsuo H, Nakazato M. Somatostatin suppresses ghrelin secretion from the rat stomach. Biochem. Biophys. Res. Commun. 2003;302(3):520–525. doi: 10.1016/s0006-291x(03)00178-5. [DOI] [PubMed] [Google Scholar]
- 155.de la Cour CD, Norlen P, Hakanson R. Secretion of ghrelin from rat stomach ghrelin cells in response to local microinfusion of candidate messenger compounds: a microdialysis study. Regul. Pept. 2007;143(1-3):118–126. doi: 10.1016/j.regpep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 156.Hagemann D, Holst JJ, Gethmann A, Banasch M, Schmidt WE, Meier JJ. Glucagon-like peptide 1 (GLP-1) suppresses ghrelin levels in humans via increased insulin secretion. Regul. Pept. 2007;143(1-3):64–68. doi: 10.1016/j.regpep.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 157.Lippl F, Kircher F, Erdmann J, Allescher HD, Schusdziarra V. Effect of GIP, GLP-1, insulin and gastrin on ghrelin release in the isolated rat stomach. Regul. Pept. 2004;119(1-2):93–98. doi: 10.1016/j.regpep.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 158.Perez-Tilve D, Gonzalez-Matias L, Alvarez-Crespo M, Leiras R, Tovar S, Dieguez C, Mallo F. Exendin-4 potently decreases ghrelin levels in fasting rats. Diabetes. 2007;56(1):143–151. doi: 10.2337/db05-0996. [DOI] [PubMed] [Google Scholar]
- 159.Zbucki RL, Sawicki B, Hryniewicz A, Winnicka MM. Cannabinoids enhance gastric X/A-like cells activity. Folia Histochem. Cytobiol. 2008;46(2):219–224. doi: 10.2478/v10042-008-0033-4. [DOI] [PubMed] [Google Scholar]
- 160.Barrachina MD, Martinez V, Wang L, Wei JY, Taché Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc. Natl. Acad. Sci. USA. 1997;94(19):10455–10460. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gourcerol G, Wang L, Wang YH, Million M, Taché Y. Urocortins and cholecystokinin-8 act synergistically to increase satiation in lean but not obese mice: involvement of corticotropin-releasing factor receptor-2 pathway. Endocrinology. 2007;148(12):6115–6123. doi: 10.1210/en.2007-0678. [DOI] [PubMed] [Google Scholar]
- 162.Gutzwiller JP, Degen L, Matzinger D, Prestin S, Beglinger C. Interaction between GLP-1 and CCK-33 in inhibiting food intake and appetite in men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287(3):R562–R527. doi: 10.1152/ajpregu.00599.2003. [DOI] [PubMed] [Google Scholar]
- 163.Bhavsar S, Watkins J, Young A. Synergy between amylin and cholecystokinin for inhibition of food intake in mice. Physiol. Behav. 1998;64(4):557–561. doi: 10.1016/s0031-9384(98)00110-3. [DOI] [PubMed] [Google Scholar]
- 164.Peters JH, Ritter RC, Simasko SM. Leptin and CCK selectively activate vagal afferent neurons innervating the stomach and duodenum. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290(6):R1544–R1549. doi: 10.1152/ajpregu.00811.2005. [DOI] [PubMed] [Google Scholar]
- 165.Wang YH, Taché Y, Sheibel AB, Go VL, Wei JY. Two types of leptin-responsive gastric vagal afferent terminals: an in vitro single-unit study in rats. Am. J. Physiol. 1997;273(2 Pt 2):R833–R837. doi: 10.1152/ajpregu.1997.273.2.R833. [DOI] [PubMed] [Google Scholar]
- 166.de Lartigue G, Dimaline R, Varro A, Dockray GJ. Cocaine-and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J. Neurosci. 2007;27(11):2876–2882. doi: 10.1523/JNEUROSCI.5508-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Feeding-dependent depression of melanin-concentrating hormone and melanin-concentrating hormone receptor-1 expression in vagal afferent neurones. Neuroscience. 2006;137(4):1405–1415. doi: 10.1016/j.neuroscience.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 168.Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J. Neurosci. 2004;24(11):2708–2715. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Burdyga G, de Lartigue G, Raybould HE, Morris R, Dimaline R, Varro A, Thompson DG, Dockray GJ. Cholecystokinin regulates expression of Y2 receptors in vagal afferent neurons serving the stomach. J. Neurosci. 2008;28(45):11583–11592. doi: 10.1523/JNEUROSCI.2493-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kobelt P, Paulitsch S, Goebel M, Stengel A, Schmidtmann M, van der Voort IR, Tebbe JJ, Veh RW, Klapp BF, Wiedenmann B, Taché Y, Mönnikes H. Peripheral injection of CCK-8S induces Fos expression in the dorsomedial hypothalamic nucleus in rats. Brain Res. 2006;1117(1):109–117. doi: 10.1016/j.brainres.2006.08.092. [DOI] [PubMed] [Google Scholar]
- 171.Price CJ, Samson WK, Ferguson AV. Nesfatin-1 inhibits NPY neurons in the arcuate nucleus. Brain Res. 2008;1230:99–106. doi: 10.1016/j.brainres.2008.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]