Abstract
Background
Compared to the open approach, randomized trials have shown that laparoscopic colectomy is associated with a shorter hospitalization without increases in morbidity or mortality rates. With broader adoption of laparoscopic colectomy for cancer in the United States, it is unclear if laparoscopic colectomy continues to be associated with shorter hospitalization and comparable morbidity.
Purpose
To determine if hospitals where a greater proportion of colon resections for cancer are approached laparoscopically (laparoscopy rate) achieve improved short-term outcomes compared to hospitals with lower laparoscopy rates.
Methods
From the 2008–2009 Nationwide Inpatient Sample, we identified hospitals where ≤12 colon resections for cancer where reported with ≥1 approached laparoscopically. We assessed the correlation between a hospital’s laparoscopy rate and risk-standardized outcomes (intra- and post-operative morbidity, in-hospital mortality rates, and average length of stay).
Results
Overall, 6,806 colon resections were performed at 276 hospitals. Variation was noted in hospital laparoscopy rates (median=52.0%, range=3.8–100%) and risk-standardized intra- (2.7%, 1.8–8.6%) and post-operative morbidity (27.8%, 16.4–53.4%), in-hospital mortality (0.7%, 0.3–42.0%), and average length of stay (7.0 days, 4.9–10.3 days). While no association was noted with in-hospital mortality, higher laparoscopy rates were correlated with lower post-operative morbidity (correlation coefficient [r]=−0.12, p=0.04) and shorter hospital stays (r=−0.23, p<0.001), but higher intra-operative morbidity (r=0.19, p<0.001) rates. This was not observed among hospitals with high procedure volumes.
Conclusions
Higher laparoscopy rates were associated with only slightly lower post-operative morbidity rates and modestly shorter hospitalizations.
Keywords: Laparoscopic colectomy, surgical outcomes
Introduction
Following early reports describing laparoscopic colon resection,1–2 widespread adoption of the technique was slowed by concerns over patient safety and oncologic effectiveness3–4 prompting a series of prospective, randomized trials.5–7 Results from these trials demonstrated that, in experienced hands, laparoscopy achieved comparable oncologic results8 with improved short-term outcomes compared to open surgery.9 Based on these findings, the laparoscopic approach to colon resection has seen a marked increase in adoption over the last several years.10 By 2009, nearly half of all colon resections performed for cancer in the United States were being approached laparoscopically.11
As laparoscopic colon resection for the treatment of cancer gains prominence, it is important to ensure that the promising outcomes of clinical trials are being realized in clinical practice.12 This is particularly important as advanced surgical procedures may be associated with more frequent intra-operative complications during early phases of adoption.13 The most recent reports evaluating outcomes in clinical practice suggested laparoscopy was associated with a substantial reduction in mortality14 and overall morbidity15–16 compared to open surgery. However, these reports may have been susceptible to bias through an inability to accurately identify laparoscopic cases. At the time of prior studies, it was estimated that fewer than 10% of cases were approached laparoscopically.17–18
With laparoscopy accounting for nearly half of all cases performed for cancer, it is unknown whether outcomes remain comparable between the open and laparoscopic approaches in clinical practice. We therefore conducted a hospital-level analysis to compare the proportion of colon resections approached laparoscopically (a hospital’s laparoscopy rate) with the risk-standardized outcomes achieved during the hospital encounter. In this manner, we determined if higher laparoscopy rates were associated with more, or less, frequent intra-operative complications, post-operative morbidity, in-hospital mortality, and hospital length of stay. Because outcomes may be related to laparoscopic colon resection-specific volume or overall colectomy volume, we evaluated these outcomes among hospitals in the highest quartile for each.
Methods
We used the 2008–2009 Nationwide Inpatient Sample (NIS) for this study. Data for the NIS are collected at the state level from hospital discharge records, reported to the Agency for Healthcare Research and Quality (AHRQ), and made publicly available via the Healthcare Cost and Utilization Project. The records are drawn from approximately 1,000 non-Federal, short-term, general and specialty hospitals, and include nearly 8 million discharges per year. Hospitals that are sampled contribute 100% of their discharges during the study period to the database, allowing for annual case volume to be determined. Each discharge abstract includes socio-demographic, hospital, and clinical variables, including up to 25 diagnostic and 15 procedure codes.19
Patient and hospital selection
First, we identified all patients aged ≥ 18 years that underwent a colon resection for a diagnosis of colon cancer (ICD-9-CM codes 153.0–153.4, 153.6–153.9, 230.3) between October 1, 2008, and October 31, 2009 during an elective admission. To be consistent with prior studies,20 we excluded patients who required a total or transverse colectomy or had concurrent procedures (hepatic, small bowel, pancreas, or bladder resection), were pregnant, or had evidence of metastatic disease. Patients were then categorized as having undergone a laparoscopic (ICD-9-CM codes 17.3, 17.31–17.39) or an open (ICD-9-CM codes 45.7, 45.71–45.79) colon resection. For the purposes of this study, cases with evidence of a procedure converted to open (ICD-9-CM codes V64.40, V64.41) were considered to have had a laparoscopic procedure and counted in this group. From this sample, we excluded patients treated at hospitals with an annual volume less than 12 cases and at hospitals where no cases were approached laparoscopically. This was done to reduce the likelihood of administrative coding errors, creating a more homogenous sample of hospitals, and allow for more reasonable approximation of a hospital’s laparoscopy rate.
Defining the outcomes
We defined four outcomes for the hospital encounter: intra-operative morbidity, postoperative morbidity, in-hospital mortality, and length of stay. To assess intra-operative and postoperative morbidity, we used a previously reported ICD-9-CM coding algorithm21 which defines complications based on diagnostic and procedural coding (Appendix 1). Intra-operative morbidity included accidental puncture or laceration of structures and hemorrhage complicating a procedure; post-operative morbidity included mechanical wound, infectious, urinary, pulmonary, gastrointestinal, and cardiovascular complications. Variables indicating in-hospital mortality and length of stay are available in the NIS data set.
Covariates
Additional covariates were collected to describe the patient population and for use in subsequent risk adjustment. Patient characteristics included age, sex, race (white, black, other, or missing), primary payer status (private insurance, Medicare, Medicaid, or other), and 23 of the 29 comorbid conditions previously defined by Elixhauser et al, including the presence of node positive or metastatic disease.22 Of the six conditions excluded, “history of a solid tumor” applied to all patients and would not contribute to risk adjustment. The remaining five conditions were present in less than 1.0% of the total population (AIDS or HIV = 0.04%; peptic ulcer disease = 0.10%; drug abuse = 0.25%; lymphoma = 0.75%; paralysis = 0.90%). In addition, we identified the anatomic resection performed (right hemi-colectomy, left hemi-colectomy, or “other”; see Appendix 1 for ICD-9-CM codes).
Statistical Analysis
First, all patient characteristics and patient-level outcomes were presented using descriptive statistics, both overall and by surgical approach. Comparisons between the open and laparoscopic groups were made with the chi-squared test (for categorical variables) or the t-test (for continuous variables). Second, we aggregated the data to the hospital level and calculated observed outcome rates (intra-operative morbidity, post-operative morbidity, in-hospital mortality) and average lengths of stay. Third, we calculated hospitals’ risk-standardized outcome rates. The hospital-level, risk-standardized rates (for intra-operative morbidity, post-operative morbidity, in-hospital mortality) were derived from hierarchical logistic regression models that adjusted for age, sex, race, primary payer status, the individual co-morbid medical conditions previously described (except for in-hospital mortality, where the conditions were categorized as 0, 1–2, or 3+), and a hospital-specific random effect. The rates are calculated as the ratio of the number of “predicted” outcomes (obtained from a model applying the hospital-specific effect) to the number of “expected” outcomes (obtained from a model applying the average effect among hospitals), multiplied by the unadjusted rate for the entire sample. We specified a binary distribution for each dichotomous outcome. Hospital-level length of stay were risk-standardized in the same way, except a negative binomial was specified in the hierarchical regression model.23 Next, the laparoscopy rate for each hospital was determined by dividing the number of laparoscopic colon resections performed for cancer (numerator) by the total number of open and laparoscopic colon resections performed for cancer (denominator). We then assessed the correlation between hospital laparoscopy rate and each risk-standardized outcome, weighting by hospital volume. The weighted correlation coefficient (r) was then squared and multiplied by 100 to express the percentage of variation in a given outcome that was explained by the laparoscopy rate.24 Finally, because procedural experience may be related to outcomes, we conducted a sensitivity analysis, we limited the data set to, 1) hospitals in the highest quartile for laparoscopic colectomy volume specifically (>15 laparoscopy cases/year); and 2) overall colectomy volume (> 30 colectomy cases) during the study period.
All analyses were conducted using SAS version 9.2 (SAS Institute, Cary, North Carolina). Hierarchical models were fitted by using the GLIMMIX procedure in SAS. All statistical tests were 2-sided and p-values <0.05 were considered significant. Because this study used publicly available data that does not include patient identifiers, it was considered exempt from review by the Yale University Human Investigations Committee.
Results
Our sample included 6,806 patients, who were treated at 276 hospitals across the United States. Sample characteristics are summarized in Table 1. On average, patients were aged 68.3 years, were predominantly white (67.5%), and underwent a right hemi-colectomy (57.4%). Over a quarter (27.5%) of patients had evidence of node positive or metastatic disease. In this sample, the surgical procedure was approached laparoscopically in 50.1% of cases (n=3,411), with 12.1% (n=414) coded as having been converted to open. Patients undergoing a laparoscopic procedure were significantly less likely to have Medicaid as their primary form of insurance (1.8% vs. 3.2%, p<0.001), to have competing medical co-morbidity (p<0.001), and to have evidence of node positive or metastatic disease (23.0% vs. 32.0%, p<0.001). In addition, compared with the open group, laparoscopic patients had significantly lower post-operative morbidity (23.3% vs. 32.1%, p<0.001), similar in-hospital mortality (0.7% vs. 1.2%, p=0.05), and shorter hospitalizations (6.0 days vs. 8.1 days, p<0.001).
Table 1.
Patient characteristics and outcomes according to surgical approach
| Overall | Open | Laparoscopic | p-value | |
|---|---|---|---|---|
| Patients, N (%) | 6,806 (100.0) | 3,395 (49.9) | 3,411 (50.1) | - |
| Age in years, mean ± SD | 68.3 ± 12.8 | 68.6 ± 13.0 | 68.1 ± 12.6 | 0.19 |
| Female (%) | 51.1 | 51.9 | 50.3 | 0.19 |
| White race (%) | 67.5 | 67.9 | 67.1 | 0.47 |
| Medicare (%) | 58.8 | 60.0 | 57.7 | 0.05 |
| Medicaid (%) | 2.5 | 3.2 | 1.8 | <0.001 |
| Comorbid medical conditions (%)a | <0.001 | |||
| No medical co-morbidity | 16.6 | 14.6 | 18.6 | |
| 1 to 2 conditions | 50.2 | 47.5 | 52.9 | |
| 3 or more conditions | 33.2 | 37.9 | 28.6 | |
| Node positive or metastatic cancer (%) | 27.5 | 32.0 | 23.0 | <0.001 |
| Anatomic Resection (%) | <0.001 | |||
| Right hemicolectomy | 57.4 | 52.6 | 62.2 | |
| Left hemi-colectomy | 31.3 | 32.6 | 30.1 | |
| Total or transverse colectomy | 11.3 | 14.9 | 7.7 | |
| Patient-level outcomes | ||||
| Intra-operative morbidityb | 3.1 | 3.3 | 3.0 | 0.55 |
| Post-operative morbidityc | 27.7 | 32.1 | 23.3 | <0.001 |
| In-hospital mortality | 1.0 | 1.2 | 0.7 | 0.05 |
| Hospital length of stay in days, mean ± SD | 7.0 ± 5.7 | 8.1 ± 6.1 | 6.0 ± 4.9 | <0.001 |
Comorbid conditions included alcohol abuse, deficiency anemias, rheumatoid arthritis, collagen vascular diseases, chronic blood loss anemia, congestive heart failure, chronic pulmonary disease, coagulopathy, depression, diabetes with and without complications, hypertension with and without complications, hypothyroidism, liver disease, fluid and electrolyte disorders, neurological disorders, obesity, peripheral vascular disease, psychoses, pulmonary circulatory disorders, renal failure, cardiac valvular disease, and recent weight loss
Includes accidental puncture, laceration, and hemorrhage complicating a procedure
Includes mechanical wound, infectious, urinary, pulmonary, gastrointestinal, and cardiovascular complications
Observed hospital laparoscopy rates and outcomes
The median hospital-level laparoscopy rate was 52.0% but varied substantially across the 276 hospitals, with a range of 96.2% (Table 2; minimum=3.8%, maximum=100.0%). Similarly, observed intra-operative morbidity (median=0%, range=23.1%), post-operative morbidity (median=25.8%, range=83.3%), in-hospital mortality (median=0.0%, range=16.7%), and average length of stay varied widely across hospitals.
Table 2.
Variation in hospital-level laparoscopy and outcome rates (N = 276 hospitals)
| Laparoscopy rate (%) | Intra-operative morbidity rate | Post-operative morbidity rate | In-hospital mortality rate | Average length of stay (days) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Observed | Risk standardized | Observed | Risk standardized | Observed | Risk standardized | Observed | Risk standardized | ||
| Max | 100.0% | 23.1% | 8.6% | 83.3% | 53.4% | 16.7% | 42.0% | 20.7 | 10.3 |
|
| |||||||||
| 75th percentile | 66.7% | 5.3% | 3.6% | 35.7% | 32.2% | 0.0% | 0.8% | 8.1 | 7.5 |
|
| |||||||||
| 50th percentile (Median) | 52.0% | 0.0% | 2.7% | 25.8% | 27.8% | 0.0% | 0.7% | 6.7 | 7.0 |
|
| |||||||||
| 25th percentile | 31.5% | 0.0% | 2.5% | 18.8% | 24.3% | 0.0% | 0.6% | 5.9 | 6.6 |
|
| |||||||||
| Minimum | 3.8% | 0.0% | 1.8% | 0.0% | 16.4% | 0.0% | 0.3% | 3.8 | 4.9 |
Association between a hospital’s risk-standardized outcomes and laparoscopy rate
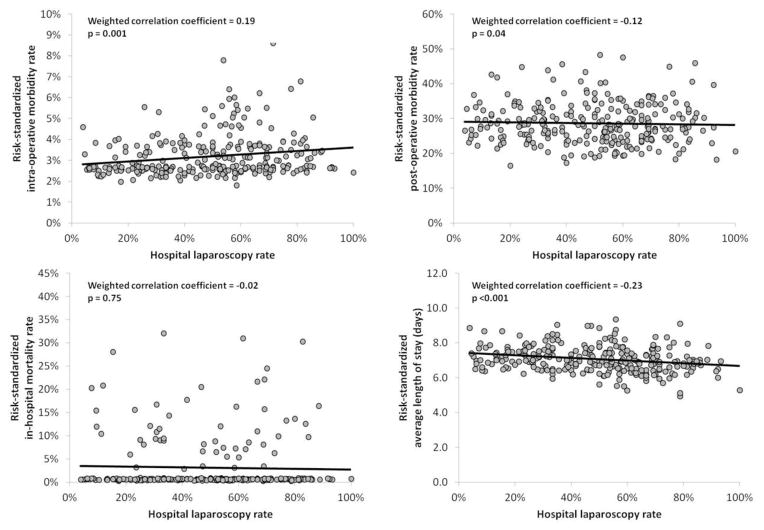
Results of the overall correlation analyses are presented in Figure 1. There was a significant, positive correlation between a hospital’s risk-standardized intra-operative morbidity rate and its laparoscopy rate (r=0.19, p=0.001). However, this correlation was weak, with the laparoscopy rate accounting for only 3.6% of the variation in hospital intra-operative morbidity rates. For example, the difference in the risk-standardized intra-operative morbidity rate between hospitals in the lowest (<31.5%) and highest quartile (≥66.7%) was small (lowest quartile=2.9% vs. highest quartile=3.3%). Additionally, there were significant correlations between higher laparoscopy rates and less frequent risk-standardized, post-operative morbidity (r= − 0.12, p=0.04), as well as shorter average length of stay (r= − 0.23, p<0.001). Again, these relationships were quantitatively small with laparoscopy rates only accounting for 1.4% and 5.3% of the variation in post-operative morbidity and length of stay respectively.
Figure 1.
Scatter plot and correlation between a hospital’s risk-standardized outcomes (y-axis) and the proportion of colectomy cases approached laparoscopically at that hospital (x-axis = laparoscopy rate) in the overall sample (N = 276 hospitals).
Sensitivity Analysis
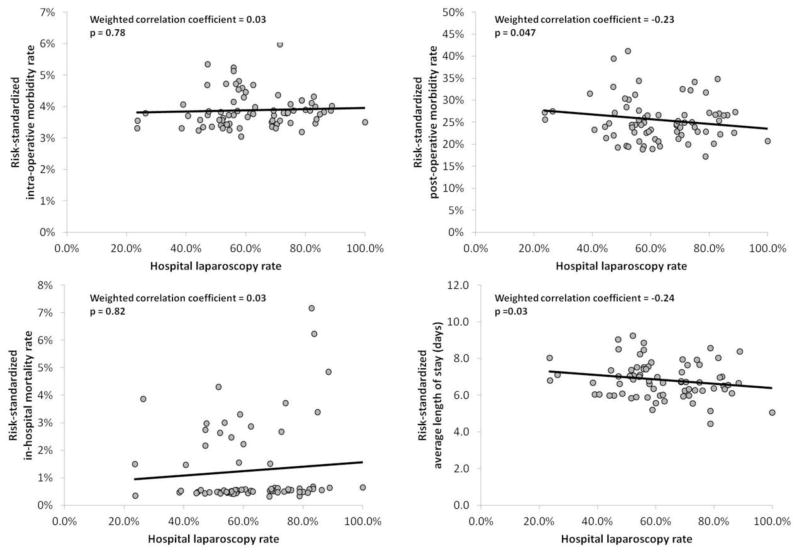
Because procedural experience may influence surgical outcomes, we restricted the analysis to hospitals in the highest volume quartile for the number of laparoscopic colon resections performed for cancer (15+ annually). Among this sub-sample of 3,156 patients treated at 74 hospitals, there was no significant correlation between laparoscopy and risk-standardized intra-operative morbidity rates (r=0.03, p=0.78). Consistent with the overall sample, the laparoscopy rate was correlated with less frequent risk-standardized, post-operative morbidity (r= − 0.23, p=0.047) and shorter, risk-standardized average length of stay (r= − 0.24, p=0.03). No correlation was noted between laparoscopy and risk-standardized, in-hospital mortality rates (see Figure 2). We noted similar findings when outcomes were evaluated among hospitals in the highest volume quartile for open and laparoscopic colon resection combined (30+ annually) with no correlation between laparoscopy rate and intra-operative morbidity or inhospital mortality, but a modest correlation with less frequent post-operative morbidity and shorter average length of stay (data not shown). Additionally, including patient-specific variables for the anatomic resection performed or hospital-specific variables for teaching status or rural-urban designation did not significantly alter these findings.
Figure 2.
Scatter plot and correlation between a hospital’s risk-standardized outcomes (y-axis) and the proportion of colectomy cases approached laparoscopically at that hospital (x-axis = laparoscopy rate) among hospitals (N=74) in the highest quartile of laparoscopic-specific.
Discussion
While nearly half of colon resections for cancer were approached laparoscopically in the overall sample, the hospital-level laparoscopy rate varied substantially. Hospitals with higher laparoscopy rates achieved modestly shorter hospital stays. However, the morbidity outcomes were mixed: hospitals with higher laparoscopy rates tended to have higher risk-standardized, intra-operative morbidity rates, but lower post-operative morbidity. However, the ascertainment of post-operative morbidity may be different in laparoscopy and open cases given the shorter hospital stays with laparoscopy. Further, when this relationship was evaluated among hospitals in the highest quartile of laparoscopic colon resection volume, the relationship was no longer significant suggesting procedure-specific experience may be related to this outcome.
These findings are comparable to outcomes achieved in clinical trials: similar overall post-operative morbidity and mortality between operative approaches with shorter hospitalizations among those treated laparoscopically.25–27 Similarly, a recent meta-analysis has suggested more frequent intra-operative complications with the laparoscopic approach.13 The fact that we did not observe a significant relationship between laparoscopy rate and higher postoperative morbidity or in-hospital mortality, is a noteworthy finding. As this procedure has moved from the more idealized environment of clinical trials to clinical practice, we observe similar results.
Prior hospital-level studies have evaluated outcomes after colon resection for cancer in terms of mortality, length of stay, and 30-day readmission rates. These studies were conducted primarily among the Medicare population in the late 1990’s and the correlation was with overall hospital volume. At that time, hospitals with higher case volumes were noted to have comparable mortality rates and length of stay, but lower 30-day readmission rates.28–30 In the current study, we found a trend toward improved, in-hospital outcomes, excluding mortality, with higher laparoscopy rates even while considering overall hospital volume. While we were unable to evaluate outcomes beyond the initial hospitalization, it has been suggested that laparoscopy may be associated with fewer hospital readmissions.31 This outcome warrants further investigation as to ensure shorter hospitalizations are not gained at the expense of more frequent readmissions.
These data suggest less dramatic benefits than have been previously described from patient-level analyses.14–16 This difference may be related to several factors. First, the identification of laparoscopy cases has changed since these prior studies were conducted. Whereas earlier studies combined diagnostic laparoscopy with open colectomy coding to define an “laparoscopic” case, newer studies can use unique coding to designate a laparoscopic case. Second, the use of observational data, particularly at the patient-level, is associated with limitations that can over-estimate treatment effects or lead to inaccurate conclusions.32–33 We believe a hospital-level analysis evaluating risk-standardized outcomes helps address some limitations of patient-level studies, including selection bias. By conducting the study at the hospital-level, patients are not selected by procedure, fewer differences are seen between the hospital populations, and hospital differences can be described using the hierarchical generalized linear models (HGLM). Further, a hospital-level analysis using HGLM helps account for variation in hospital performance which can be important predictors of outcomes in addition to patient-level factors.
The results of this study should be interpreted with several limitations in mind. First, although administrative data sets based on ICD-9-CM coding now allow for the identification of laparoscopic colon resections, more specific detail does not exist to indicate what proportion used hand-assist, single-incision, or were only partly completed laparoscopically. Therefore, we cannot comment on how the specific laparoscopic technique may influence outcomes. Second, we cannot determine cause and effect in this cross-sectional data, but rather suggest an association between the laparoscopy rate and outcomes. Further, we are unable to determine whether laparoscopic surgery improves outcomes beyond the initial admission using the current data set. Understanding how longer-term outcomes are influenced by the surgical approach, including readmission rates, emergency department visit rates, incisional hernias, or obstruction, should be further evaluated. Finally, the source of variation in outcomes seen across hospitals is multi-factorial. We examined only one factor which may contribute to this variation. Future studies should evaluate other factors so that best practices can be identified.
In conclusion, despite broad utilization of laparoscopic colon resection for cancer outcomes remain favorable with trends toward mildly lower risk-standardized postoperative complication rates and modestly shorter hospitalizations. While there was also a small increase in risk-standardized intra-operative morbidity, this did not appear to impact the patient’s overall hospital course and was not seen among hospitals with high procedure volume.
Acknowledgments
The authors would like to acknowledge the contributions of Zhenqiu Lin, PhD at the Center for Outcomes Research and Evaluation, Yale–New Haven Hospital, New Haven CT, for his contributions to this studies statistical analysis.
Appendix 1. ICD-9-CM Coding to Define Key Variables
| Surgical Variables | |
|---|---|
| Surgical approach | |
| Laparoscopic | 17.3, 17.31–17.39 |
| Open | 45.7, 45.71–45.79 |
| Conversion | V64.40, V64.41 |
| Anatomic resection | |
| Right hemi-colectomy | 17.32, 17.33, 45.72, 45.73 |
| Left hemi-colectomy | 17.35, 17.36, 45.75, 45.76 |
| Other colectomy | |
| Total colectomy | 45.8, 45.81–45.83 |
| Transverse colectomy | 17.34, 45.74 |
| Multiple segment | 17.31, 45.71 |
| Not specified resection | 17.39, 45.79 |
| Other diagnostic and procedural variables | |
| Colon cancer | 230.3, 153.0–153.9 (excluding 153.5) |
| In-situ disease | 230.3 |
| Node positive disease | 196, 196.0–196.9 |
| Metastatic disease | 197, 197.0–197.8, 198, 198.0–198.8, 198.81, 198.82, 198.89, 199.1 |
| Intra- and post-operative morbidity | |
| Post-operative morbidity | |
| Mechanical Wound | 998.12, 998.13, 998.31, 998.32, 998.6, 998.83 |
| Infectious | 038.0–038.9, 790.7, 998.5, 998.51, 998.59 |
| Urinary | 997.5 |
| Pulmonary | 512.2, 518.5, 518.4, 518.81, 518.82, 997.3 |
| Gastrointestinal | 560.1, 997.4 |
| Cardiovascular | 410.0–410.9, 415.11, 427.5, 453.40–453.42, 453.8–453.9, 997.02, 997.1, 998 |
| Intra-operative morbidity | |
| Accidental puncture or laceration during a procedure | 998.2 |
| Hemorrhage complicating a procedure | 998.11 |
Footnotes
Conflict of Interest Disclosure: Drs. Krumholz and Gross are the recipients of a research grant from Medtronic, Inc. through Yale University, Dr. Krumholz is chair of a cardiac scientific advisory board for UnitedHealth, and Dr. Gross is a member of the Scientific Advisory Committee for Fair Health, Inc.
Disclaimers: The views expressed in this article are those of the authors and do not reflect the official policy of the United States Air Force, the Department of Defense, or the U.S. Government.
Financial Disclosure: All authors are affiliated with the Clinical Scholars Program, which is supported by the Robert Wood Johnson Foundation. Dr. Krumholz is supported by grant U01 HL105270-02 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute.
References
- 1.Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy) Surgical laparoscopy & endoscopy. 1991;1(3):144–50. [PubMed] [Google Scholar]
- 2.Schlinkert RT. Laparoscopic-assisted right hemicolectomy. Diseases of the colon and rectum. 1991;34(11):1030–1. doi: 10.1007/BF02049971. [DOI] [PubMed] [Google Scholar]
- 3.Fusco MA, Paluzzi MW. Abdominal wall recurrence after laparoscopic-assisted colectomy for adenocarcinoma of the colon. Report of a case. Diseases of the Colon and Rectum. 1993;36(9):858–61. doi: 10.1007/BF02047384. [DOI] [PubMed] [Google Scholar]
- 4.Ota DM, Nelson H, Weeks JC. Controversies regarding laparoscopic colectomy for malignant diseases. Current opinion in general surgery. 1994:208–13. [PubMed] [Google Scholar]
- 5.COLOR: a randomized clinical trial comparing laparoscopic and open resection for colon cancer. Digestive surgery. 2000;17(6):617–622. doi: 10.1159/000051971. [DOI] [PubMed] [Google Scholar]
- 6.A comparison of laparoscopically assisted and open colectomy for colon cancer. The New England journal of medicine. 2004;350(20):2050–9. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 7.Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(21):3061–8. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 8.Kuhry E, Schwenk W, Gaupset R, et al. Long-term outcome of laparoscopic surgery for colorectal cancer: a cochrane systematic review of randomised controlled trials. Cancer Treatment Reviews. 2008;34(6):498–504. doi: 10.1016/j.ctrv.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Schwenk W, Haase O, Neudecker J, et al. Short term benefits for laparoscopic colorectal resection. Cochrane Database of Systematic Reviews (Online) 2005;(3):CD003145. doi: 10.1002/14651858.CD003145.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang CY, Halabi WJ, Luo R, et al. Laparoscopic Colorectal Surgery: A Better Look Into the Latest Trends. Arch Surg. 2012 doi: 10.1001/archsurg.2012.358. archsurg.2012.358. [DOI] [PubMed] [Google Scholar]
- 11.Fox J, Gross CP, Longo W, et al. Laparoscopic colectomy for the treatment of cancer has been widely adopted in the United States. Diseases of the colon and rectum. 2012;55(5):501–8. doi: 10.1097/DCR.0b013e318249ce5a. [DOI] [PubMed] [Google Scholar]
- 12.Etzioni DA. Laparoscopy...For all? Diseases of the colon and rectum. 2012;55(5):499–500. doi: 10.1097/DCR.0b013e318249d993. [DOI] [PubMed] [Google Scholar]
- 13.Sammour T, Kahokehr A, Srinivasa S, et al. Laparoscopic colorectal surgery is associated with a higher intraoperative complication rate than open surgery. Annals of Surgery. 2011;253(1):35–43. doi: 10.1097/sla.0b013e318204a8b4. [DOI] [PubMed] [Google Scholar]
- 14.Cone MM, Herzig DO, Diggs BS, et al. Dramatic decreases in mortality from laparoscopic colon resections based on data from the nationwide inpatient sample. Archives of Surgery. 2011;146(5):594–9. doi: 10.1001/archsurg.2011.79. [DOI] [PubMed] [Google Scholar]
- 15.Bilimoria KY, Bentrem DJ, Merkow RP, et al. Laparoscopic-assisted vs. open colectomy for cancer: comparison of short-term outcomes from 121 hospitals. Journal of Gastrointestinal Surgery. 2008;12(11):2001–9. doi: 10.1007/s11605-008-0568-x. [DOI] [PubMed] [Google Scholar]
- 16.Kemp JA, Finlayson SR. Outcomes of laparoscopic and open colectomy: a national population-based comparison. Surgical Innovation. 2008;15(4):277–83. doi: 10.1177/1553350608327171. [DOI] [PubMed] [Google Scholar]
- 17.Rea JD, Cone MM, Diggs BS, et al. Utilization of Laparoscopic Colectomy in the United States Before and After the Clinical Outcomes of Surgical Therapy Study Group Trial. Annals of Surgery. 2011;254(2):281–8. doi: 10.1097/SLA.0b013e3182251aa3. [DOI] [PubMed] [Google Scholar]
- 18.Robinson CN, Chen GJ, Balentine CJ, et al. Minimally invasive surgery is underutilized for colon cancer. Annals of Surgical Oncology. 18(5):1412–8. doi: 10.1245/s10434-010-1479-0. [DOI] [PubMed] [Google Scholar]
- 19.HCUP. Nationwide Inpatient Sample (NIS) [Accessed March 6, 2012];Healthcare Cost and Utilization Project (HCUP) 2005–2008. 2009 Available at: www.hcup-us.ahrq.gov/nisoverview.jsp.
- 20.Bonjer HJ, Hop WC, Nelson H, et al. Laparoscopically assisted vs open colectomy for colon cancer: a meta-analysis. Archives of surgery (Chicago, Ill : 1960) 2007;142(3):298–303. doi: 10.1001/archsurg.142.3.298. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan GG, McCarthy EP, Ayanian JZ, et al. Impact of hospital volume on postoperative morbidity and mortality following a colectomy for ulcerative colitis. Gastroenterology. 2008;134(3):680–7. doi: 10.1053/j.gastro.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Medical Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC, Ghali WA, Tu JV. A comparison of several regression models for analysing cost of CABG surgery. Statistics in Medicine. 2003;22(17):2799–815. doi: 10.1002/sim.1442. [DOI] [PubMed] [Google Scholar]
- 24.Norman G, Streiner D. Biostatistics: The Bare Essentials. Lewiston, NY: BC Decker, Inc; 2008. Part 3: Regression and Correlation; pp. 131–232. [Google Scholar]
- 25.Nelson H, Sargent D, Wieand S, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. New England Journal of Medicine. 2004;350(20):2050–9. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 26.Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncology. 2005;6(7):477–84. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 27.Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365(9472):1718–26. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 28.Goodney PP, Stukel TA, Lucas FL, et al. Hospital volume, length of stay, and readmission rates in high-risk surgery. Annals of Surgery. 2003;238(2):161–7. doi: 10.1097/01.SLA.0000081094.66659.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. New England Journal of Medicine. 2002;346(15):1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 30.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. The New England journal of medicine. 2011;364(22):2128–37. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendren S, Morris AM, Zhang W, et al. Early discharge and hospital readmission after colectomy for cancer. Diseases of the Colon and Rectum. 2011;54(11):1362–7. doi: 10.1097/DCR.0b013e31822b72d3. [DOI] [PubMed] [Google Scholar]
- 32.Hemmila MR, Birkmeyer NJ, Arbabi S, et al. Introduction to propensity scores: A case study on the comparative effectiveness of laparoscopic vs open appendectomy. Archives of Surgery. 2010;145(10):939–45. doi: 10.1001/archsurg.2010.193. [DOI] [PubMed] [Google Scholar]
- 33.Stukel TA, Fisher ES, Wennberg DE, et al. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA. 2007;297(3):278–85. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]