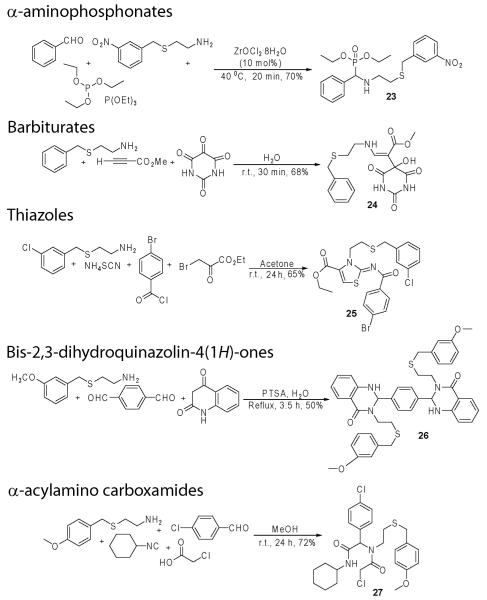
Abstract
Here we describe a new experimental approach to the synthesis of the β-benzylmercaptoethylamine functionality and illustrate its synthetic utility in multi-component reactions. Although prevalent in modern organic synthesis, no general methods have been described for this functionality. Using a carefully developed LiOH-water-ethanol reaction mixture we were able to produce a diverse collection of β-benzylmercaptoethylamines containing a range of sensitive functional groups in excellent yields. To further illustrate their utility in molecular library synthesis, we also report the use of β-benzylmercaptoethylamines in five different multi-component reactions.
The β-benzylmercaptoethylamine functionality and its derivatives are widely utilized with applications in organic,1 inorganic,2 medicinal,3 and cosmetic chemistry.4 For example, this functionality played a critical role in the synthesis of pantetheine (a cysteamine derivative of pantothenic acid–Vitamin B5).3a Reisner reported the synthesis of D/L-γ-sulfamyl-α-amino acids as potential anti-metabolites utilizing β-benzylmercaptoethylamine as a key intermediate.3b When Okarvi et al. was exploring an alternative of 131I-hippuran (a renal radiopharmaceutical), they synthesized a number of S-protected derivatives of MAG3 (mercaptoacetyltriglycine) and discovered that the p-methoxy derivative of β-benzylmercaptoethylamine was a key starting material.3l Finally, in order to prove the necessity of the disulfide unit present in a series of psammaplin A type antibacterial agents (prepared by a novel combinatorial disulfide exchange strategy), Nicolaou et al. used β-benzylmercaptoethylamine as a thioether containing primary amine coupling partner.3k
Despite the wide reaching implications of this functionality, the disparate methods for producing it have afforded varying yields, and a systematic synthetic study is lacking. Some conditions that have been utilized with varying degrees of success include NaOMe-methanol,3a hydrazine hydrate-methanol,3b thiourea-NaOH-ethanol,1e ethyleneimine-ethanol,1b K2CO3-ethanol,1c,3k NaOEt-ethanol,1d Na/liq. NH3,1h NaI-NaOEt,2c TFA-CH2Cl2,2f and NaOH-ethanol.3i These methods suffer from various disadvantages such as long reaction times,1b,2f,3i,k refluxing conditions,1b,3a,b the use of moisture sensitive solvents and reagents,1h extensive work-up procedures,2f or a two step reaction procedure.1e
During the course of synthesizing a small library of molecules to modulate the secretion of the Aβ peptide by a neuronal cell line, we became interested in a specific molecule that contained the β-benzylmercaptoethylamine functionality. It was during these studies that we identified the need to develop the method described here.
In our initial consideration of this reaction, and after a careful evaluation of previously reported reaction conditions, we hypothesized that the general reaction (depicted in Scheme 1) could possibly be proceeding via a borderline/SN1-type reaction mechanism, in contrast to the prototypical SN2-type pathway. Thus, we envisioned that a careful optimization of both the solvent conditions and the base used would be critical for developing a superior method. Towards the first point, and lending support to our borderline mechanism hypothesis, we were able to identify mixtures of water and ethanol as ideal for smooth conversions. Further lending support to our hypothesis, we found that simple ionic bases further facilitated smooth conversions (Scheme 1).
Scheme 1.
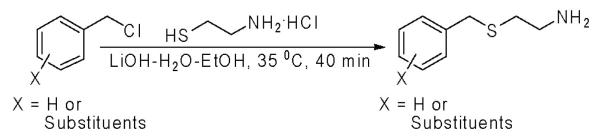
General synthesis of β-benzylmercaptoethylamine and its derivatives using a combination of LiOH-water-ethanol.
Here we report a LiOH-water-ethanol reaction system that, with the corresponding benzyl chlorides/bromides and cysteamine hydrochloride, affords excellent yields, rapid reaction times, and a facile workup.5 We have subsequently tested this methodology utilizing a wide range of substrates, and we illustrate the utility of these β-benzylmercaptoethylamines in combinatorial chemistry and diversity oriented synthesis through five separate multi-component reactions (MCRs).
Table 1 illustrates a sampling of the reaction conditions we studied. Our initial exploration focused primarily on alcoholic solvents; however we quickly determined that varying amounts of water played an important role in both reaction time and yield. It is likely that the 3:1 ratio of ethanol:water provided a balance between solvent dielectric for the reaction pathway while still allowing the reagents to be soluble in the media. Keeping the ethanol:water ratio fixed, we subsequently screened the effect of various alkali metal and organic bases to find LiOH the most effective.
Table 1.
A systematic evaluation of solvent systems and bases for the formation of β-benzylmercaptoethylamines. The reported yields are for reaction 7 from Table 2. All entries reacted for 40 minutes at 35°C.
| Base used | Solvent used | Yield |
|---|---|---|
| LiOH | EtOH | 72% |
| LiOH | EtOH-H2O (3:1) | 86% |
| LiOH | H2O | 20% |
| NaOH | EtOH | 66% |
| NaOH | EtOH-H2O (3:1) | 78% |
| KOH | EtOH-H2O (3:1) | 75% |
| CsOH | EtOH-H2O (3:1) | 75% |
| K2CO3 | EtOH | 30% |
| K2CO3 | EtOH-H2O (3:1) | 50% |
| Et3N | EtOH | 25% |
| Et3N | DCM | 5% |
Using this approach, not only were our yields superior to those previously reported, but we were also able to accomplish the conversions in a minimal amount of time (approximately 40 minutes) and at low temperatures (35°C). For the reactions listed in Table 1 we utilized 3-chlorobenzyl chloride as the representative benzyl halide mainly due to its importance in the molecules we were synthesizing to modulate Aβ secretion.
After our careful optimization, we applied this methodology to a wide range of substrates summarized in Table 2 using a uniform set of reaction conditions.5 Interestingly, we obtained comparatively lower yields for the nitro derivatives. Among all other nitro derivatives, p-nitro (entries 12 and 13, Table 2) gave the lowest yield, further substantiating a borderline/SN1-type mechanism, where at least a partial charge builds at the benzylic position. Further, the best yields were obtained for the substrates containing an electron donating functional group such as -OCH3 (entry 3, Table 2). It is worth pointing out that the reaction was highly compatible with a number of functional groups including -OCH3, -NO2, halogens, -CN, -CO2CH3, -CH3, -CF3. Benzyl chlorides and bromides gave similar yields (compare entries 1 and 2, 5 and 6, 12 and 13: Table 2).
Table 2.
Reaction between various benzyl halides and cysteamine hydrochloride using a combination of LiOH-H2O-EtOH.
| Entry | Benzyl halidea | Product | Yieldb (%) |
|---|---|---|---|
|
|
||
| 1 | Z = H, X = Br | Z = H | 90 |
| 2 | Z = H | Z = H | 90 |
| 3 | Z = 4-OMe | Z = 4-OMe | 94 |
| 4 | Z = 3-OMe | Z = 3-OMe | 85 |
| 5 | Z = 3-F, X = Br | Z = 3-F | 83 |
| 6 | Z = 3-F | Z = 3-F | 82 |
| 7 | Z = 3-Cl | Z = 3-Cl | 86 |
| 8 | Z = 3-Br | Z = 3-Br | 85 |
| 9 | Z = 3-I | Z = 3-I | 85 |
| 10 | Z = 2-NO2 | Z = 2-NO2 | 78 |
| 11 | Z = 3-NO2 | Z = 3-NO2 | 82 |
| 12 | Z = 4-NO2, X = Br |
Z = 4-NO2 | 75 |
| 13 | Z = 4-NO2 | Z = 4-NO2 | 75 |
| 14 | Z = 3-CO2Me | Z = 3-CO2Me | 60c |
| 15 | Z = 3-CN | Z = 3-CN | 80d |
| 16 | Z = 3-Me | Z = 3-Me | 82 |
| 17 | Z = 3-CF3 | Z = 3-CF3 | 80 |
| 18 | Z = 4-t-Butyl | Z = 4-t-Butyl | 82 |
| 19 | Z = 2,3-di-OMe | Z = 2,3-di-OMe | 81 |
| 20 | Z = 2,6-di-Cl | Z = 2,6-di-Cl | 80 |
| 21 | Z = 3-Cl-5- OMe |
Z = 3-Cl-5-OMe | 78 |
| 22 | Z = 2-CN-3-Cl, X = Br |
Z = 2-CN-3-Cl | 80 |
Unless otherwise stated, X = Cl and reaction time 40 min
= Isolated yield.
Reaction time was 15 min.
Reaction time was 30 min
In conjunction with our efforts to synthesize small libraries that modulate Aβ-peptide excretion we became further interested in exploiting β-benzylmercaptoethylamines in MCRs, as they create significant possibilities for molecular diversity in one step, affording more economical synthetic approaches as compared to linear syntheses.6 We explored five separate MCRs with aims of generating a broad swath of diverse small molecules quickly (Scheme 2). We first explored α-aminophosphonates due, in large part, to their well documented, diverse biological activities.7 As a first trial, we synthesized the novel α-aminophosphonate 23 by following a literature procedure utilizing 2-(3-nitrobenzylsulfanyl)-ethylamine as the amine component. The reaction proceeded smoothly, affording a similar yield (70%) to those previously reported utilizing the Zr(IV) catalyst.8 Because of the well known therapeutic value of barbiturates,9 next we turned our attention towards the synthesis of 24 via a three component reaction using 2-benzylsulfanyl-ethylamine. Our 68% yield is in line with the 72%-90% yields previously reported in the water-based solvent system.10 Due to the prevalence of the thiazoles across diverse pharmaceutical targets,11 we utilized the methodology of Yavari et al.12 to synthesize 25 via a four component reaction with 2-(3-chlorobenzylsulfanyl)-ethylamine.12 The reaction proceeded with reasonable yield (65%) after 24 hours. We further explored the synthesis of novel bis-2,3-dihydroquinazolin-4(1H)-one derivatives 26 utilizing a three component reaction following a literature procedure.13 As compared to precedent, our substrate proved as effective a reagent in the p-toluenesulfonic acid catalyzed reaction affording 26 in 50% yield. Finally, since its discovery, the ‘Ugi four-component reaction’ is one of the best known, and most popular multicomponent reactions. In this reaction an aldehyde/ketone, an amine, a carboxylic acid, and an isocyanide form an α-acylamino carboxamide through a one pot condensation.14 The final application is illustrated by the formation of 27, derived from 2-(4-methoxybenzylsulfanyl)-ethylamine, in 72% yeild.14c
Scheme 2.
The use of β-benzylmercaptoethylamine and its derivatives in various multicomponent reactions.
Supplementary Material
Acknowledgments
This work was supported by the NIH (AG015885 & HL053315).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material: Experimental procedure, NMR spectra, high resolution mass spectra and compound characterization data are available for all molecules.
References
- 1(a).Marshall K. Can. J. Chem. 1959;37:325. [Google Scholar]; (b) Chu SH, Mautner HG. J. Org. Chem. 1961;26:4498. [Google Scholar]; (c) Johnston TP, Gallagher A. J. Org. Chem. 1963;28:1305. [Google Scholar]; (d) Carroll FI, Dickson HM, Wall ME. J. Org. Chem. 1965;30:33. [Google Scholar]; (e) Aroyan AA, Ovsepyan TR. Armyanskii Khimicheskii Zhurnal. 1968;21:858. [Google Scholar]; (f) Bewick A, Mellor JM, Owton WM. J. Chem. Soc., Perkins Trans. 1. 1985:1039. [Google Scholar]; (g) Vyas DM, Benigni D, Partyka RA, Doyle TW. J. Org. Chem. 1986;51:4307. [Google Scholar]; (h) Weintraub PM, Miller FP, Wiech NL. Heterocycles. 1987;26:1503. [Google Scholar]; (i) Walker MA, Heathcock CH. J. Org. Chem. 1992;57:5566. [Google Scholar]; (j) Kumar S, Saini R, Sing H. J. Chem. Soc., Perkins Trans. 1. 1992:2011. [Google Scholar]; (k) Akaji K, Kuriyama N, Kiso Y. J. Org. Chem. 1996;61:3350. [Google Scholar]; (l) Tan DS, Foley MA, Shair MD, Schreiber SL. J. Am. Chem. Soc. 1998;120:8565. [Google Scholar]; (m) Tan DS, Foley MA, Stockwell BR, Shair MD, Schreiber SL. J. Am. Chem. Soc. 1999;121:9073. [Google Scholar]; (n) Zhang Z, Martell AE, Motekaitis RJ, Fu L. Tetrahedron Lett. 1999;40:4615. [Google Scholar]; (o) Petra DGI, Kamer PCJ, Spek AL, Schoemaker HE, van Leeuwen PWNM. J. Org. Chem. 2000;65:3010. doi: 10.1021/jo991700t. [DOI] [PubMed] [Google Scholar]; (p) Rombouts FJR, Fridkin G, Lubell WD. J. Comb. Chem. 2005;7:589. doi: 10.1021/cc050002l. [DOI] [PubMed] [Google Scholar]; (q) Angelosante JK, Lewis BJ, Cooper LE, Swanson RA, Daley CJA. Phosphorus, Sulfur, and Silicon. 2006;181:2263. [Google Scholar]
- 2(a).Kennard GJ, Deutsch E. Inorg. Chem. 1978;17:2225. [Google Scholar]; (b) Mirza SA, Pressler MA, Kumar M, Day RO, Maroney MJ. Inorg. Chem. 1993;32:977. [Google Scholar]; (c) Hay RW, Galyer AL. Transition Met. Chem. 1997;22:97. [Google Scholar]; (d) Gallo V, Mastrorilli P, Nobile CF, Braunstein P, Englert U. Dalton Trans. 2006:2342. doi: 10.1039/b514787e. [DOI] [PubMed] [Google Scholar]; (e) Carson EC, Lippard SJ. J. Inorg. Biochem. 2006;100:1109. doi: 10.1016/j.jinorgbio.2005.11.019. [DOI] [PubMed] [Google Scholar]; (f) Smith AL, Day CS, Que L, Jr., Zhou Y, Bierbach U. Inorg. Chim. Acta. 2007;360:2824. doi: 10.1016/j.ica.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3(a).Walton E, Wilson AN, Holly FW, Folkers K. J. Am. Chem. Soc. 1954;76:1146. [Google Scholar]; (b) Reisner DB. J. Am. Chem. Soc. 1956;78:5102. [Google Scholar]; (c) Moffatt JG, Khorana HG. J. Am. Chem. Soc. 1961;83:663. [Google Scholar]; (d) Tucker H, Coope JF. J. Med. Chem. 1978;21:769. doi: 10.1021/jm00206a010. [DOI] [PubMed] [Google Scholar]; (e) Sterk GJ, Van der Goot H, Henk T. Eur. J. Med. Chem. 1986;21:305. [Google Scholar]; (f) Sami SM, Remers WA, Bradner WT. J. Med. Chem. 1989;32:703. doi: 10.1021/jm00123a036. [DOI] [PubMed] [Google Scholar]; (g) Hardy GW, Lowe LA, Mills G, Sang PY, Simpkin DSA, Follenfant RL, Shankley C, Smith TW. J. Med. Chem. 1989;32:1108. doi: 10.1021/jm00125a028. [DOI] [PubMed] [Google Scholar]; (h) Buschauer A, Lachenmayr F, Schunack W. Pharmazie. 1991;46:840. [PubMed] [Google Scholar]; (i) Oya S, Plössl K, Kung MP, Stevenson DA, Kung HF. Nucl. Med. Biol. 1998;25:135. doi: 10.1016/s0969-8051(97)00153-4. [DOI] [PubMed] [Google Scholar]; (j) Zhuang ZP, Kung MP, Mu M, Hou C, Kung HF. Bioconjugate Chem. 1999;10:159. doi: 10.1021/bc970207q. [DOI] [PubMed] [Google Scholar]; (k) Nicolaou KC, Hughes R, Pfefferkorn JA, Barluenga S. Chem. Eur. J. 2001;7:4296. doi: 10.1002/1521-3765(20011001)7:19<4296::aid-chem4296>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]; (l) Okavi SM, Torfs P, Adriaens P, Verbruggen AM. J. Label. Compd. Radiopharm. 2002;45:407. [Google Scholar]; (m) Zhang Y, Dai X, Kallmes DF, Pan D. Tetrahedron Lett. 2004;45:8673. [Google Scholar]; (n) Masip I, Ferrándiz-Huertas C, García-Martínez C, Ferragut JA, Ferrer-Montiel A, Messeguer A. J. Comb. Chem. 2004;6:135. doi: 10.1021/cc030002q. [DOI] [PubMed] [Google Scholar]; (o) Cleynhens BJ, de Groot TJ, Vanbilloen HP, Kieffer D, Mortelmans L, Bormans GM, Verbruggen AM. Bioorg. Med. Chem. 2005;13:1053. doi: 10.1016/j.bmc.2004.11.036. [DOI] [PubMed] [Google Scholar]; (p) Zhuang ZP, Kung MP, Hou C, Ploessl K, Kung HF. Nucl. Med. Biol. 2005;32:171. doi: 10.1016/j.nucmedbio.2004.10.002. [DOI] [PubMed] [Google Scholar]; (q) Kieffer DM, Vanbilloen HP, Cleynhens BJ, Terwinghe CY, Mortelmans L, Bormans GM, Verbruggen AM. Nucl. Med. Biol. 2006;33:125. doi: 10.1016/j.nucmedbio.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 4(a).LaPorte G. Parfumerie, Cosmetique, Savons. 1968;11:516. [Google Scholar]; (b) LaPorte G. American Perfumer and Cosmetics. 1970;85:47. [Google Scholar]
- 5.Typical reaction procedure: LiOH (0.245 g, 10.2 mmol) was dissolved in 5 mL of water and 15 mL of ethanol was added. The resulting solution was added to a flask containing cysteamine hydrochloride (0.568 g, 5 mmol) followed by the dropwise addition of benzyl halides (5 mmol) with continuous stirring. The reaction mixture was stirred for 40 min at 35 °C, and ethanol was removed in vacuo. 20 mL of water was subsequently added and the mixture was extracted with dichloromethane (3 × 30 mL), dried over anhydrous Na2SO4, concentrated in vacuo, and purified via column chromatography over silica gel (60Å, 230 to 400 mesh, SiliCycle) using a mobile phase consisting of a suitable mixture of dichloromethane-methanol (gradient from 2% v/v methanol/dichloromethane to 20% v/v methanol/dichloromethane) to afford the chromatographically pure desired β-benzylmercaptoethylamine derivatives.
- 6(a).Gallop MA, Barrett RW, Dower WJ, Fodor SPA, Gordon EM. J. Med. Chem. 1994;37:1233. doi: 10.1021/jm00035a001. [DOI] [PubMed] [Google Scholar]; (b) Gordon EM, Barrett RW, Dower WJ, Fodor SPA, Gallop MA. J. Med. Chem. 1994;37:1385. doi: 10.1021/jm00036a001. [DOI] [PubMed] [Google Scholar]
- 7(a).Pratt RF. Science. 1989;246:917. doi: 10.1126/science.2814513. [DOI] [PubMed] [Google Scholar]; (b) Allen MC, Fuhrer W, Tuck B, Wade R, Wood JM. J. Med. Chem. 1989;32:1652. doi: 10.1021/jm00127a041. [DOI] [PubMed] [Google Scholar]; (c) Bonarska D, Kleszczyńska H, Sarapuk J. Cell. Mol. Biol. Lett. 2002;7:929. [PubMed] [Google Scholar]; (d) Grembecka J, Mucha A, Cierpicki T, Kafarski P. J. Med. Chem. 2003;46:2641. doi: 10.1021/jm030795v. [DOI] [PubMed] [Google Scholar]; (e) Skropeta D, Schwörer R, Schmidt RR. Bioorg. Med. Chem. Lett. 2003;13:3351. doi: 10.1016/s0960-894x(03)00672-3. [DOI] [PubMed] [Google Scholar]
- 8.Bhagat S, Chakraborti AK. J. Org. Chem. 2008;73:6029. doi: 10.1021/jo8009006. [DOI] [PubMed] [Google Scholar]
- 9(a).Bojarski JT, Mokrosz JL, Bartoń HJ, Paluchowska MH. Adv. Heterocycl. Chem. 1985;38:229. [Google Scholar]; (b) Foley LH, Palermo R, Dunten P, Wang P. Bioorg. Med. Chem. Lett. 2001;11:969. doi: 10.1016/s0960-894x(01)00104-4. [DOI] [PubMed] [Google Scholar]
- 10.Teimouri MB, Abbasi T, Mivehchi H. Tetrahedron. 2008;64:10425. [Google Scholar]
- 11(a).Kumar Y, Green R, Borysko KZ, Wise DS, Wotring LL, Townsend LB. J. Med. Chem. 1993;36:3843. doi: 10.1021/jm00076a012. [DOI] [PubMed] [Google Scholar]; (b) Miwatashi S, Arikawa Y, Kotani E, Miyamoto M, Naruo K–I, Kimura H, Tanaka T, Asahi S, Ohkawa S. J. Med. Chem. 2005;48:5966. doi: 10.1021/jm050165o. [DOI] [PubMed] [Google Scholar]; (c) Papadopoulou C, Geronikaki A, Hadjipavlou-Litina D. Farmaco. 2005;60:969. doi: 10.1016/j.farmac.2005.06.014. [DOI] [PubMed] [Google Scholar]; (d) Pereira R, Gaudon C, Iglesias B, Germain P, Gronemeyer H, de Lera AR. Bioorg. Med. Chem. Lett. 2006;16:49. doi: 10.1016/j.bmcl.2005.09.060. [DOI] [PubMed] [Google Scholar]
- 12.Yavari I, Hossaini Z, Shirgahi-Talari F, Seyfi S. Synlett. 2008:1631. [Google Scholar]
- 13.Baghbanzadeh M, Salehi P, Dabiri M, Kozehgary G. Synthesis. 2006:344. [Google Scholar]
- 14(a).Ugi I, Meyer R, Fetzer U, Steinbrückner C. Angew. Chem. 1959;71:386. Ugi I, Steinbrückner C. Angew. Chem. 1960;72:267. Marcaccini S, Torroba T. Nat. Protoc. 2007;2:632. doi: 10.1038/nprot.2007.71. and references cited therein.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.