Abstract
Programs to help people living with HIV/AIDS practice safer sex are needed to prevent transmission of HIV and other sexually transmitted infections. We sought to assess the impact of SafeTalk, a multicomponent motivational interviewing-based safer sex program, on HIV-infected patients’ risky sexual behavior. We enrolled sexually active adult HIV-infected patients from one of three clinical sites in North Carolina and randomized them to receive the 4-session SafeTalk intervention versus a hearthealthy attention-control. There was no significant difference in the proportion of people having unprotected sex between the two arms at enrollment. SafeTalk significantly reduced the number of unprotected sex acts with at-risk partners from baseline, while in controls the number of unprotected sex acts increased. Motivational interviewing can provide an effective, flexible prevention intervention for a heterogeneous group of people living with HIV.
Keywords: AIDS, HIV, Motivational interviewing, Safer sex, SafeTalk
Introduction
Since the advent of highly active antiretroviral therapy, people infected with HIV are living longer, healthier lives that include sexual relationships [1-5]. Most people discontinue risky sexual behavior when diagnosed with HIV. While the degree of safer sex practiced for each individual may vary over time [1, 6], on average, about 30% continue to engage in risky behavior after diagnosis [1, 7-10]. Of concern, high rates of sexually transmitted infections have been noted among some samples of people living with HIV [11-16].
Prior research has identified several factors that are associated with practicing risky sexual practices among people living with HIV. These include health beliefs that HIV treatment reduces transmission [17, 18], lower self-efficacy to practice safer sex [19, 20], better health status [20, 21], substance use [19, 22], stressful life events [20, 23], perceived stigma [24], and having sex in a committed relationship [17, 25]. Additionally, engaging in unsafe sexual practices has been associated with having low behavioral control over condom use and a lack of communication with sex partners [19]. These factors can be considered when developing programs to reduce risky practices [1, 8-10].
Not only has headway been made in understanding the factors associated with HIV risk, but recent meta-analyses have also shown that HIV prevention programs targeting people living with HIV can be effective [26]. Moreover, programs found to be most effective were based on theories of behavior change; targeted HIV transmission risk behaviors; included skills-building; delivered by counselors or medical providers; were intensive; lasted longer than 3 months; and were conducted where clients received medical care [27, 28].
Clearly, programs must consider multiple risk factors and multiple components of intervention efficacy. Motivational interviewing (MI), an effective theory-based, client-centered counseling approach, is individualized in nature and a particularly promising strategy for addressing the complex, multidimensional drivers of safer sex practices among people living with HIV [29]. Using a conceptual model based on a comprehensive literature review, formative research, and social cognitive theory [30] to guide our intervention development, we developed SafeTalk—an MI-based, multi-component program to enhance safer sex practices among people living with HIV. Previously, we demonstrated that the SafeTalk intervention was feasible; patients found it important, acceptable, and potentially useful [31]. In the present study, we report its effect on patients’ sexual HIV transmission risk behavior.
Methods
Overall Study Design
The intervention was evaluated in a two-armed, block-randomized, controlled trial of: (1) an intervention group whose members received SafeTalk, a four-session MI-based safer sex program, or (2) a control group whose members received A New Leaf, a four-session heart-healthy attention-matched control program [32]. The intervention assignment was concealed from those collecting data. Our goal was to deliver the sessions monthly whenever possible for both treatment groups during the first 4 months of study participation; however, we allowed flexible windows to accommodate patients with extenuating scheduling needs. Most counseling sessions were conducted in person, although in December 2007 we introduced the option of undergoing counseling by telephone to participants who reported difficulty travelling severe enough to hinder their ability to complete the intervention. Of the 110 to whom it was offered at study consent, 64 participants elected to have some sessions by phone (6 participants had all 4 sessions by phone, 7 people had 3 phone sessions total, 19 had a total of 2 phone sessions, and 32 had only one session by phone). A baseline and three follow-up evaluations were conducted at 4-month intervals.
All study procedures were approved by the University of North Carolina at Chapel Hill’s Office of Human Research Ethics that is responsible for ethical and oversight of research that involves human subjects.
Sample
Study Sites
The study was conducted at three HIV clinics in North Carolina. Recruitment began in July, 2006 at a university-affiliated infectious diseases (ID) clinic; in September, 2006 at an urban county health department HIV clinic; and in March, 2008 at a community health center HIV clinic in a smaller city.
Participant Enrollment and Follow-up
Eligible participants were: (1) at least 18 years old; (2) HIV-infected; (3) receiving HIV treatment at one of our three study sites; (4) English-speaking; (5) cognitively able to complete consent and counseling; and (6) self-reported having oral, anal, or vaginal sex in the last 12 months. Potential participants were excluded if it was their first visit to the clinic, they or their provider deemed them too sick, they knew that they would be leaving the clinic before the duration of study participation, or they had participated in another safer sex MI program within 6 months. At the community health center, we recruited only men to prevent co-enrollment with another safer sex study.
To recruit, the university-affiliated ID clinic utilized a research screener who provided the study staff with a list of scheduled patients who had been prescreened for eligibility and previously provided informed consent to be approached for any potential research projects. Over 90% of all clinic patients had pre-consented to be approached for research. At the other two sites, medical providers and clinic staff prescreened patients when they came to clinic visits. The prescreened eligible and interested patients were referred to study staff for further screening. We also posted fliers and screening forms at all three clinics and an affiliated AIDS service agency.
At all three sites, all patients who expressed potential interest were taken to a private room to complete eligibility screening, provide Informed consent and give extensive contact information. Patients who declined participation that day but expressed interest in participating in the future were approached at their next clinic visit.
Subjects received a gift card to a popular local grocery store worth $25.00 for each survey and $15 for each counseling session in addition to vouchers for parking, bus, and meals when needed.
Intervention Program: SafeTalk
Details of the SafeTalk intervention and its development have been described elsewhere [31]. Briefly, we refined a standardized, 13-step MI safer sex program that we had previously tested for feasibility in a demonstration project at one of our study sites [33]. The enhanced safer sex program SafeTalk incorporated several elements identified as important for effective “prevention with positives” programs and involved the following: four consecutive monthly, one-on-one MI sessions, with a series of five CD-booklet pairs that helped prepare patients for each MI session and provided tailored safer sex information; and four booster letters, each linked to the content of the preceding MI session. We included four sessions because we believed this was the minimum number that would allow a first session to primarily establish rapport, a second to identify and set initial goals and strategies, and the third and fourth to follow-up on, and then boost, the original goals, respectively. Four monthly sessions allowed the duration of the program to give adequate time for behavior change to take place and be able to be assessed in the designated time frame of our study measures (3 months).
Attention-Matched Control Program: New Leaf
We adapted an intervention entitled “A New Leaf… Choices for Healthy Living” [32, 34, 35], a structured nutrition and physical activity counseling program to prevent cardiovascular disease among low-income adults with limited literacy residing in the southeastern United States as the attention-matched control. We adapted the program to include information relevant for HIV-positive individuals and to mirror the SafeTalk format and materials.
Program Delivery and Quality Control
To ensure intervention quality, each SafeTalk MI counselor: (a) had a Masters level degree in Social Work, Counseling, or Health Behavior and Health Education; (b) underwent a 2.5 day training in HIV counseling and testing; (c) completed a manualized 5 day SafeTalk training in motivational interviewing conducted by a MINT trainer (CEG) that integrated advanced MI skills with HIV “prevention with positives” counseling that included intensive practice using the 13-step protocol, and a review of intervention fidelity.
Participants were asked to complete the four monthly SafeTalk or New Leaf counseling sessions within the first 4 months following study enrollment. Each session was intended to last 40–60 min, although 5% of them lasted less than 15 min and another 5% lasted longer than 60 min. Sessions were audio-recorded when participants granted consent (151 intervention participants agreed to be recorded and had at least one session recorded; a total of 275 sessions were recorded). Counselors also completed a detailed data recording sheet immediately following each session to document: (1) session content (e.g. topics selected for discussion, goals set, strategies identified) to be used for the individualized booster letters and; (2) degree of fidelity maintained for each of the 13 steps. To enhance intervention quality, each counselor reviewed her sessions weekly with a licensed, trained clinical counselor, and the entire team of counselors met monthly together with the clinical supervisor and the principal investigator to discuss counseling sessions. These reviews focused on improving MI technique, managing difficult cases, and resolving ethical dilemmas.
Data Collection Procedures
Participants completed an audio computer-assisted self interview (ACASI) (Nova Research Co) at baseline, and then at 4, 8 and 12 months after enrollment. Using headphones, respondents selected and entered responses into the computer unless they requested help from study staff. This approach allowed increased privacy to reduce the social desirability bias of responses to sensitive questions [36, 37]. Each survey lasted 30–60 min and assessed participants’ risky sexual behavior, demographic and clinical characteristics, psychosocial factors, attitudes, beliefs, and sexual relationships. We assessed CD4 counts from medical records.
Measures
Outcomes—Risky Sexual Behaviors
The interview gathered detailed information about sexual behaviors over a 3-month recall period. Separate but equivalent versions were used for men and women, allowing the language and questions to be consistent with respondents’ genders and the reported gender(s) of their sex partners. Participants were asked, by partner gender and for HIV-positive, HIV-negative, and HIV serostatus-unknown partner types, respectively, how many of each partner type they had and how many times they had had insertive or receptive vaginal or anal intercourse with this type of partner in the last 3 months, as well as how many times they had used a condom. Based on this information, for each participant we calculated how many times he or she had had unprotected vaginal or anal sex with ANY partner (UAVI = Unprotected Anal/Vaginal Intercourse) as well as with any AT-RISK (HIV-negative or unknown serostatus) partner (TRB = Transmission Risk Behavior).
Primary outcome
As the a priori primary test of efficacy of the intervention, we used TRB in the 3 months before the 8-month survey. We selected TRB as our primary outcome because it represented the behavioral outcome most likely to contribute to a reduction in HIV incidence. We selected 8 months because it was the first time point following the end of the intervention and thus allowed us to observe an effect of the entire intervention and yet to assess later, at 12 months, whether this effect attenuated.
Secondary outcomes
We also assessed: (1) the number of UAVI acts reported at 8 months post baseline and (2) the number of UAVI and TRB acts at 4 and 12 months post baseline.
Other Measures
To describe our sample, we measured several other independent variables. These are presented in Table 1.
Table 1.
Baseline descriptive characteristics of sample by treatment group
| New leaf control N (%) | SafeTalk intervention N (%) | Total sample N (%) | |
|---|---|---|---|
| Sample size | 242 (49.39) | 248 (50.61) | 490 (100) |
| Demographics | |||
| Age N (Mean, Std) | 241 (42.7, 8.4) | 246 (42.6, 9.7) | 487 (42.6, 9.0) |
| Race/ethnicity | |||
| Black/African-American | 165 (68.46) | 182 (73.68) | 347 (71.11) |
| White, non-Hispanic | 54 (22.41) | 46 (18.62) | 100 (20.49) |
| Other | 22 (9.13) | 19 (7.69) | 41 (8.40) |
| Sex preference subgroup | |||
| MSM | 97 (40.59) | 88 (36.07) | 185 (38.30) |
| MSW | 63 (26.36) | 67 (27.46) | 130 (26.92) |
| WSM | 75 (31.38) | 86 (35.25) | 161 (33.33) |
| WSW | 4 (1.67) | 3 (1.23) | 7 (1.45) |
| Education | |||
| Less than high school | 63 (26.14) | 57 (23.08) | 120 (24.59) |
| High school graduate or GED | 77 (31.95) | 85 (34.41) | 162 (33.20) |
| At least some college or technical school training | 101 (41.91) | 105 (42.51) | 206 (42.21) |
| Income | |||
| $10,000 or less | 128 (55.90) | 139 (59.15) | 267 (57.54) |
| $10,001 to $40,000 | 82 (35.81) | 78 (33.19) | 160 (34.48) |
| More than $40,000 | 19 (8.30) | 18 (7.66) | 37 (7.97) |
| Employment | |||
| Working part- or full-time | 82 (33.88) | 83 (33.47) | 165 (33.67) |
| Not working | 160 (66.12) | 165 (66.53) | 325 (66.33) |
| Had health insurance | 157 (68.26) | 170 (69.11) | 327 (68.70) |
| Relationship status | |||
| Single, not living with a partner | 119 (49.38) | 118 (47.97) | 237 (48.67) |
| Single, living with a partner | 58 (24.07) | 41 (16.67) | 99 (20.33) |
| Married | 26 (10.79) | 37 (15.04) | 63 (12.94) |
| Separated/divorced/widowed | 38 (15.77) | 50 (20.33) | 88 (18.07) |
| Sexual identity | |||
| Straight/heterosexual | 128 (53.33) | 145 (59.18) | 273 (57.47) |
| Gay/homosexual | 83 (34.58) | 70 (28.57) | 153 (32.21) |
| Bisexual | 19 (7.92) | 19 (7.76) | 38 (8.00) |
| Other/not sure | 10 (4.16) | 11 (4.49) | 21 (4.42) |
| Clinical characteristics | |||
| Had undetectable viral load in last 6 months | 128 (52.89) | 121 (48.99) | 249 (50.92) |
| Duration of diagnosis | |||
| Less than one year | 16 (6.69) | 21 (8.57) | 37 (7.64) |
| 1–9 years | 112 (46.86) | 120 (48.98) | 232 (47.93) |
| 10 years or more | 111 (46.45) | 104 (42.45) | 215 (44.42) |
| Currently on HAART | 192 (79.34) | 197 (79.44) | 389 (79.39) |
| CD4 count <200 | 45 (20.64) | 35 (15.63) | 80 (18.1) |
| Psychosocial characteristics | |||
| Sexual abuse | 105 (43.57) | 103 (41.53) | 208 (42.54) |
| Incarceration | 130 (53.72) | 145 (58.47) | 275 (56.12) |
| Emotional well-being N (Mean, Std) | 241 (2.6, 0.9) | 245 (2.5, 0.8) | 486 (2.6, 0.9) |
| Behavioral characteristics | |||
| Binge drinking in past 3 months | 100 (40.98) | 92 (39.15) | 192 (40.08) |
| Crack/cocaine use in past 3 months | 50 (21.01) | 39 (17.03) | 89 (19.06) |
| Number of sex partners N (Mean, Std) | 236 (1.4, 2.0) | 243 (1.3, 1.4) | 479 (1.4, 1.8) |
| Had main sex partner | 131 (55.74) | 139 (57.44) | 270 (56.60) |
Demographic Factors
We assessed participants’ age, race/ethnicity, gender, education level, income, employment status, health insurance coverage and current relationship status. We determined participants’ sex preference subgroup (men who have sex with men (MSM), men who have sex with women (MSW), or women who have sex with men (WSM) from their gender and the gender of their reported sexual partners at baseline. Those who did not report any sex partners at baseline were categorized based on their gender and self-identified sexual orientation. Men who reported either male and female sex partners or bisexual orientation were categorized as MSM.
Clinical Characteristics
We assessed reported undetectable viral load in the last 6 months, time since HIV diagnosis, current antiretroviral use, and chart-abstracted CD4 count.
Psychosocial Factors
We asked participants if they had “ever been in prison or jail?” or “ever been molested, sexually attacked, raped, sexually abused or forced to have sex?” We assessed emotional well-being using 7 items from the SF-36 Health Survey [38]. The Cronbach’s alpha for the scale was 0.860.
Behavioral Factors
We asked how frequently participants binge drank (≥5 drinks in a single day for men, ≥4 drinks for women), which we dichotomized as any or no binge drinking. Participants were also asked whether or not they had used each of 12 substances in the past 3 months. Because few participants reported substance use other than crack/cocaine during the past 3 months, we categorized each participant as having used or not used crack/cocaine.
We asked participants the number of men and women with whom they had had oral, vaginal, or anal sex in the past 3 months. We assessed whether or not participants were in a “primary relationship” defined as “someone you have lived with or seen a lot, and to whom you have felt a special emotional commitment.”
Analyses
We performed a two-sample test of equal proportions to determine whether the dropout rate of respondents differed by treatment arm. We conducted descriptive analyses to examine the characteristics of the whole sample and of each treatment group. Because some subjects failed to complete information at all four time points (baseline, 4, 8, and 12 months) we had missing data during the follow-up months (90 participants missing at 4-month follow-up, 120 at 8 months, and 183 at 12 months); therefore, we used multiple imputation procedures to handle the missing data [39]. The SAS procedures PROC MI and MIANALYZE were used for this purpose (SAS Institute, Cary, NC). With these imputed data we also conducted descriptive analysis of UAVI and TRB for each intervention group at each time point. Four respondents reported a large outlying value for the number of unprotected sex acts (for example, 417 UAVI acts over a 3-month period). In a sensitivity analysis we set these large values as missing and reanalyzed the data with the multiple imputation procedures.
To conduct our primary test of efficacy, we compared the SafeTalk and New Leaf groups regarding the number of TRB acts they had at 8 months, controlling for baseline TRB count. Because the outcome variables are count variables, Poisson regression model was used to test the difference in the rate of occurrence of sexual acts in the two intervention groups. A significant regression coefficient (β) for SafeTalk indicates the difference in the rate of occurrence of TRB in the two treatment arms. The quantity “exp (β)” represents the ratio of occurrence of the mean number of sexual activities (TRB) between the two groups. One can also interpret 100* (exp (β)) as the percentage change in expected number of sexual acts between the two intervention arms. Because intervention delivery site was used as a stratification variable in treatment randomization, it was included in the model as a control variable. We conducted the same analysis for our secondary outcome variable, UAVI at 8 months.
To determine the treatment effects on the two outcomes, UAVI and TRB acts, over time, we used the longitudinal information available for these variables. To take into account the correlation among these longitudinal measures, we used the Generalized Estimating Equation (GEE) procedure to test the difference between the treatment groups in the value at each time point and trend over time using the SAS procedure PROC GENMOD (SAS Institute, Cary, NC). The Poisson regression model contained time (as three dummy variables with baseline as reference), treatment, and treatment and time interaction. Significant treatment and time interactions would indicate that the treatment effect varied by time.
Results
Study Participants and Participation
During the recruitment period, 1,278 patients were eligible to be screened for the study based on eligibility criteria (at least 18 years old, English-speaking, HIV-infected, and receiving HIV treatment at one of our three study sites). Of these, 376 declined further screening and 67 were missed. Of the 835 approached and screened further, 179 were not eligible based on sexual behavior, intending to leave the clinic, or cognitive inability to provide consent, and 164 declined participation in the study. Of the 164 declining, 97 (59%) did not have time for the study, 15 (9%) were not interested, 20 (12%) had travel or transportation difficulties prohibiting study participation, 2 (1%) were too sick, and 30 (18%) declined for other reasons. Of 492 participants who enrolled in the study, two were unable to complete a baseline survey at enrollment and withdrew from the study at a later date, leaving a baseline study sample of 490 participants. Of these: 296 were from the university-affiliated infectious diseases (ID) clinic; 165 from the urban county health department HIV clinic; and 31 from the community health center HIV clinic. After completing the baseline survey, 248 were randomized to the intervention arm and 242 were randomized to the control arm (Fig. 1).
Fig. 1.
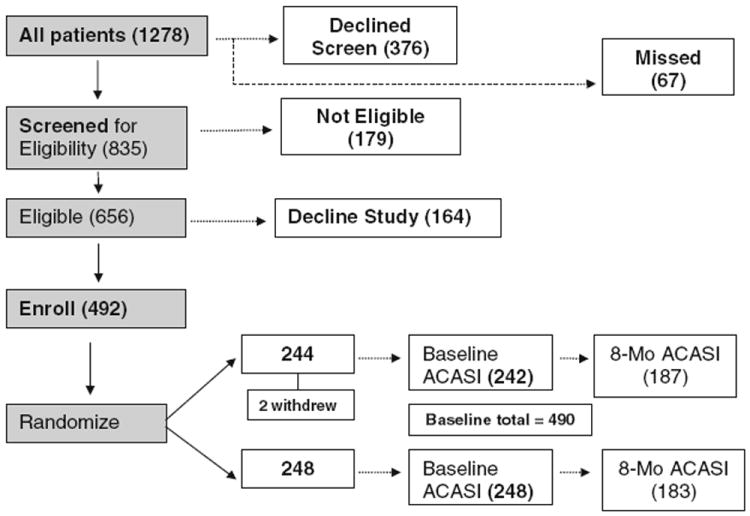
Eligibility and enrollment for a 2-arm randomized, controlled trial of SafeTalk
Sample characteristics (Table 1) were similar in both groups, with a mean age of 43 years, a majority being African American, about one-third being women, about one-fourth having less than a high school education, about 60% making less than $10,000 annually, and nearly two-thirds being unemployed. About 80% were on antiretroviral therapy and half had an undetectable viral load. Approximately 60% had a main partner, with a mean number of partners of 1.3 (SafeTalk) and 1.4 (New Leaf).
Retention and Study Sample
Of the 490 enrolled at baseline, 410 (84%) were retained at 4-month follow-up, 370 (76%) at 8 months, and 307 (63%) at 12 months. The proportion of participants selecting some phone counseling was evenly distributed by intervention arm. Between the 64 individuals who elected to receive some counseling by phone and all other participants, we found no statistically significant differences in race/ethnicity, education, income, housing stability, insurance, or relationship status between those who did and did not receive phone counseling. Defining dropout as those participants who did not complete the 8-month survey, the dropout rate was 23% for those randomized to New Leaf and 26% for those randomized to SafeTalk, with no statistically significant difference in these proportions (P = 0.37). Three quarters of those who dropped out at 8 months simply were lost to follow-up. Among those who we reached who did not complete an 8-month survey, reasons for this include the following: death (2%); illness (2%), withdrawal (2%); lack of time (2%); incarceration (8%); transportation difficulties (4%); migration (1%); work conflicts (2%); feeling the study was no longer relevant (2%); discontinuing clinical care at the study site (3%); personal challenges (1%); and no reason given (1%). The analysis sample (using imputed values) included observations at all time points for all 490 individuals.
SafeTalk Intervention Effects on Outcomes
Unadjusted Values for TRB and UAVI by Treatment Group. Table 2 shows the observed mean number of UAVI and TRB acts by treatment group at baseline and each follow up. Of note, there was no statistically significant difference in unprotected sex between the two arms at enrollment.
Table 2.
Baseline and follow-up unadjusted outcomes by treatment group
| Baseline | 4-month | 8-month | 12-month | |
|---|---|---|---|---|
| UAVI count | ||||
| SafeTalk Mean (SD) | 3.86 (27.35) | 1.68 (6.22) | 1.10 (3.21) | 1.30 (7.10) |
| New Leaf Mean (SD) | 3.32 (13.75) | 3.66 (29.60) | 3.54 (32.64) | 2.31 (16.12) |
| TRB count | ||||
| SafeTalk Mean (SD) | 2.66 (26.91) | 0.57 (3.72) | 0.37 (1.33) | 0.17 (0.81) |
| New Leaf Mean (SD) | 1.44 (9.03) | 2.19 (28.97) | 2.58 (32.23) | 0.53 (4.04) |
UAVI unprotected anal/vaginal intercourse with any partner, TRB transmission risk behavior, UAVI with At–Risk Partner
Primary Test of Efficacy (TRB) and of Test for Change in UAVI (Table 3). Controlling for baseline TRB and site, SafeTalk reduced the average number of TRB acts at 8-month follow-up by 87% compared with controls (P < 0.0001). Controlling for baseline UAVI and site, SafeTalk reduced the number of UAVI acts at 8-month follow-up by 73% compared with controls (P < 0.0001).
Table 3.
Adjusted parameter estimates for Transmission Risk Behavior (TRB) and Unprotected Anal/Vaginal Intercourse (UAVI) at eight months and using longitudinal Generalized Estimating Equation (GEE)
| TRB parameter estimate | Std error | P-value | UAVI parameter estimate | Std error | P-value | |
|---|---|---|---|---|---|---|
| Test of efficacy | ||||||
| 8-month outcome | −2.05 | 0.39 | <0.0001 | −1.28 | 0.27 | <0.0001 |
| GEE results | ||||||
| SafeTalk effect | 0.60 | 0.76 | 0.43 | 0.14 | 0.52 | 0.79 |
| Visit2 | 0.42 | 0.95 | 0.66 | 0.09 | 0.58 | 0.87 |
| Visit3 | 0.55 | 0.92 | 0.55 | 0.06 | 0.63 | 0.92 |
| Visit4 | −1.01 | 0.59 | 0.09 | −0.38 | 0.41 | 0.37 |
| Arm * visit 2 | −1.99 | 1.22 | 0.10 | −0.96 | 0.77 | 0.21 |
| Arm * visit 3 | −2.54 | 1.14 | 0.03 | −1.32 | 0.79 | 0.10 |
| Arm * visit 4 | −1.86 | 0.92 | 0.04 | −0.71 | 0.70 | 0.32 |
Longitudinal Analyses (Table 3 and Fig. 2a, b)
Fig. 2.
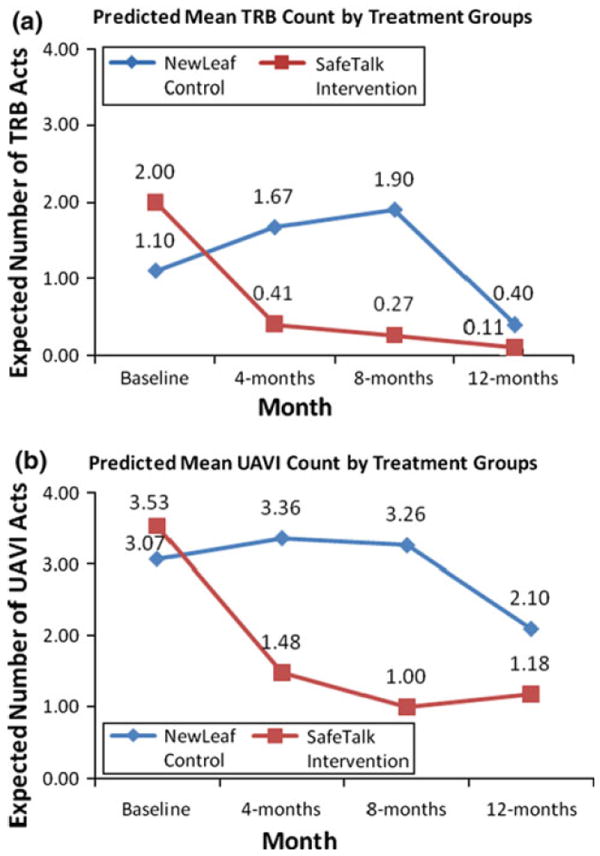
a Predicted mean counts of Transmission Risk Behavior (TRB). b Predicted mean counts of Unprotected Anal/Vaginal Intercourse (UAVI)
The regression coefficients in Table 3 indicate that the relative risks of occurrence of TRB (rate of occurrence of TRB in the SafeTalk group in relation to New Leaf) are 1.81, 0.25, 0.14, and 0.28, respectively at baseline, 4, 8, and 12 months. The P-values indicate that these differences were not significant at baseline (P = .43) and at 4 months (P = .10). However, SafeTalk significantly reduced TRB act rates at 8 and 12 months. The predicted means in Fig. 2a show that at 8 months, the average occurrence of TRB in the SafeTalk group is 0.27 (reduced from a level of 2.00 at baseline), whereas in the New Leaf group, the average TRB acts at 8 months was 1.9 (an increase from 1.10 at baseline).
The number of UAVI acts decreased in the SafeTalk arm from the baseline to follow-up surveys at all time points while increasing in the New Leaf control arm at all but the 12-month time point; however, the difference in UAVI between the two groups was not statistically significant.
Discussion
This study demonstrates that a multi-component, one-on-one MI-based program for a heterogeneous group of people living with HIV can effectively reduce the amount of risky sexual behavior with at-risk partners at 8 months follow-up compared with an attention-matched control condition, our primary test of efficacy. Moreover, this reduction was maintained at 12 months follow-up. The effect size of these changes is consistent with other studies of successful behavioral interventions [27]. Further, the number of unprotected acts with any partner also improved in the SafeTalk arm while it worsened among controls, although the difference between groups only achieved statistical significance in the 8-month Poisson regression model but not in the longitudinal GEE analysis.
The effect of the intervention on the number of risky sex acts is consistent with the harm reduction approach of the SafeTalk MI counseling style. This approach emphasizes setting achievable goals. Reducing the average number of unprotected sex acts with at-risk partners from 2.0 to 0.5 has the potential to reduce transmission of HIV to these partners.
It is also interesting that the intervention’s impact on a reduction in the number of unprotected sex acts with at-risk partners was significant over time at 8 and 12 months, but not for changes that occurred in unsafe sex with any partner. At-risk partners in this study were defined as partners of HIV-negative or unknown serostatus, implying that sexual behavior with HIV-positive partners accounts for the difference in significance between these two outcomes. This finding is consistent with the focus of the Safe Talk intervention, which was on reducing HIV transmission. It is also possible that patients are willing to modify their (and their partners’) behavior to avoid exposing uninfected partners, but that avoiding exposing partners who are already infected with HIV to additional HIV strains is not as strong a motivation for behavior change.
The efficacy of the SafeTalk intervention for unprotected vaginal or anal intercourse with at-risk partners may have resulted from several factors. First, we based the program on MI principles and on social cognitive theory with an emphasis on enhancing self-efficacy and skills-building, factors known to improve healthy behaviors [31]. Skills-building in the SafeTalk intervention included not only condom use skills, but also communication skills such as condom negotiation and serostatus disclosure, which are critical for a “prevention with positives” program. Second, the intervention was delivered by professional counselors who were trained in MI and met regularly with a clinical supervisor to review their techniques and taped sessions to ensure quality control and fidelity. Third, a counseling protocol that focused on inviting participants to identify their specific needs related to safer sex (such as addressing environmental factors) allowed counselors to address issues most salient to participants in a heterogeneous sample that included MSM, MSW, and women from both rural and urban areas in the southeastern United States. Fourth, the use of booklet/CD pairs and booster letters to support the MI sessions likely served to enhance the effects of counseling. In particular, the use of realistic characters and actual patient vignettes that were vetted in formative work and refined based on input from other individuals living with HIV [31] likely facilitated participants’ comfort with the program. Offering the program through clinics where participants already had established trusting relationships with clinic personnel may have facilitated program acceptance and rapport-building with the counselors. These findings are consistent with those of meta-analyses identifying a number of intervention features characteristic of successful prevention with positives interventions [27]. Finally, our longitudinal study design allowed us to demonstrate the extent to which changes were maintained over time.
Our results may not be applicable to HIV positive individuals with different risk profiles, such as injection drug users or persons from urban settings living in the Northeast or on the West coast of the United States, because these populations were not represented in our study. Although the measurement of sexual behavior using ACASI represents a methodological improvement over interviewer-administered data which can be biased by socially desirable responses and recall errors [36, 37], some reporting error may be differential in nature, biasing results away from the null. For example, participants receiving safer sex counseling may be more likely to understate their risky sexual behaviors. In another study, however, we did find a correlation between our ACASI-reported behaviors and sexually transmitted infection (STI) outcomes (Quinlivan EB, Golin CE, Patel S, et al. Trichomonas vaginalis infections among HIV-infected individuals receiving medical care in North Carolina. In preparation). However, reporting bias in the other direction is equally possible; that is, those receiving the MI counseling might be more likely to report risky behavior than those not receiving it because its nonjudgmental nature may make patients feel more comfortable admitting to risky behaviors. If this type of reporting bias were present, our current findings would underestimate the effect size.
Conclusions
In conclusion, MI delivered to HIV positive patients monthly in clinic over a 4-month period and enhanced by audiovisual materials can provide an effective prevention intervention for a heterogeneous group of people living with HIV. The individualized nature of MI and the tailored nature of the SafeTalk materials allowed the counselors to target the program to each client’s unique needs and behaviors. In response to the growing number of people living with HIV, a compelling need exists to support effective prevention programs such as SafeTalk that can be easily disseminated and incorporated into the clinical setting. In this study, SafeTalk relied on training Masters level counselors. However, widely disseminating it to more resource-limited settings may require retesting its implementation, training and using less highly educated counselors to carry it out. We believe that the use of relatively low tech audio and visual materials can facilitate and further standardize this process.
Acknowledgments
We would like to thank Sarah Przybyla, LaToya White, Meheret Mamo, Megan Kays, Robert Michael, Jessica Kadis, and Kathy Ramsey for their assistance in data collection, cleaning, and management. We would also like to thank Dr. Ronald Strauss, DDM, PhD for providing consultation in survey development as well as Carol Carr, Beth Fowler, and Regina McCoy for assistance with materials development. We would like to acknowledge Roger Akers for his technical assistance designing our data management system. We thank Niasha Brown, Rebecca Davis, Tyndall Harris, and Katherine Tiller for their delivery of counseling to study participants, Andrea Wong for statistical support, and Ross Oglesbee for her outstanding administrative assistance and support. This work would not have been possible without the enthusiastic support from the clinic staff in which the study was conducted. This work was supported by National Institute of Health (NIH) grants R01-MH069989, DK56350 and AI50410.
Contributor Information
Carol E. Golin, Email: Carol_Golin@unc.edu, Division of General Medicine and Clinical Epidemiology, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Department of Health Behavior and Health Education, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; University of North Carolina Center for AIDS Research, Chapel Hill, NC, USA; UNC Sheps Center for Health Services Research, 725 MLK Jr. Blvd., Campus Box 7590, Chapel Hill, NC 27599-7590, USA.
Jo Anne Earp, Department of Health Behavior and Health Education, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Catherine A. Grodensky, Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Shilpa N. Patel, Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Chirayath Suchindran, Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Megha Parikh, Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Seth Kalichman, Department of Psychology, University of Connecticut, Storrs, CT, USA.
Kristine Patterson, Division of Infectious Diseases, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Heidi Swygard, Division of Infectious Diseases, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
E. Byrd Quinlivan, University of North Carolina Center for AIDS Research, Chapel Hill, NC, USA; Division of Infectious Diseases, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Kemi Amola, Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Zulfiya Chariyeva, Department of Health Behavior and Health Education, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Jennifer Groves, Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
References
- 1.Kalichman SC. Positive prevention: reducing HIV Transmission among people living with HIV/AIDS. New York: Plenum; 2004. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Advancing HIV prevention: new strategies for a changing epidemic, United States, 2003. MMWR. 2003;52:329–32. [PubMed] [Google Scholar]
- 3.Global HIV Prevention Working Group. HIV prevention in the era of expanded treatment. Washington, DC: Kaiser Family Foundation; 2004. [Google Scholar]
- 4.Gordon CM, Stall R, Cheever LW. Prevention interventions with persons living with HIV/AIDS: challenges, progress, and research priorities. J Acquir Immune Defic Syndr. 2004;37:S53–7. doi: 10.1097/01.qai.0000142321.27136.8b. [DOI] [PubMed] [Google Scholar]
- 5.Janssen RS, Valdiserri RO. HIV prevention in the United States: increasing emphasis on working with those living with HIV. J Acquir Immune Defic Syndr. 2004;37:S119–21. doi: 10.1097/01.qai.0000140610.82134.e3. [DOI] [PubMed] [Google Scholar]
- 6.Eaton LA, Kalichman SC. Changes in transmission risk behaviors across stages of HIV disease among people living with HIV. J Assoc Nurses AIDS Care. 2009;20(1):39–49. doi: 10.1016/j.jana.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golin CE, Marks G, Wright J, et al. Psychosocial characteristics and sexual behaviors of people in care for HIV infection: an examination of men who have sex with men, heterosexual men and women. AIDS Behav. 2009;13(6):1129–42. doi: 10.1007/s10461-009-9613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39:446–53. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 9.Weinhardt LS. HIV diagnosis and risk behavior. In: Kalichman SC, editor. Positive prevention. New York: Plenum; 2004. pp. 29–64. [Google Scholar]
- 10.Schiltz MA, Sandfort TG. HIV-positive people, risk and sexual behaviour. Soc Sci Med. 2000;50:1571–88. doi: 10.1016/s0277-9536(99)00466-9. [DOI] [PubMed] [Google Scholar]
- 11.Celum CL. Sexually transmitted infections and HIV: epidemiology and interventions. Top HIV Med. 2010;18:138–42. [PubMed] [Google Scholar]
- 12.Kalichman SC, Rompal D, Cage M. Sexually transmitted infections among HIV seropositive men and women. Sex Transm Infect. 2000;76:350–4. doi: 10.1136/sti.76.5.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erbelding EJ, Stanton D, Quinn TC, et al. Behavioral and biologic evidence or persistent high-risk behavior in an HIV primary care population. AIDS. 2000;14:297–301. doi: 10.1097/00002030-200002180-00012. [DOI] [PubMed] [Google Scholar]
- 14.Brewer TH, Metsch LR, Zenilman JM. Use of a public sexually transmitted disease clinic by known HIV-positive adults: decreased self-reported risk behavior and increased disease incidence. J Acquir Immune Defic Syndr. 2002;29:289–94. doi: 10.1097/00126334-200203010-00010. [DOI] [PubMed] [Google Scholar]
- 15.Kissinger P, Clark R, Dumestre J, et al. Incidence of three sexually transmitted diseases during a safer sex promotion program for HIV-infected women. J Gen Intern Med. 1996;11:750–2. doi: 10.1007/BF02598989. [DOI] [PubMed] [Google Scholar]
- 16.Capps L, Peng G, Doyle M, et al. Sexually transmitted infections in women infected with the human immunodeficiency virus. Sex Transm Dis. 1998;25:443–7. doi: 10.1097/00007435-199809000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Milam J, Richardson JL, Espinoza L, Stoyanoff S. Correlates of unprotected sex among adult heterosexual men living with HIV. J Urban Health. 2006;83(4):669–81. doi: 10.1007/s11524-006-9068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalichman SC, Eaton L, White D, et al. Beliefs about treatments for HIV/AIDS and sexual risk behaviors among men who have sex with men, 1997–2006. J Behav Med. 2007;30(6):497–503. doi: 10.1007/s10865-007-9123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crepaz N, Hart T, Marks G. Highly active antiretroviral therapy and sexual risk behavior: a meta-analytic review. JAMA. 2004;292:224–36. doi: 10.1001/jama.292.2.224. [DOI] [PubMed] [Google Scholar]
- 20.van Kesteren NM, Hospers HJ, Kok G. Sexual risk behavior among HIV-positive men who have sex with men: a literature review. Patient Educ Couns. 2007;65:5–20. doi: 10.1016/j.pec.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Stall R, Mills TC, Williamson J, Hart T, et al. Association of co-occurring psychosocial health problems and increased vulnerability to HIV/AIDS among urban men who have sex with men. Am J Public Health. 2003;93(6):939–42. doi: 10.2105/ajph.93.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metsch LR, Pereyra M, Messinger S, et al. HIV transmission risk behaviors among HIV infected persons who are successfully linked to care. Cl Infect Dis. 2008;47(4):577–84. doi: 10.1086/590153. [DOI] [PubMed] [Google Scholar]
- 23.Folkman S, Chesney M, Pollack L, Coates T. Stress, control, coping, and depressive mood in human immunodeficiency virus-positive and -negative gay men in San Francisco. J Nerv Ment Dis. 1993;181(7):409–16. doi: 10.1097/00005053-199307000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Wolitski RJ, Pals SL, Kidder DP, Courtenay-Quirk C, Holtgrave D. The effects of HIV stigma on health, disclosure of HIV status, and risk behavior of homeless. AIDS Behav. 2008 Sep 4; doi: 10.1007/s10461-008-9455-4. serial online. [DOI] [PubMed] [Google Scholar]
- 25.Kalichman SC. Psychological and social correlates of high-risk sexual behaviour among men and women living with HIV/AIDS. AIDS Care. 1999;11(4):415–27. doi: 10.1080/09540129947794. [DOI] [PubMed] [Google Scholar]
- 26.Crepaz N, Passin WF, Herbst JH, et al. Meta-analysis of cognitive-behavioral interventions on HIV-positive persons’ mental health and immune functioning. Health Psychol. 2008;27(1):4–14. doi: 10.1037/0278-6133.27.1.4. [DOI] [PubMed] [Google Scholar]
- 27.Crepaz N, Lyles CM, Wolitski RJ, et al. Do prevention interventions reduce HIV risk behaviours among people living with HIV? A meta-analytic review of controlled trials. AIDS. 2006;20(2):143–57. doi: 10.1097/01.aids.0000196166.48518.a0. [DOI] [PubMed] [Google Scholar]
- 28.Noar SM. Behavioral interventions to reduce HIV-related sexual risk behavior: review and synthesis of meta-analytic evidence. AIDS Behav. 2008;12(3):335–53. doi: 10.1007/s10461-007-9313-9. [DOI] [PubMed] [Google Scholar]
- 29.DiIorio C, McCarty F, Resnicow K, et al. Using motivational interviewing to promote adherence to antiretroviral medications: a randomized controlled study. AIDS Care. 2008;20(3):273–83. doi: 10.1080/09540120701593489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bandura A. Self-efficacy: the exercise of control. New York: W.H. Freeman and Company; 1997. [Google Scholar]
- 31.Golin CE, Davis RA, Przybyla SM, et al. SafeTalk, a multi-component, motivational interviewing-based, safer sex counseling program for people living with HIV/AIDS: a qualitative assessment of patients’ views. AIDS Patient Care STDS. 2010;24(4):237–45. doi: 10.1089/apc.2009.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosamond WD, Ammerman AS, Holliday JL, et al. Cardiovascular disease risk factor intervention in low-income women: the North Carolina WISEWOMAN project. Prev Med. 2000;31(4):370–9. doi: 10.1006/pmed.2000.0726. [DOI] [PubMed] [Google Scholar]
- 33.Golin CE, Patel S, Tiller K, Quinlivan EB, Grodensky CA, Boland M. Start talking about risks: development of a motivational interviewing-based safer sex program for people living with HIV. AIDS Behav. 2007;11(5 Suppl):S72–83. doi: 10.1007/s10461-007-9256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keyserling TC, Samuel-Hodge CD, Jilcott SB, et al. Randomized trial of a clinic-based, community-supported, lifestyle intervention to improve physical activity and diet: the North Carolina enhanced WISEWOMAN project. Prev Med. 2008;46:499–510. doi: 10.1016/j.ypmed.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Jilcott SB, Keyserling TC, Samuel-Hodge CD, et al. Linking clinical care to community resources for cardiovascular disease prevention: the North Carolina enhanced WISEWOMAN project. J Women’s Health. 2006;15(5):569–83. doi: 10.1089/jwh.2006.15.569. [DOI] [PubMed] [Google Scholar]
- 36.Gribble JN, Miller HG, Rogers SM, Turner CF. Interview mode and measurement of sexual behaviors: methodological issues. J Sex Res. 1999;36:16–24. doi: 10.1080/00224499909551963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner CF, Ku L, Rogers SM, Lindberg LD, Pleck JH, Sonenstein FL. Adolescent sexual behavior, drug use, and violence: increased reporting with computer survey technology. Science. 1998;280(5365):867–73. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- 38.Ware JE, Sherbourne CD. The MOS 36-items short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 39.Rubin DB. Multiple imputations for nonresponse in surveys. New York: J.Wiley & Sons, Inc.; 1987. [Google Scholar]


