Summary
Background
Microtubules are built from linear polymers of α-β tubulin dimers (protofilaments) that form a tubular quinary structure. Microtubules assembled from purified tubulin in vitro contain between ten and sixteen protofilaments, however such structural polymorphisms are not found in cells. This discrepancy implies that factors other than tubulin constrain microtubule protofilament number, but the nature of these constraints is unknown.
Results
Here, we show that acetylation of MEC-12 α tubulin constrains protofilament number in C. elegans touch receptor neurons (TRNs). Whereas the sensory dendrite of wild-type TRNs is packed with a cross-linked bundle of long, 15-protofilament microtubules, mec-17;atat-2 mutants lacking α tubulin acetyltransferase activity have short microtubules, rampant lattice defects, and variable protofilament number both between and within microtubules. All-atom molecular dynamics simulations suggest a model in which acetylation of lysine 40 promotes the formation of inter-protofilament salt bridges, stabilizing lateral interactions between protofilaments and constraining quinary structure to produce stable, structurally uniform microtubules in vivo.
Conclusions
Acetylation of α tubulin is an essential constraint on protofilament number in vivo. We propose a structural model in which this post-translational modification promotes the formation of lateral salt bridges that fine-tune the association between adjacent protofilaments and enable the formation of uniform microtubule populations in vivo.
Introduction
Microtubules have diverse functions and are needed for cell division, migration, and intracellular transport. They are fundamental elements of the eukaryotic cytoskeleton composed of linear polymers of α-β dimers (protofilaments) assembled into hollow tubes. It has been known for decades that the number of protofilaments that compose a microtubule varies significantly among cells [1, 2], despite a remarkable degree of tubulin sequence conservation both within and across species [3]. Such architectural polymorphisms are a pervasive feature of microtubules and are strikingly recapitulated among microtubules assembled in vitro from tissues that contain uniform microtubules [4]. This classic observation implies that lateral interactions between protofilaments are flexible enough to accommodate a wide range of bending angles and that factors present in cells constrain microtubule architecture and help to define the quinary structure of native microtubules.
Complex, microtubule-based structures are a hallmark of many mechanoreceptor neurons, including the C. elegans touch receptor neurons (TRNs), which are low-threshold mechanoreceptor neurons. The TRNs are filled with dense, cross-linked arrays of as many as fifty 15-protofilament (pf) microtubules [5]. Some cells in mammals [6, 7] and other invertebrates [8] also contain 15-pf microtubules. In C. elegans TRNs, these distinctive microtubules serve multiple functions, including the regulation of gene and protein expression [9], but are not essential for neurite outgrowth [10–12] or for activation of the mechano-electrical transduction channels that initiate touch sensation [13, 14]. Not all C. elegans cells contain 15-pf microtubules [5], however. Thirteen-pf microtubules are found in sensory cilia and 11-pf microtubules are the norm in most C. elegans cells, including other neurons [5]. Thus, different C. elegans cells form microtubules composed of 11, 13, or 15 protofilaments in a cell-specific manner.
The factors that regulate microtubule quinary structure and account for the well-known structural polymorphisms observed in vitro and in vivo have yet to be identified. One hypothesis is a tubulin code in which specific tubulin isoforms form specific microtubules [15]. Consistent with this idea, the TRNs co-express mec-7 β tubulin and mec-12 α tubulin and depend on both tubulin genes to form large-diameter, 15-pf microtubules [10–12]. However, while MEC-7 and MEC-12 are expressed at high levels in TRNs, both are also expressed in neurons that lack 15-pf microtubules [12, 16]. Thus, while these tubulins are needed to form 15-pf microtubules, they may not be sufficient. Post-translational modifications are another potential source of structural variation. Indeed, α tubulin acetylation has been hypothesized to confer structural and thus functional heterogeneity to microtubules [17, 18], as it does to histones [19]. C. elegans expresses two α tubulin acetyltransferases (αTATs), mec-17 and its paralog atat-2 [20, 21], that catalyze acetylation of lysine 40 on α tubulin. Both enzymes are expressed in TRNs [20, 21] where they acetylate MEC-12 α tubulin, the sole K40-bearing α tubulin in C. elegans [12]. The function of K40 acetylation, however, remains poorly understood.
Here, we combine genetic dissection, in vivo structure-function analysis, and serial-section, high-pressure freezing electron microscopy to demonstrate that α tubulin K40 acetylation is needed to constrain microtubule pf number and quinary structure in TRNs in vivo. Based on all-atom molecular dynamics simulations, we propose a structural model in which α tubulin K40 acetylation favors inter-protofilament salt bridges and modulates lateral interactions in the microtubule lattice. This investigation is a vital first step towards a full definition of the factors that control the quinary structure of native microtubules in cells.
Results
We used serial-section electron microscopy of high pressure frozen animals to analyze microtubules with nanometer resolution [22] and to investigate how loss of αTAT activity affects microtubule architecture. Individual 15-pf microtubules are approximately 20 µm long and organized in a staggered array that fills the entire neurite [5, 22], which is 500 µm long in wild-type adults. Microtubules in wild-type TRNs are heavily acetylated [23], as revealed by intense labeling with an antibody that specifically recognizes acetylated lysine 40 of α tubulin (αK40Ac) [23, 24] and supported by the observation that both αTATs are expressed in TRNs [20] and localize to the TRN cytoplasm (Figure S1). In mec-12 null mutants, TRNs are devoid of 15-pf microtubules (Table 1, Refs. [14, 25]) and αK40Ac is undetectable [20, 21].
TABLE 1.
Microtubule architecture in wild type and αTAT mutants.
| Genotype | Transgene | n | MTs/section | MT length (µm)§ | pf number† |
|---|---|---|---|---|---|
| wild typea | none | 3 | 62 ± 7 | 20.1 ± 0.4 | wild type |
| mec-12 | none | 3 | 0±0 | 0±0 | n/a |
| atat-2 | none | 3 | 37 ± 17 | 15±2.4 | wild type |
| mec-17 | none | 3 | 8.8 ± 1.6 | 7.5±2.8 | variable |
| mec-17;atat-2 | none | 3 | 7.1 ± 0.6 | 2.29±0.06 | variable |
n = the number of TRNs serially sectioned and examined for each genotype. Each serial section data set consisted of at least 60 ultrathin sections; individual sections were 50-nm thick.
Data derived from an HPF-FS serial section dataset reported in Ref. 22.
Average microtubule length was calculated using the formula L = 2Na/T where L is average microtubule length, N is the average number of microtubules per section, a is the length of the series, and T is the total number of microtubule terminations [5].
wild type = most microtubules have 15 protofilaments, though rare 11-pf microtubules are present; variable = microtubules have 9 to 16 protofilaments.
We counted the number of microtubules in our serial section data sets and used these counts to estimate the average microtubule length, as described previously [5]. Loss of αTAT activity decreased both microtubule number and length (Table 1), suggesting that αK40Ac enables the formation and retention of long microtubules. Microtubules in wild-type TRNs are linked into a bundle by fine filaments [22]. Despite the decrease in microtubule number, inter-microtubule links were retained in αTAT mutants: the average length of such links was 7.8 ± 2.0 nm (mean ± s.d., n = 173) and 7.3 ± 1.9 nm (n = 185) in wild-type and mec-17;atat-2 double mutants, respectively (p = 0.02, Student’s t-test). Taken together, these findings indicate that αTAT enzymes and αK40 acetylation are needed to stabilize microtubule length and number and that the factors regulating individual microtubules are distinct from those organizing the bundle.
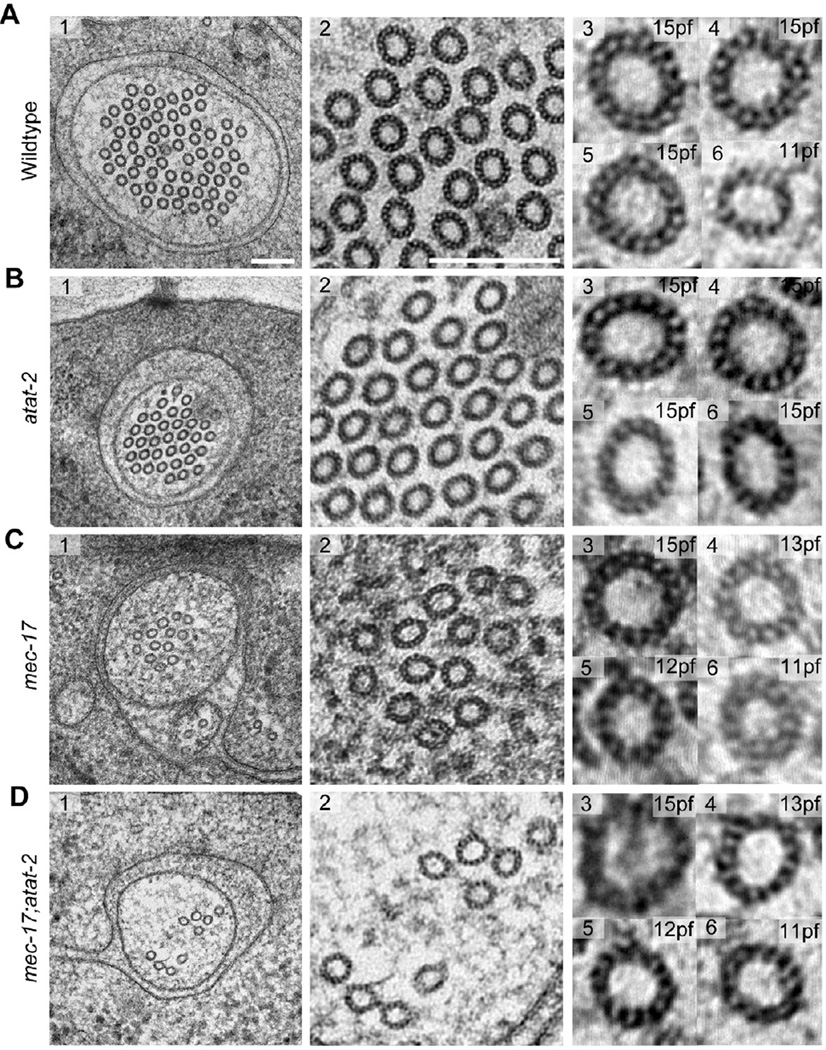
Disrupting the mec-17 gene also diversified the number of protofilaments contained in each microtubule (Figure 1C, D). To examine this in detail, we developed a novel post-staining technique (see Methods) and applied it to our high-pressure frozen, freeze-substituted (HPF-FS) samples. This technique allowed for direct visualization of microtubule protofilaments and provided resolution comparable to classical, tannic acid-based staining methods applied to thin sections. In agreement with previous studies [5, 22], the vast majority (>95%) of microtubules in wild-type TRNs have 15 protofilaments and the remainder have 11 protofilaments (Figure 1A). Microtubules in atat-2 mutants also consisted primarily of 15-pf microtubules (Figure 1B), suggesting that the modest reduction in TRN αK40Ac levels associated with this mutant [20] does not affect protofilament number. In mec-17 and mec-17;atat-2 mutants where αK40Ac levels in TRNs are essentially undetectable [20], we found polymorphic microtubules consisting of as few as 10 and as many as 16 protofilaments (Figure 1C, D). Until now, such polymorphisms in microtubule quinary structure have only been observed in vitro [4, 26]. Thus, α tubulin K40 acetylation is required to regulate protofilament number in vivo.
Figure 1. Alpha K40 acetylation is required for the formation of the distinctive 15-pf microtubules present in TRNs and for proper cylindrical shape.
High-resolution electron micrographs of 50-nm thin sections of touch receptor neurons in wild type (A), atat-2(ok2415) (B), mec-17(ok2109) (C), and mec-17;atat-2 (D) animals. Scale bars are 150 nm. See also Figure S1 for alpha tubulin acetyltransferase expression pattern in TRNs.
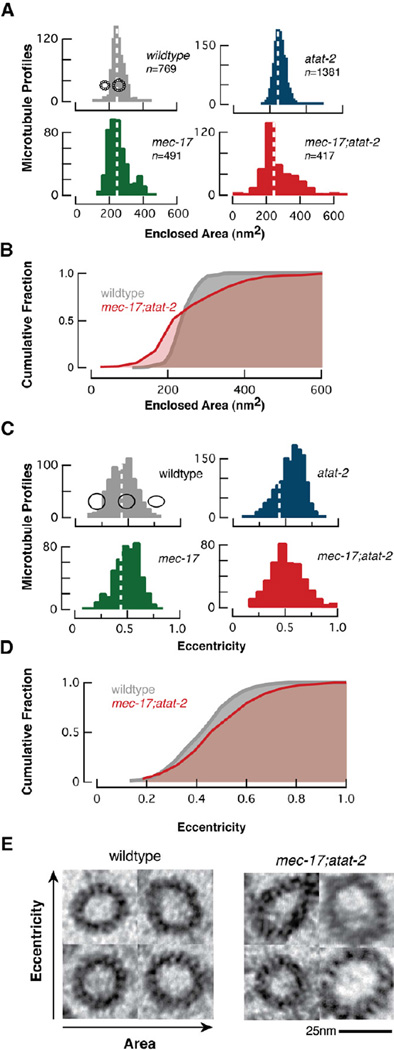
We observed differences in microtubule cross-section shape and area in all mutants. To quantify this variation, we developed a semi-automatic image processing workflow capable of measuring the area and eccentricity of microtubule profiles (see Experimental Procedures). Microtubule profiles were defined by finding a curve running approximately through the centers of the protofilaments; inner and outer circumferences were calculated from this curve assuming the microtubule formed an annulus of 6.5-nm thick protofilaments. Microtubule profiles in wild-type TRNs were essentially circular and had dimensions consistent with previous measurements of 15-pf microtubules in both fixed and frozen preparations [22, 27].
Microtubule size, but not shape, was similar in wild type and atat-2 animals (Figure 2A, C and Figure S2). We found an increase in the number of elliptical microtubule profiles in atat-2 mutants (Figure 2C, Figure S2). To eliminate the possibility that such profiles arose from sectioning cylindrical profiles at oblique angles, we restricted our analysis to data sets in which the TRN plasma membrane could be resolved. We note that our analysis algorithm also excluded individual elliptical microtubule profiles that were not in sharp focus. This establishes that our thin sections are perpendicular to the long axis of the TRNs and the microtubule bundle. Both microtubule size and shape were variable in mec-17 and mec-17;atat-2 mutants (Figure 2, Figure S2). Narrowing our focus to the subset of large microtubules that retained 15 pfs in mutant TRNs revealed an increase in average eccentricity as well as an increase in the proportion of elliptical profiles (Figure 2E). Thus, partial loss of αK40 acetylation may weaken interprotofilament interactions and alter microtubule shape, while complete loss destabilizes quinary structure and enables the formation of microtubules with a variable number of protofilaments.
Figure 2. Loss of αK40Ac alters the size and shape of TRN microtubules.
(A) Histograms showing the distribution of the area enclosed by microtubules in TRNs as a function of genotype. Dotted lines are at 244 nm2, the mean area in wild-type TRNs. Schematics show 15-pf and 11-pf microtubules positioned along the x-axis according to their predicted internal area.
(B) Cumulative distribution functions comparing the size of wild-type and αTAT-deficient mec-17;atat-2 double mutants. αTAT-deficient TRNs have an increase in smaller microtubules and a concomitant decrease in larger ones. The size distribution of mutant microtubules differs from wild type (p<0.001, KS test).
(C) Histograms of microtubule eccentricity as a function of genotype. Dotted lines are at 0.44, the mean eccentricity for wild type microtubules. Profiles show the shape of ellipses with eccentricity values of 0.25, 0.5 and 0.75.
(D) Cumulative distribution functions comparing the shape of wild-type and αTAT-deficient mec-17;atat-2 double mutants. αTAT-deficient microtubules have more elliptical cross-sections.
(E) Fifteen-pf microtubules in wild type (left) and αTAT-deficient double mutants (right). Area increases from left to right; eccentricity increases from bottom to top. The scale bar applies to both panels. See also Figure S2.
Based on the variations in microtubule shape, size, and protofilament number in αTAT mutants (Figures 1, 2), we propose that αK40Ac stabilizes interprotofilament interactions and the bending angle between protofilaments. To investigate the structural basis of the proposed effect of αK40 acetylation on lateral interactions between protofilaments, we generated a homology model of two neighboring MEC-12 α tubulin monomers based on the work of Wells and Aksimentiev [28] (Figure S3A). This static model places αK40 close to αE55 in the H1’-S2 loop, suggesting a salt bridge could form between these residues (Figure 3A). The model also reveals that αE55 could form a salt bridge with αH283 in the M-loop of the neighboring α tubulin (Figure 3B), as suggested previously [29]. Upon lattice polymerization, αE55 could thus form a salt bridge either within a single α tubulin monomer (with αK40) or between neighboring α tubulins across the inter-protofilament interface (with αH283). We propose a model in which acetylation of αK40 reduces the probability that αE55 forms an intra-monomer salt bridge and increases the probability that it forms a salt bridge with the neighboring αH283 (Figure 3C). In this model (Figure 3D), αK40Ac strengthens lateral interactions between α tubulins by promoting formation of inter-protofilament salt bridges between adjacent α tubulins. This loop-loop interaction is part of a flexible hinge that permits a wide range of interprotofilament angles required to accommodate different protofilament configurations [30–32]
Figure 3. MEC-12 α-tubulin dimer homology model incorporated into a microtubule lattice.
Two adjacent MEC-12 monomers are colored in light and dark blue and all views are from the luminal side of the inter-protofilament interface. Key resides are shown in space-filling representation: αK40 (green); αE55 and αE90 (red); αK280 and αH283 (orange).
(A, B) αE55 can form an intra-monomer salt bridge with αK40 (A) or an inter-monomer salt bridge with H283 of the neighboring monomer (B).
(C) Following acetylation, αK40 can no longer form a salt bridge and the inter-monomer salt bridge between αE55 and αH283 is favored.
(D) Schematic of the proposed model for αK40 acetylation-mediated regulation of the protofilament lattice. See also Figure S3 and Movies S1–S6 for salt bridge stability probed by molecular dynamics simulations.
We used all-atom molecular dynamics simulations to investigate the feasibility of this structural model. In these simulations, we introduced a salt bridge for each pair of residues by bringing the two residues together with an external force. This force was gradually reduced to zero. A salt bridge was considered to be stable if the two residues remained in sufficient proximity to form a salt bridge after the force was released (Figure S3B). Using this strategy and this criterion for stability, we found that αE55 can form a stable intra-monomer salt bridge with αK40 and a stable inter-monomer salt bridge with αH283 (Movies S1, S2). Following acetylation of αK40, only αE55 and αH283 maintained their electrostatic interaction (Movies S3, S4), providing a structural substrate for interprotofilament stabilization. As a control, we also tested the stability of the inter-monomer salt bridge between αE55 and αK280, since the latter residue is hypothesized to interact with αE90 [32]. As expected, αE55 and αK280 separated within 5 ns of the force being released, confirming that these two residues are unlikely to form a salt bridge (Figure S3B and Movies S5, S6). Formation of the αE55-αH283 inter-monomer salt bridge also increased the contact surface area between the MEC-12 subunits (Figure S3C), suggesting more stabilized interprotofilament interactions in the αK40-modified conformation.
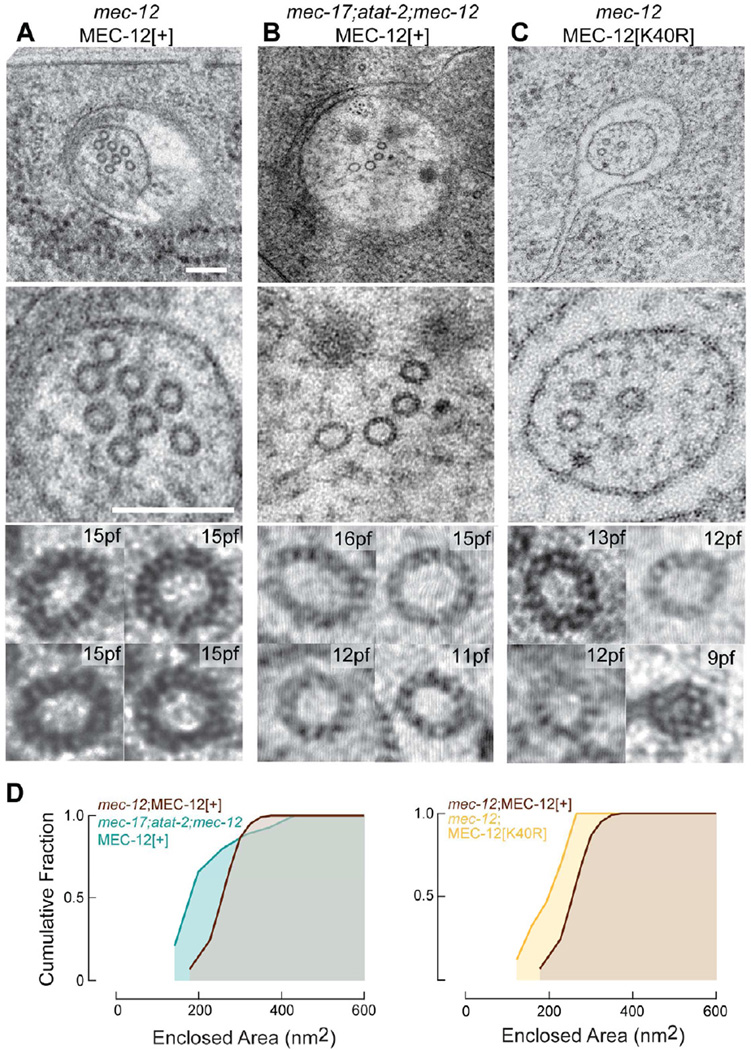
To test whether acetylation of MEC-12[K40] could account for the observed effects on microtubule quinary structure, we used our HPF-FS technique to analyze microtubules in transgenic mutants expressing wild type and mutant MEC-12 isoforms selectively in the touch receptor neurons [20]. As reported previously [14, 25], mec-12 null mutants are devoid of 15-pf microtubules (Table 1). Transgenic expression of MEC-12[+] allowed microtubules containing mostly 15 protofilaments to form in mec-12 mutants (Figure 4A, 4D), but not in mec-17;atat-2;mec-12 triple mutants (Figure 4B, 4D). Thus, transgenic expression of MEC-12[+] enables 15-pf microtubules to form only if the αTAT genes, mec-17 and atat-2, are intact. Despite the recapitulation of wild-type quinary structure in transgenic mec-12;MEC-12[+] animals, we observed significantly fewer microtubules in transgenic than in wild-type TRNs (Tables 1, 2). The exact reason for this difference between intact and transgenic control TRNs is unclear, but a likely possibility is over-expression of UNC-86::MEC-3 binding sites in the mec-17 promoter used to selectively express MEC-12 in the touch receptor neurons [20].
Figure 4. Loss of αTAT genes or αK40 diversifies microtubule quinary structure.
(A) Transgenic expression of MEC-12[+] in mec-12(e1607) null mutants restores essential uniform 15-pf quinary structure. Scale bars are 150 nm.
(B) Transgenic expression of MEC-12[+] in mec-17;atat-2;mec-12 triple mutants results in polymorphic TRN microtubules.
(C) Transgenic MEC-12[K40R] induces polymorphic TRN microtubules in mec-12 mutants.
(D) Cumulative distribution function of microtubule size in transgenic TRNs. Measurements obtained from 3–5 three serial-section datasets. The size distribution of mec-17;atat-2;mec-12;MEC-12[+] (n=115) and mec-12;MEC-12[K40R] (n=90) differ significantly from mec-12;MEC-12[+] (n=205) (p < 0.001, KS test)
TABLE 2.
Microtubule architecture depends on the acetylation state of MEC-12 αK40.
| Genotype | Transgene | n | MTs/section | MT length (µm)§ | pf number† |
|---|---|---|---|---|---|
| mec-12 | MEC-12[+] | 3 | 6.0 ± 0.7 | 14.7±0.7 | wild type |
| mec-12;mec-17;atat-2 | MEC-12[+] | 3 | 3.0 ± 0.7 | 7.9±1.6 | variable |
| mec-12 | MEC-12[K40R] | 5 | 2.2 ± 1.0 | 6.8±3.4 | variable |
n = the number of TRNs serially sectioned and examined for each genotype. Each data set consisted of at least 60 ultrathin (50-nm) serial sections.
Average microtubule length was calculated using the formula L = 2Na/T where L is average microtubule length, N is the average number of microtubules per section, a is the length of the series, and T is the total number of microtubule terminations [5].
wild type = most microtubules have 15 protofilaments, though rare 11-pf microtubules are present; variable = microtubules have 9 to 16 protofilaments.
Reminiscent of the microtubule phenotype observed in αTAT-deficient mec-17;atat-2 double mutants, mec-12 null mutant TRNs expressing MEC-12[K40R] harbored polymorphic microtubules (Figure 4C, 4D). Because this substitution replaces lysine 40 with a positively charged residue that cannot be acetylated [33], this finding suggests that the post-translational modification per se is crucial for the formation or stability of 15-pf microtubules. The polymorphic microtubules in mec-12 mutants expressing MEC-12[K40R] and in mec-17;atat-2;mec-12 triple mutants expressing MEC-12[+] were similar in length and abundance. Both populations of polymorphic microtubules were approximately half as long and three times less abundant than the 15-pf microtubules found in mec-12;MEC-12[+] transgenic animals (Table 2). Collectively, these findings imply that α tubulin acetylation is required to constrain microtubule quinary structure to 15-pf in touch receptor neurons.
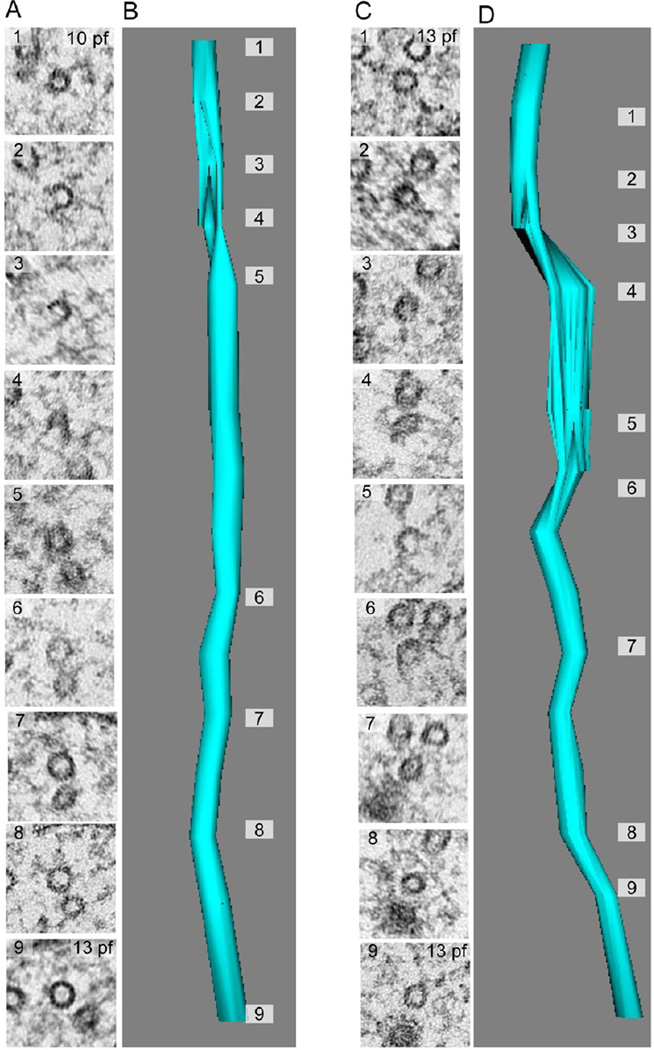
Three-dimensional reconstructions of microtubules from serial sections of mec-17 and mec-17;atat-2 mutants revealed openings in the microtubule lattice (Figure 5) in half (18 out of 35) of the microtubules present in these mutants. Such openings have not been observed in >4,000 EM sections from over a dozen high-pressure frozen, freeze-substituted wild-type TRNs (not shown). While microtubule lattice openings have been reported in vitro [34, 35], to our knowledge, this is the first reported observation of microtubule lattice openings in vivo. Remarkably, many of these lattice openings extend for >150 nm, a length equivalent to nearly twenty tubulin dimers. Lattice defects were detected distal (~1µm) to microtubule endpoints and were frequently associated with a change in protofilament number (Figure 5A). Thus, in the absence of α tubulin acetyltransferase activity, microtubule lattice openings can occur in vivo and the lattice is sufficiently flexible to incorporate multiple quinary structures within a single microtubule. Future studies are needed to discern whether lattice openings and changes in protofilament number arise primarily from defects in polymerization, destabilization of lateral interactions between adjacent protofilaments, or a combination of both factors.
Figure 5. Loss of αTAT activity reveals lattice defects and variations in protofilament number in individual microtubules.
(A, B) Example of a microtubule lattice defect associated with a change in protofilament number on opposite sides of the defect (10 vs. 13).
(C, D) Example of a microtubule lattice defect with no change in protofilament number. Images were selected from two serial-section reconstructions consisting of seventeen (17) consecutive thin (50-nm) sections. The datasets were used to reconstruct two TRN microtubules in mec-17;atat-2 double mutants.
Discussion
Microtubules can contain fewer than ten or as many as sixteen protofilaments and are the target of numerous post-translational modifications. We investigated the contribution of one post-translational modification to microtubule architecture: the transfer of acetyl groups to lysine 40 of α tubulin. This study leveraged two unique features of C. elegans: 1) MEC-12 α tubulin is required to form bundles of distinctive, 15-pf microtubules in TRNs and 2) of the twelve α tubulins encoded by the C. elegans genome, MEC-12 is the only one with lysine at position 40. We report that disrupting the α tubulin acetyltransferase genes mec-17 and atat-2 results in the formation of short, polymorphic microtubules in TRNs and that eliminating lysine 40 by mutating the residue to arginine likewise promotes the formation of short, polymorphic microtubules.
How might αK40Ac regulate protofilament number? Motivated by all-atom molecular dynamics simulations, we propose the following model: transfer of an acetyl group to αK40 disrupts an intramonomer salt bridge (αE55 to αK40) and favors a salt bridge between adjacent α tubulin monomers (αE55 to αH283). The intermonomer salt bridge alters lateral interactions between α-tubulins, leading to inter-protofilament angles consistent with 15-pf.microtubules. In the absence of αK40Ac or if αK40 is replaced with arginine, αE55 might engage in either an intramonomer salt bridge (with αK40 or αR40) or an intermonomer salt bridge (with αH283) and, under these conditions, inter-protofilament angle can assume a wider range of values, leading to elliptical microtubule profiles and variation in protofilament number both within and between microtubules. In principle, the lateral salt bridge could be formed at any α-α tubulin interface since most, if not all α tubulins have either a glutamate or aspartate at position 55 and a histidine at position 283.
To conclude, we have established that loss of α tubulin K40 acetylation removes an essential in vivo constraint on microtubule architecture and used all-atom molecular dynamics simulations to develop a structural model of the mechanism of how enzymatic modification of a protein interface could alter quinary structure. However, since not all cells that co-express αTAT enzymes and K40-bearing α tubulins form 15-protofilament microtubules, additional cell-specific factors that fine-tune inter-protofilament angles and allow cells to count protofilaments remain to be discovered.
Experimental Procedures
Growth conditions and strains
Worms were raised on OP50 E. coli-seeded NGM plates at 20°C, following standard methods [36]. The following wild type and mutant C. elegans strains were used: wild-type (N2), CB1607 mec-12(e1607) III, GN232 mec-17(ok2109) IV, GN233 atat-2(ok2415) X, GN234 mec-17(ok2109) IV; atat-2(ok2415) X, GN239 mec-12(e1607) III; mec-17(ok2109) IV; atat-2(ok2415) X. The following transgenic strains, derived as described previously [20], were used: GN280 mec-12(e1607) III; pgEx46[Pmec-17::MEC-12(+), Punc-122::RFP], GN283 mec-12(e1607) III; pgEx49[Pmec-17::MEC-12(K40R), Punc-122::RFP], GN295 mec-12(e1607) III; mec-17(ok2109) IV; atat-2(ok2415) X; pgEx58[Pmec-17::MEC-12(+), Punc-122::RFP].
Serial-section electron microscopy
Adult nematodes were prepared for electron microscopy as previously described [22]. Briefly, animals were frozen using an EMPACT2 high-pressure freezer system (Leica, Vienna, Austria). A Leica AFS freeze substitution apparatus was used to preserve and embed nematodes in 2% glutaraldehyde plus 1% osmium tetroxide and in Eponate 12/Araldite 502. Serial, ultrathin (50 nm) sections were cut with a diamond knife on a Leica Ultracut S microtome and collected on Formvarcoated copper-slot grids. To enhance contrast, sections were post-stained in 3.5% uranyl acetate (30 sec) and Reynold’s lead citrate preparation (3 min). The grids were imaged on a transmission electron microscope (JEOL TEM 1230, Tokyo, Japan) and images were acquired with an 11-megapixel bottom mounted cooled CCD camera (Orius SC1000, Gatan, Pleasanton, California). Three-dimensional models were generated from serial sections with Reconstruct [37]. Images of consecutive sections were aligned manually.
Computational determination of microtubule geometries
Segmentation proceeded in two steps: first, approximate outlines of the microtubules were identified; second, the outlines were refined based on local intensity distributions to accurately quantify area, perimeter, and eccentricity. EM images were filtered to smooth variations at length scales larger than a typical microtubule and to attenuate structures smaller than the width of a protofilament. Each image was then contrast-adjusted and a Gaussian filter was applied to smooth the image and remove pixel-to-pixel variation. All further operations were performed on the negative of the image so that the microtubules appeared as bright regions. The background was subtracted using morphological opening with a disk element of a similar size to the protofilament width and the image was thresholded to create a binary image. Objects in the binary image were then thinned to lines and spur pixels were removed until no further changes occurred. The remaining closed curves were filtered based on size to identify microtubules. Microtubules that exhibited significant bending within the section appeared blurred and hence were ignored. All image processing was performed in Matlab (The Mathworks, Natick, MA).
The eccentricity of microtubules was calculated to provide a measure of the noncircularity of microtubules. The eccentricity of an ellipse is defined as (1 − r2minor/r2major)1/2, where rminor and rmajor are the minor and major axes, respectively. The eccentricity is invariant upon uniform scaling of the shape, and is bounded by 0 (a circle) and 1 (a line).
Homology model construction and molecular dynamics simulations
The atomic model of C. elegans MEC-12 was constructed based on a previous model of B. taurus α-tubulin dimer [28]. This B. taurus α-tubulin dimer model incorporated available crystallographic structures [30, 38, 39], as well as the structural information in a cryo-EM map of a microtubule at 8 Å resolution [32]. The B. taurus α-tubulin and C. elegans MEC-12 share more than 90% sequence identity, therefore homology modeling was straightforward. After the necessary mutations were introduced in the B. taurus α-tubulin dimer model [28] to transform it into a C. elegans MEC-12 dimer model, energy minimization was performed for 5000 steps with the molecular dynamics package NAMD [40] to avoid clashes between side chains. Construction of the homology model was performed with the molecular rendering software VMD [41]. All molecular dynamics simulations were performed using NAMD [40]with protein and ion molecules described by the CHARMM27 force field with CMAP corrections [42–44], and water molecules described with the TIP3P model [45]. Standard simulation parameters were used [40].
In each simulation, an external force was used to bring the two residues into proximity and was gradually reduced to zero at 4.5 ns, followed by nearly 5 ns of simulation in the absence of any force to determine if the amino acid pair maintained its interaction. To reduce the degrees of freedom of the simulated systems, most protein Cα atoms were constrained except the following amino acids: residues 35 to 60 (residing on the flexible H1’-S2 loop and not resolved in crystal structure [30, 38] and residues 272 to 287 (residing on the M loop, which required structural modification to match an EM density [46]). Molecular trajectories of the simulations are included as Movies in Supplemental Information.
Supplementary Material
Highlights.
αTAT mutants have short microtubules and variable protofilament number
α tubulin K40 acetylation promotes inter-protofilament salt bridges
Acetylation of α tubulin K40 is a key constraint on protofilament number in native microtubules
Acknowledgements
We thank C. Gao for worm injection; S. Watanabe and E. Jorgensen (University of Utah) for high-pressure freezing some samples while Stanford’s equipment was being repaired; the Stanford Cell Sciences Imaging Facility for their EM facility; M. Chalfie and the Caenorhabditis Genetics Center for strains. Work was supported by NIH grants RO1NS047715 (MBG), RO1EB006745 (MBG), and DP2OD006466 (KCH), NSF grant EF1038697 (KCH), and a Stanford University School of Medicine Dean’s Postdoctoral Fellowship (JH). Molecular dynamics simulations performed using the Extreme Science and Engineering Discovery Environment (XSEDE), supported by the NSF OCI-1053575, under allocation number TG-MCB110056 (JH and KCH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burton PR, Hinkley RE, Pierson GB. Tannic acid-stained microtubules with 12, 13, and 15 protofilaments. J. Cell Biol. 1975;65:227–233. doi: 10.1083/jcb.65.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tilney LG, Bryan J, Bush DJ, Fujiwara K, Mooseker MS, Murphy DB, Snyder DH. Microtubules: evidence for 13 protofilaments. J. Cell Biol. 1973;59:267–275. doi: 10.1083/jcb.59.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKean PG, Vaughan S, Gull K. The extended tubulin superfamily. J. Cell. Sci. 2001;114:2723–2733. doi: 10.1242/jcs.114.15.2723. [DOI] [PubMed] [Google Scholar]
- 4.Pierson GB, Burton PR, Himes RH. Alterations in number of protofilaments in microtubules assembled in vitro. J. Cell Biol. 1978;76:223–228. doi: 10.1083/jcb.76.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalfie M, Thomson JN. Organization of neuronal microtubules in the nematode Caenorhabditis elegans. J. Cell Biol. 1979;82:278–289. doi: 10.1083/jcb.82.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito K, Hama K. Structural diversity of microtubules in the supporting cells of the sensory epithelium of guinea pig organ of Corti. J Electron Microsc (Tokyo) 1982;31:278–281. [PubMed] [Google Scholar]
- 7.Kikuchi T, Takasaka T, Tonosaki A, Watanabe H, Hozawa K, Shinkawa H, Wada H. Microtubule subunits of guinea pig vestibular epithelial cells. Acta Otolaryngol Suppl. 1991;481:107–111. doi: 10.3109/00016489109131359. [DOI] [PubMed] [Google Scholar]
- 8.Mogensen MM, Tucker JB. Evidence for microtubule nucleation at plasma membrane-associated sites in Drosophila. J. Cell. Sci. 1987;88(Pt 1):95–107. doi: 10.1242/jcs.88.1.95. [DOI] [PubMed] [Google Scholar]
- 9.Bounoutas A, Kratz J, Emtage L, Ma C, Nguyen KC, Chalfie M. Microtubule depolymerization in Caenorhabditis elegans touch receptor neurons reduces gene expression through a p38 MAPK pathway. Proc. Natl. Acad. Sci. U.S.A. 2011;108:3982–3987. doi: 10.1073/pnas.1101360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalfie M, Sulston J. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev. Biol. 1981;82:358–370. doi: 10.1016/0012-1606(81)90459-0. [DOI] [PubMed] [Google Scholar]
- 11.Savage C, Hamelin M, Culotti JG, Coulson A, Albertson DG, Chalfie M. mec-7 is a beta-tubulin gene required for the production of 15-protofilament microtubules in Caenorhabditis elegans. Genes & Development. 1989;3:870–881. doi: 10.1101/gad.3.6.870. [DOI] [PubMed] [Google Scholar]
- 12.Fukushige T, Siddiqui ZK, Chou M, Culotti JG, Gogonea CB, Siddiqui SS, Hamelin M. MEC-12, an alpha-tubulin required for touch sensitivity in C. elegans. J. Cell. Sci. 1999;112(Pt 3):395–403. doi: 10.1242/jcs.112.3.395. [DOI] [PubMed] [Google Scholar]
- 13.O'Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat. Neurosci. 2004;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- 14.Bounoutas A, Hagan RO, Chalfie M. The multipurpose 15-protofilament microtubules in C. elegans have specific roles in mechanosensation. Curr. Biol. 2009:1–6. doi: 10.1016/j.cub.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wade RH. On and around microtubules: an overview. Mol. Biotechnol. 2009;43:177–191. doi: 10.1007/s12033-009-9193-5. [DOI] [PubMed] [Google Scholar]
- 16.Hamelin M, Scott IM, Way JC, Culotti JG. The mec-7 beta-tubulin gene of Caenorhabditis elegans is expressed primarily in the touch receptor neurons. EMBO J. 1992;11:2885–2893. doi: 10.1002/j.1460-2075.1992.tb05357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verhey KJ, Gaertig J. The tubulin code. Cell Cycle. 2007;6:2152–2160. doi: 10.4161/cc.6.17.4633. [DOI] [PubMed] [Google Scholar]
- 18.Janke C, Kneussel M. Tubulin post-translational modifications: encoding functions on the neuronal microtubule cytoskeleton. Trends in Neurosciences. 2010;33:362–372. doi: 10.1016/j.tins.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Lee J-S, Smith E, Shilatifard A. The language of histone crosstalk. Cell. 2010;142:682–685. doi: 10.1016/j.cell.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV. The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc. Natl. Acad. Sci. U.S.A. 2010;107:21517–21522. doi: 10.1073/pnas.1013728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, Dougan ST, Kipreos ET, Gaertig J. MEC-17 is an α-tubulin acetyltransferase. Nature. 2010;467:218–222. doi: 10.1038/nature09324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cueva JG, Mulholland A, Goodman MB. Nanoscale organization of the MEC-4 DEG/ENaC sensory mechanotransduction channel in Caenorhabditis elegans touch receptor neurons. J. Neurosci. 2007;27:14089–14098. doi: 10.1523/JNEUROSCI.4179-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddiqui SS, Aamodt E, Rastinejad F, Culotti J. Anti-tubulin monoclonal antibodies that bind to specific neurons in Caenorhabditis elegans. Journal of Neuroscience. 1989;9:2963–2972. doi: 10.1523/JNEUROSCI.09-08-02963.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piperno G, Fuller MT. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J. Cell Biol. 1985;101:2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chalfie M, Au M. Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons. Science. 1989;243:1027–1033. doi: 10.1126/science.2646709. [DOI] [PubMed] [Google Scholar]
- 26.Unger E, Böhm KJ, Vater W. Structural diversity and dynamics of microtubules and polymorphic tubulin assemblies. Electron Microsc. Rev. 1990;3:355–395. doi: 10.1016/0892-0354(90)90007-f. [DOI] [PubMed] [Google Scholar]
- 27.Chalfie M, Thomson JN. Structural and functional diversity in the neuronal microtubules of Caenorhabditis elegans. J. Cell Biol. 1982;93:15–23. doi: 10.1083/jcb.93.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells DB, Aksimentiev A. Mechanical properties of a complete microtubule revealed through molecular dynamics simulation. Biophysical Journal. 2010;99:629–637. doi: 10.1016/j.bpj.2010.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, DeRosier DJ, Nicholson WV, Nogales E, Downing KH. Microtubule structure at 8 A resolution. Structure. 2002;10:1317–1328. doi: 10.1016/s0969-2126(02)00827-4. [DOI] [PubMed] [Google Scholar]
- 30.Löwe J, Li H, Downing KH, Nogales E. Refined structure of alpha beta-tubulin at 3.5 A resolution. J. Mol. Biol. 2001;313:1045–1057. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- 31.Nogales E, Whittaker M, Milligan R, Downing K. High-resolution model of the microtubule. Cell. 1999;96:79–88. doi: 10.1016/s0092-8674(00)80961-7. [DOI] [PubMed] [Google Scholar]
- 32.Sui H, Downing KH. Structural basis of interprotofilament interaction and lateral deformation of microtubules. Structure. 2010;18:1022–1031. doi: 10.1016/j.str.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaertig J, Cruz MA, Bowen J, Gu L, Pennock DG, Gorovsky MA. Acetylation of lysine 40 in alpha-tubulin is not essential in Tetrahymena thermophila. J. Cell Biol. 1995;129:1301–1310. doi: 10.1083/jcb.129.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chrétien D, Metoz F, Verde F, Karsenti E, Wade RH. Lattice defects in microtubules: protofilament numbers vary within individual microtubules. J. Cell Biol. 1992;117:1031–1040. doi: 10.1083/jcb.117.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chrétien D. Microtubules switch occasionally into unfavorable configurations during elongation1. J. Mol. Biol. 2000;298:663–676. doi: 10.1006/jmbi.2000.3696. [DOI] [PubMed] [Google Scholar]
- 36.Stiernagle T. Maintenance of C. elegans. WormBook. 2006:1–11. doi: 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiala JC. Reconstruct: a free editor for serial section microscopy. J Microsc. 2005;218:52–61. doi: 10.1111/j.1365-2818.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- 38.Nogales E, Wolf SG, Downing KH. Structure of the alpha beta tubulin dimer by electron crystallography. Nature. 1998;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 39.Ravelli RBG, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature. 2004;428:198–202. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 40.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, Schulten K. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–38. 27–28. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 42.MacKerell AD, Bashford D, Bellott, Dunbrack RL, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 43.Foloppe N, Mackerell AD., Jr All-atom empirical force field for nucleic acids: I. Parameter optimization based on small molecule and condensed phase macromolecular target data. J Comput Chem. 2000;21:86–104. [Google Scholar]
- 44.Mackerell AD, Jr, Feig M, Brooks CL., III Extending the treatment of backbone energetics in protein force fields: Limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J Comput Chem. 2004;25:1400–1415. doi: 10.1002/jcc.20065. [DOI] [PubMed] [Google Scholar]
- 45.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983;79:926–935. [Google Scholar]
- 46.Sui H, Downing KH. Molecular architecture of axonemal microtubule doublets revealed by cryo-electron tomography. Nature. 2006;442:475–478. doi: 10.1038/nature04816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.