Abstract
Objective
Disease-modifying osteoarthritis drugs (DMOADs) are under development. Our goal was to determine efficacy, toxicity, and cost thresholds under which DMOADs would be a cost-effective knee OA treatment.
Design
We used the Osteoarthritis Policy Model, a validated computer simulation of knee OA, to compare guideline-concordant care to strategies that insert DMOADs into the care sequence. The guideline-concordant care sequence included conservative pain management, corticosteroid injections, total knee replacement (TKR), and revision TKR. Base case DMOAD characteristics included: 50% chance of suspending progression in the first year (resumption rate of 10% thereafter) and 30% pain relief among those with suspended progression; 0.5%/year risk of major toxicity; and costs of $1,000/year. In sensitivity analyses, we varied suspended progression (20–100%), pain relief (10–100%), major toxicity (0.1–2%), and cost ($1,000–$7,000). Outcomes included costs, quality-adjusted life expectancy, incremental cost-effectiveness ratios (ICERs), and TKR utilization.
Results
Base case DMOADs added 4.00 quality-adjusted life years (QALYs) and $230,000 per 100 persons, with an ICER of $57,500/QALY. DMOADs reduced need for TKR by 15%. Cost-effectiveness was most sensitive to likelihoods of suspended progression and pain relief. DMOADs costing $3,000/year achieved ICERs below $100,000/QALY if the likelihoods of suspended progression and pain relief were 20% and 70%. At a cost of $5,000, these ICERs were attained if the likelihoods of suspended progression and pain relief were both 60%.
Conclusions
Cost, suspended progression, and pain relief are key drivers of value for DMOADs. Plausible combinations of these factors could reduce need for TKR and satisfy commonly cited cost-effectiveness criteria.
INTRODUCTION
Knee osteoarthritis (OA) is a prevalent and costly disease characterized by structural changes in cartilage, bone, synovium, and other joint structures1. Symptomatic knee OA is a leading cause of disability, afflicting more than 9.3 million US adults2. The risk for knee OA is growing substantially due to the aging population, obesity epidemic, and an increasing rate of knee injuries in young, active individuals1,3–7.
Current guidelines for knee OA care focus on pain relief and functional improvement8–10. Pharmacologic therapies are only modestly efficacious and have significant associated toxicities. For example, non-steroidal anti-inflammatory drugs (NSAIDs) pose gastrointestinal and cardiovascular risks11–13. There are no currently approved OA treatments capable of slowing OA-related structural progression or delaying the need for total knee replacement (TKR). Several large pharmaceutical companies are in the late stages of developing and testing disease-modifying OA drugs (DMOADs), and promising agents that may both halt progression and provide symptom relief are currently being studied14–17.
In light of ongoing efforts to develop DMOADs, we sought to address several key questions: Can DMOADs be cost-effective, and if so, at what levels of efficacy, toxicity, and cost? How early in the course of treatment should DMOADs be initiated? Do DMOADs have the potential to reduce TKR utilization? To address these key issues, we propose a novel framework in which model-based evaluations of cost-effectiveness can be used to pre-evaluate new treatment strategies before the treatments are actually in widespread use. Estimating the effects of particular features of a medication on that medication’s cost-effectiveness can inform the design of trials and provide performance targets.
METHODS
Analytic Overview
We used the Osteoarthritis Policy (OAPol) Model, a validated state-transition computer simulation model, to compare clinical outcomes and costs between subjects receiving guideline-concordant treatments (the standard of care) and subjects receiving standard of care and DMOADs18,19. Outcomes included costs, quality-adjusted life expectancy (QALE), incremental cost-effectiveness ratios (ICERs, the ratio of change in costs to change in QALE), and TKR utilization. In conformity with accepted practice, strategies that increased cost while not increasing QALE relative to an alternative treatment strategy were referred to as “Dominated.” We performed the analysis from the health systems perspective (indirect costs were not included), with costs and QALE discounted at a rate of 3%/year, as recommended by the Panel on Cost-Effectiveness in Medicine20.
The OAPol Model
The OAPol Model is a Monte Carlo simulation with a one-year cycle length and health states defined by knee OA severity, presence of knee pain, comorbidities, and obesity18,19. Each year, subjects may develop a comorbid condition, increase in BMI, progress in OA severity, and/or die. Progression of OA is defined as an increase by one Kellgren-Lawrence (K-L) radiographic grade and is dependent on obesity status and sex21. The model considers five comorbid conditions: coronary heart disease, diabetes mellitus, chronic obstructive pulmonary disease, cancer, and musculoskeletal disorders other than OA. The prevalences of these comorbid conditions depend on age, sex, race/ethnicity, and obesity22–24. Each subject is followed until death, which may occur in any health state. The OAPol Model uses underlying mortality rates from US life tables with excess mortality due to specific comorbid conditions removed25. Individuals with comorbid conditions have greater risk of death26,27. Subjects with knee OA may receive OA treatments, which are characterized by the ability to relieve pain and suspend OA progression, toxicity, and cost. OA treatments may carry major (e.g. gastrointestinal bleeding) and minor (e.g. dyspepsia, rash) toxicities, both of which decrease quality of life and increase costs. Major toxicities lead to regimen discontinuation and may also cause death.
Each year, subjects accrue costs and changes in quality of life due to OA or OA-related treatments and other underlying medical conditions. Quality of life weights are assigned to capture preferences for health states; a value of 1.0 denotes a state of perfect health while a value of zero denotes health states that are preferentially equivalent to death28. Annual medical costs not directly attributable to knee OA are based on comorbidities and age22,23,29,30. These data are presented in Table 1. Running tallies of survival, quality-adjusted survival, and costs are maintained for each individual and then aggregated to compute average values for the cohort31. The following paragraphs describe the means of modeling the standard of care and DMOAD regimens.
Table 1.
Select OAPol Model inputs
| Age at treatment initialization (mean ± standard deviation) | 53.54 ± 14.39 | Losina 201236 | |||||
| OSTEOARTHRITIS PROGRESSION (annual likelihood, %) | |||||||
| K-L 2 to K-L 3 | K-L 3 to K-L 4 | ||||||
| Obesity Group | Male | Female | Male | Female | Holt 201119 | ||
| Non-Obese | 5.58 | 4.00 | 1.29 | 1.95 | |||
| Obese | 12.26 | 8.95 | 2.94 | 4.27 | |||
| QUALITY OF LIFE UTILITIES | |||||||
| Utility for subjects with severe OA (K-L 3 or 4) | 0.690 | Losina 200943 | |||||
| Non-Obese | Obese | ||||||
| Number of Comorbidities | Age Group | OA Pain | No OA Pain | OA Pain | No OA Pain | ||
| 0 – 1 | 25–44 | 0.814 | 0.955 | 0.781 | 0.921 | ||
| 45–64 | 0.806 | 0.952 | 0.773 | 0.918 | |||
| 65+ | 0.884 | 0.943 | 0.850 | 0.909 | |||
| 2 –3 | 25–44 | 0.721 | 0.903 | 0.688 | 0.870 | NHANES 2005–822,23 | |
| 45–64 | 0.713 | 0.901 | 0.679 | 0.867 | |||
| 65+ | 0.791 | 0.891 | 0.757 | 0.858 | |||
| > 3 | 0.662 | 0.662 | 0.662 | 0.662 | |||
| ANNUAL DIRECT MEDICAL COSTS (USD 2010) | |||||||
| Number of Comorbidities | Age Group | OA Pain | No OA Pain | ||||
| 0–1 | 25–34 | $1,506 | $1,302 | ||||
| 35–44 | $2,018 | $1,814 | |||||
| 45–49 | $2.635 | $2,431 | |||||
| 50–54 | $2,636 | $2,432 | |||||
| 55–59 | $3,443 | $3,239 | |||||
| 60–64 | $4,144 | $3,940 | |||||
| 65–69 | $4,401 | $4,198 | |||||
| 70–74 | $5,092 | $4,888 | |||||
| 75–79 | $5,916 | $5,712 | |||||
| 80+ | $7,709 | $7,505 | |||||
| 2–3 | 25–34 | $6,856 | $6,652 | Pope 200437 NHANES 2005–822,23 CPI 201038 MCBS 200639 Red Book 201030 |
|||
| 35–44 | $7,368 | $7,165 | |||||
| 45–49 | $7,958 | $7,755 | |||||
| 50–54 | $7,959 | $7,755 | |||||
| 55–59 | $8,436 | $8,232 | |||||
| 60–64 | $9,136 | $8,933 | |||||
| 65–69 | $9,060 | $8,856 | |||||
| 70–74 | $9,750 | $9,547 | |||||
| 75–79 | $10,575 | $10,371 | |||||
| 80+ | $12,367 | $12,163 | |||||
| >3 | 25–34 | $12,710 | $12,506 | ||||
| 35–44 | $13,223 | $13,019 | |||||
| 45–49 | $11,954 | $11,751 | |||||
| 50–54 | $11,955 | $11,751 | |||||
| 55–59 | $13,105 | $12,902 | |||||
| 60–64 | $13,806 | $13,602 | |||||
| 65–69 | $15,570 | $15,366 | |||||
| 70–74 | $16,260 | $16,056 | |||||
| 75–79 | $17,084 | $16,881 | |||||
| 80+ | $18,877 | $18,673 | |||||
| STANDARD OF CARE TREATMENTS | |||||||
| First Year | Subsequent Year Failure | ||||||
| Regimen 1: NSAIDs, Acetaminophen, Physical Therapy, Assistive Devices | Pain Relief (annual, %)†† | 64.00 | 24.00 | Scott 200034 | |||
| First Year | Subsequent Years | ||||||
| Major Toxicity (annual, %) | 0.38 | 0.38 | Solomon 200511, Goldstein 199933 | ||||
| Minor Toxicity (annual, %) | 2.95 | 2.24 | Bensen 199935, Scott 200034, Silverstein 199555 | ||||
| Cost (USD 2010) | $643 | $483 | Medicare 201056–58, Redbook 201030, MCBS 200639, Van Der Esch 200359, Grindrod 201060 | ||||
| First Year | Subsequent Year Failure | ||||||
| Regimen 2: Intra-articular Injections | Pain Relief (annual, %)†† | 64.00 | 19.00 | Raynauld, 200361 | |||
| First Year | Subsequent Years | ||||||
| Major Toxicity (annual, %) | 0.00 | 0.00 | Ayral, 200162 | ||||
| Minor Toxicity (annual, %) | 24.00 | 24.00 | Ayral, 200162 | ||||
| Cost†† (USD 2010) | $437 | $437 | Medicare 201056–58, MCBS 200639 | ||||
| First Year | Subsequent Year Failure | ||||||
| Regimen 3: Primary TKR | Pain Relief (annual, %)††† | 86.20 | 4.00 | Katz 200763 | |||
| First Year | Subsequent Years | ||||||
| Major Toxicity (annual, %) | 1.33 | 0.00 | Paxton 201064, Katz 200465 | ||||
| Minor Toxicity (annual, %) | 2.94 | 0.00 | Katz 200465 | ||||
| Cost†† (USD 2010) | $19,065 | $90 | Medicare 201056–58, HCUP 200951, Buntin 200566, CPI 201038, Teeny 200367 | ||||
| First Year | Subsequent Year Failure | ||||||
| Regimen 4: Revision TKR | Pain Relief (annual, %)††† | 74.30 | 5.60 | Katz 200763 | |||
| First Year | Subsequent Years | ||||||
| Major Toxicity (annual, %) | 0.96 | 0.00 | Paxton 201064, Katz 200465 | ||||
| Minor Toxicity (%) | 3.64 | 0.00 | Katz 200465 | ||||
| Cost†† (USD 2010) | $24,631 | $90 | Medicare 201056–58, HCUP 200951, Buntin 200566, CPI 201038, Teeny 200367 | ||||
| DMOADS‡ | |||||||
| 1st Year | Subsequent Years | ||||||
| Annual Costs (Base Case) (USD 2010) | |||||||
| Overall | $1,000 – $7,000 ($1,000) | ||||||
| Office Visits | $132 | $93 | 2010 Medicare Data56–58 | ||||
| Efficacy (Base Case) %, Annual | 1st Year | Subsequent Year Failure | |||||
| Halted Progression (K-L 2 – 3) ‡‡ | 20 – 100 (50) | 1 – 10 (10) | |||||
| Pain Relief* (K-L 2 – 3) ‡‡ | 10 – 100 (30) | 1 – 10 (1) | |||||
| Toxicity (Base Case) %, Annual | 1st Year | Subsequent Years | |||||
| Major | 0.5 – 2.0 (0.5) | ||||||
| Minor | 9.50 | 7.27 | Scott 200034, Bensen 199935 | ||||
| Toxicity Outcomes | |||||||
| Major | Cardiovascular | Likelihood | 32.3 | Solomon 200511 | |||
| Mortality | 6.02 | HCUP 200868 | |||||
| Utility** | 0.778 | Sullivan 200669, NHANES 05–0822,23 | |||||
| Cost** | $18,478 | HCUP 200868, CPI 201038 | |||||
| Gastrointestinal | Likelihood | 67.7 | Goldstein 200033 | ||||
| Mortality | 2.93 | HCUP 200868 | |||||
| Utility | 0.859 | Jansen 200770, NHANES 05–0822,23 | |||||
| Cost | $9,408 | HCUP 200868, CPI 201038 | |||||
| Minor | General Minor Events | Likelihood | 100 | ||||
| Mortality | 0 | ||||||
| Utility | 0.923 | Jansen 200770, NHANES 05–0822,23 | |||||
| Cost | $47 | Kamath 200371, CPI 201038 | |||||
The lowest utility associated with the subject’s health state was used by the model; for example, a 45 year-old subject with severe OA and one comorbidity would have a utility of 0.690, whereas, the same subject with 3 comorbidities would have a utility of 0.662.
Efficacy for Regimens 1 and 2 applies only to individuals who are at K-L grade 2.
Only pain relief efficacy associated with TKR is shown. TKR technical efficacy (e.g. stability of the implant) was greater than 98% for primary and revision TKR.
Sensitivity analysis ranges for each parameter have been presented; base case values appear in bold within parentheses.
Pain relief and suspended progression were 0% for subjects who have progressed to K-L grade 4. (K-L 4 represents the most severe level of knee OA, thus patients cannot progress beyond it.)
Pain relief only occurred if there was also suspended progression.
Toxicity utilities and costs (USD 2010) were applied only in the year that the event occurred.
Abbreviations: OA, osteoarthritis; K-L, Kellgren-Lawrence grade; NHANES, National Health and Nutrition Examination Survey; CPI, consumer price inflation calculator; MCBS, Medicare Current Beneficiary Survey; HCUP, Healthcare Cost and Utilization Project; DMOADs, disease-modifying osteoarthritis drugs
Guideline-concordant OA care (standard of care)
The standard of care consists of four, sequentially more invasive regimens: conservative pain management, including NSAIDs, acetaminophen, supportive devices, and physical therapy (Regimen 1); corticosteroid injections (Regimen 2); primary TKR (Regimen 3); and revision TKR (Regimen 4)8–10. Subjects progress to the next regimen in the sequence only when the current treatment fails or if a major toxicity occurs. Failure of each regimen is assumed to be detected in the year it occurs. Fundamental treatment characteristics for the standard of care are presented in Table 1 and described in detail in the Technical Appendix.
DMOADs
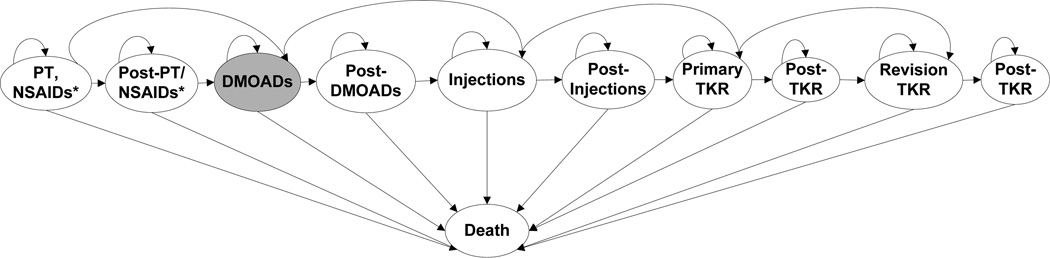
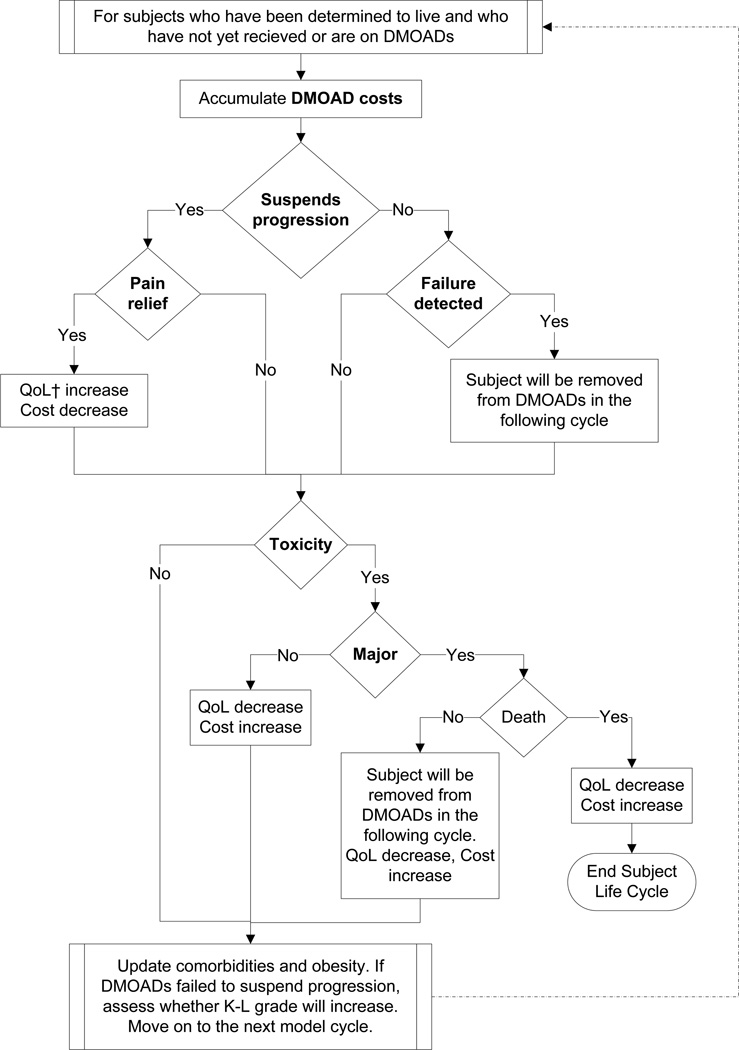
We evaluated treatment strategies where DMOADs were used after the first standard of care regimen and before the second standard of care regimen. Figure 1 illustrates the treatment sequence for individuals receiving DMOADs. There are two measures of DMOAD efficacy: structural efficacy and pain relief. Structural efficacy is defined by a relative reduction in the probability of progressing from one K-L grade to the next. Subjects for whom DMOADs suspend OA progression (i.e. DMOADs exhibit structural efficacy) remain at their current K-L grade. Subjects in whom structural progression is suspended may also experience pain relief and improvement in quality of life. To ensure a conservative approach with respect to the clinical value of DMOADs, we assumed that DMOAD-related pain relief is restricted to subjects in whom knee OA progression is suspended. Delaying progression at earlier stages of the disease prevents decrements in quality of life associated with advanced OA (K-L grade 3 or 4). Subjects experiencing toxicity (major or minor) have a decrement in quality of life for that year and incur costs to treat the toxicity. Major toxicity carries a small risk of death. Subjects are removed from DMOADs and move on to the next treatment in the sequence if DMOADs fail to suspend progression and that failure is detected, or if a major toxicity occurs. Figure 2 shows the OAPol Model process for subjects receiving DMOADs.
Figure 1. The OAPol Model Treatment Sequence.
This figure shows the treatment sequence that each model subject will receive. Initially, subjects are on the first regimen, which consists of NSAIDs, acetaminophen, and physical therapy. Each year on the regimen, subjects are evaluated for regimen failure and for major toxicity. If the regimen fails or a major toxicity occurs, the subject will be removed from the regimen and will move on either to the next regimen or to a post-treatment waiting period. Subjects will remain in the post-treatment waiting period until they are determined to be eligible for the next treatment. Subjects in the DMOADs cohorts are eligible to receive DMOADs after the first regimen (subjects not in the DMOADs cohort move on to corticosteroid injections). Once DMOADs fail to relieve pain or a major toxicity occurs, subjects move on to receive corticosteroid injections either immediately, or after a waiting period. This process continues through to TKR. Each year, subjects are evaluated for death; a subject may die at any point.
* This regimen includes physical therapy, NSAIDs, and acetaminophen
Figure 2. DMOADs in the OAPol Model.
This figure depicts the pathway of a hypothetical subject in the OAPol Model receiving DMOADs. When DMOADs are discontinued, subjects will be evaluated for the treatment immediately following DMOADs.
†QoL – quality of life
Base Case DMOAD Characteristics and Assumptions
As recommended by the Panel in Cost-effectiveness Analyses in Health and Medicine, we chose a set of “base case” estimates of DMOAD efficacy, cost, and toxicity, to reflect the most likely set of parameters of DMOADs based on a review of available literature when possible and otherwise based on extensive discussions with clinicians20. In the base case, we assumed that DMOADs suspended OA progression in 50% of subjects. Among those in whom DMOADs succeeded in suspending progression in the first year, there was a 10% failure rate of maintaining the suspension of progression in every subsequent year. We further assumed that once disease progression resumed, it could no longer be suspended via DMOADs. For the base case analysis we chose to anchor pricing for DMOADs at $1,000/year, similar to the cost of prescription NSAIDs30. In addition to the baseline cost of DMOADs, we also considered the cost of one office visit per year: $132 in the first year and $93 in subsequent years (reflecting higher costs for new patient visits)32.
In practice, monitoring for drug failure is typically triggered when patients report the persistence or recurrence of pain. Since drug failures to suspend disease progression would be accompanied by pain, we therefore assumed that all DMOAD failures would be detected in the year they occurred, resulting in discontinuation of DMOADs and allowing subjects to advance to the next treatment regimen. We assumed in the base case that the likelihood of pain relief was 30% given that progression was suspended (that is, 15% overall likelihood of pain relief). Among patients whose structural progression had been suspended due to DMOADs and who experienced initial pain relief, there was a 1%/year chance of losing pain relief. The failure to sustain pain relief reflects a multitude of factors, including suboptimal adherence and accumulation of additional risk factors such as injury.
We anchored values for both major and minor toxicities of DMOADs to NSAID toxicity characteristics. The cohort of individuals eligible to receive DMOADs will be similar to the population currently utilizing NSAIDs for OA pain; thus, acceptable DMOAD adverse event rates are likely to be comparable to those of NSAIDs. The likelihood of major toxicity was assumed to be 0.5% per year based on the major toxicity risks of Cox-2 selective NSAIDs11,33. DMOAD minor toxicity was modeled after the toxicity of non-selective NSAIDs, with 9.50% risk in the first year, and 7.27% risk in all subsequent years34,35.
Cohort Characteristics
We considered cohorts with a mean age of 53.5 years (standard deviation 14.4 years) based on estimates of the average age of OA diagnosis in the US36. Race/ethnicity, sex, and obesity distributions for persons with diagnosed knee OA were derived from the National Health Interview Survey 2007–200824. In the absence of efficacious DMOADs, annual OA progression rates (percentage of subjects who worsened in K-L grade in a year) ranged from 1.29% for non-obese, K-L grade 3 males to 12.26% for obese, K-L grade 2 males19. Annual underlying (not related to OA management) medical costs (USD 2010) ranged from $1,302 for young subjects with at most one comorbid condition to $18,877 for older subjects with greater than three comorbid conditions22,23,30,37–39. Quality of life weights were derived by converting responses to general health status questions in the National Health and Nutrition Examination Survey (NHANES) 2005–2008 to health status ratings on a scale of zero to 1.022,23,40,41. These ratings were then transformed to preference-based utilities42. The values ranged from 0.95 for young, healthy subjects with no OA pain to 0.66 for older subjects with several comorbidities and knee pain. Advanced knee OA (defined as symptomatic K-L grades 3 or 4) had a quality of life weight of 0.6943. Prevalence of comorbid conditions were derived from NHANES 2005–200822,23. Table 1 summarizes select cohort input characteristics; additional details have been published elsewhere18,19 or are presented in the Technical Appendix.
Sensitivity Analyses
Two-way Sensitivity Analyses of DMOAD Characteristics
We conducted 21 sets of two-way sensitivity analyses, varying likelihood of suspending OA progression, pain relief, major toxicity, loss of pain relief and/or resumption of OA progression, and costs. We tested the sensitivity of DMOAD cost-effectiveness to variations in the initial likelihood of suspended progression (20% – 100%), failure to suspend progression in subsequent years (1% – 10%), initial pain relief (10% – 100%), failure to relieve pain in subsequent years (1% – 10%), cost ($1,000 – $7,000), and major toxicity (0.1% – 2%) in a series of two-way sensitivity analyses. By modeling DMOADs with low levels of pain relief (10%), we incorporated the possibility that DMOADs may not necessarily provide pain relief, even if they suspend progression. These ranges were chosen to cover the spectrum of possible DMOAD characteristics. Costs and toxicity were anchored to known values for NSAIDs, based on recommendations from experts in the field.
Additional Sensitivity Analyses
In addition to varying levels of DMOAD efficacy, toxicity, and cost, we varied the timing of DMOAD administration, defined by where DMOADs are inserted in the current standard of care sequence. We also varied the placement of the regimens by switching the order of Regimen 1 (NSAIDs, physical therapy, acetaminophen) and Regimen 2 (corticosteroid injections). We also tested the effect of removing Regimen 2 (corticosteroid injections) from the treatment sequence.
In a separate sensitivity analysis, we examined the value of DMOADs while varying the baseline K-L grade distribution: (1) initialized with 100% K-L grade 1 OA, and (2) initialized with 50% K-L grade 1 and 50% K-L grade 2 OA.
Finally, we conducted a sensitivity analysis using data for doxycycline, which has been suggested to have disease-modifying properties. The published study showed that doxycycline could reduce progression by up to 40% while doxycycline has not been shown to have any effect on symptoms. We modeled minor gastrointestinal toxicities (the most significant toxicity reported in the study) occurring at a rate of 7% annually. Costs were estimated at $200 annually according to the Red Book30.
RESULTS
Base Case Analysis (Table 2, top row)
Table 2.
Two-way sensitivity analysis of DMOAD pain relief and suspended progression
| Suspended Progression |
Pain Relief | Treatment Strategy |
Avg. QALE |
Avg. Cost |
ICER | Proportion of Cohort Receiving Primary TKR |
|---|---|---|---|---|---|---|
| 50% |
Base Case 30% 15% overall‡ |
Standard of Care* | 14.21 | $115,800 | 52.37% | |
| SoC + DMOADs** | 14.25 | $118,100 | $57,500 | 44.35% | ||
|
40% 20% overall |
Standard of Care | 14.21 | $115,800 | 52.37% | ||
| SoC + DMOADs | 14.28 | $118,000 | $31,400 | 44.34% | ||
|
50% 25% overall |
Standard of Care | 14.21 | $115,800 | 52.37% | ||
| SoC + DMOADs | 14.32 | $118,000 | $20,000 | 44.33% | ||
| 60% |
30% 18% overall |
Standard of Care | 14.21 | $115,800 | 52.37% | |
| SoC + DMOADs | 14.26 | $118,400 | $52,000 | 42.82% | ||
|
40% 24% overall |
Standard of Care | 14.21 | $115,800 | 52.37% | ||
| SoC + DMOADs | 14.30 | $118,300 | $27,800 | 42.82% | ||
|
50% 30% overall |
Standard of Care | 14.21 | $115,800 | 52.37% | ||
| SoC + DMOADs | 14.35 | $118,200 | $17,100 | 42.83% | ||
| 70% |
30% 21% overall |
Standard of Care | 14.21 | $115,800 | 52.37% | |
| SoC + DMOADs | 14.28 | $118,600 | $40,000 | 41.31% | ||
|
40% 28% overall |
Standard of Care | 14.21 | $115,800 | 52.37% | ||
| SoC + DMOADs | 14.33 | $118,600 | $23,300 | 41.31% | ||
|
50% 35% overall |
Standard of Care | 14.21 | $115,800 | 52.37% | ||
| SoC + DMOADs | 14.38 | $118,500 | $15,900 | 41.31% |
Standard of care sequence: conservative pain management (NSAIDs, acetaminophen, physical therapy), corticosteroid injections, primary TKR, revision TKR
Standard of care + DMOADs sequence: conservative pain management, DMOADs, corticosteroid injections, primary TKR, revision TKR
Overall pain relief is calculated as (% pain relief given suspended progression) × (% suspended progression); the top row of this table corresponds with 30% pain relief given suspended progression, 50% suspended progression, and thus 15% overall pain relief.
Abbreviations: ICER, incremental cost-effectiveness ratio; TKR, total knee replacement; SoC, standard of care
Clinical Benefits of DMOADs
The QALE among persons with knee OA who received the standard of care was estimated at 14.21 quality-adjusted life years (QALYs) discounted (22.22 QALYs undiscounted). Adding base case DMOADs as the second-line regimen in the treatment sequence (after NSAIDs and physical therapy but before corticosteroid injections) led to an estimated QALE of 14.25 QALYs.
Among knee OA patients receiving the current standard of care, 11.00% underwent TKR within 10 years of treatment initiation, with a 52.37% lifetime risk of primary TKR. Adding base case DMOADs as the second-line regimen reduced the 10-year risk of TKR by 46%, with 5.99% of the DMOADs cohort receiving TKR within 10 years of treatment initiation. Moreover, DMOADs reduced lifetime risk of TKR by 15%, with 44.35% of the DMOADs cohort receiving primary TKR.
Cost-effectiveness of DMOADs
Priced at $1,000 annually, the cost-effectiveness of DMOADs offered as the second-line regimen for those diagnosed with knee OA was estimated at $57,500/QALY gained.
Guidance for the Prospective Evaluation of DMOADs Regimens
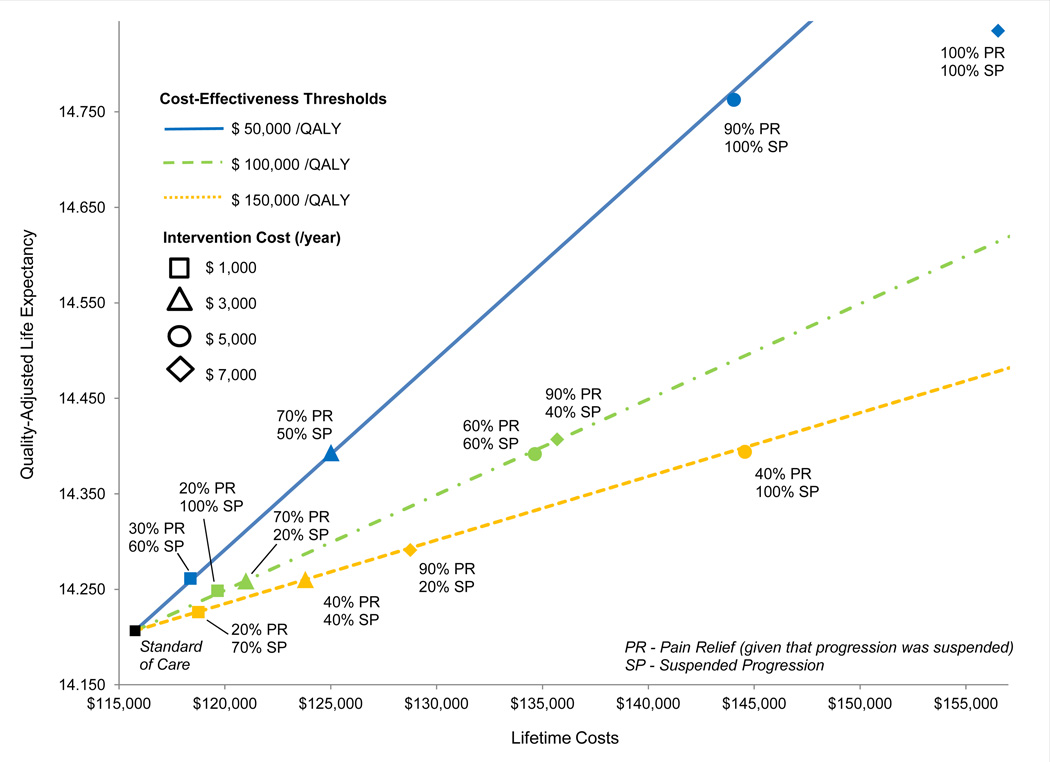
Figure 3 shows the minimum degree of structural OA progression suspension and pain relief at which DMOADs might be considered cost-effective using three different cost-effectiveness thresholds: $50,000/QALY, $100,000/QALY, and $150,000/QALY. Assuming DMOADs are associated with 0.5% risk of major toxicity and failure of DMOADs is diagnosed in the year it occurs, DMOADs costing $1,000/person/year would achieve ICERs below $50,000/QALY if they could suspend OA progression by at least 60% and provide concurrent pain relief in at least 30% of those with suspended OA progression. DMOADs that cost $3,000 or $5,000 would attain ICERs below $100,000/QALY if they could suspend OA progression/lead to pain relief by at least 20%/70% or 60%/60%. ICERs below $150,000/QALY could be achieved by DMOADs costing $7,000/person/year if they could suspend structural progression by at least 20% and lead to concomitant pain relief in at least 90% of those with suspended OA progression. Figure 3 shows that DMOADs costing $1,000, suspending progression in 100% of cases, and leading to 20% pain relief would provide similar value as more expensive DMOADs ($3,000/person/year) that suspend progression in 20% of cases, and relieve pain in 70% of cases. The same value would also be achieved by a more expensive DMOAD ($5,000) with pain relief and suspended progression at 60%. DMOADs costing $7,000 were unlikely to attain ICERs of $50,000/QALY, even if they were 100% effective in both suspending structural progression and relieving pain.
Figure 3.
Threshold efficacy, cost, and life expectancy associated with DMOADs treatment. This figure describes threshold efficacy for alternative willingness-to-pay thresholds, shown in blue ($50,000/QALY), green ($100,000/QALY), and yellow ($150,000/QALY). Squares represent efficacy thresholds for DMOADs costing $1,000/person/year, triangles -- $3,000/person/year, circles -- $5,000/person/year, and diamonds -- $7,000/person/year. The vertical axis shows the per person discounted quality-adjusted life expectancy and the horizontal axis shows the per person discounted lifetime cost. The black square in the lower left corner represents the per person life expectancy and lifetime cost in a program with no DMOADs intervention.
Sensitivity Analyses
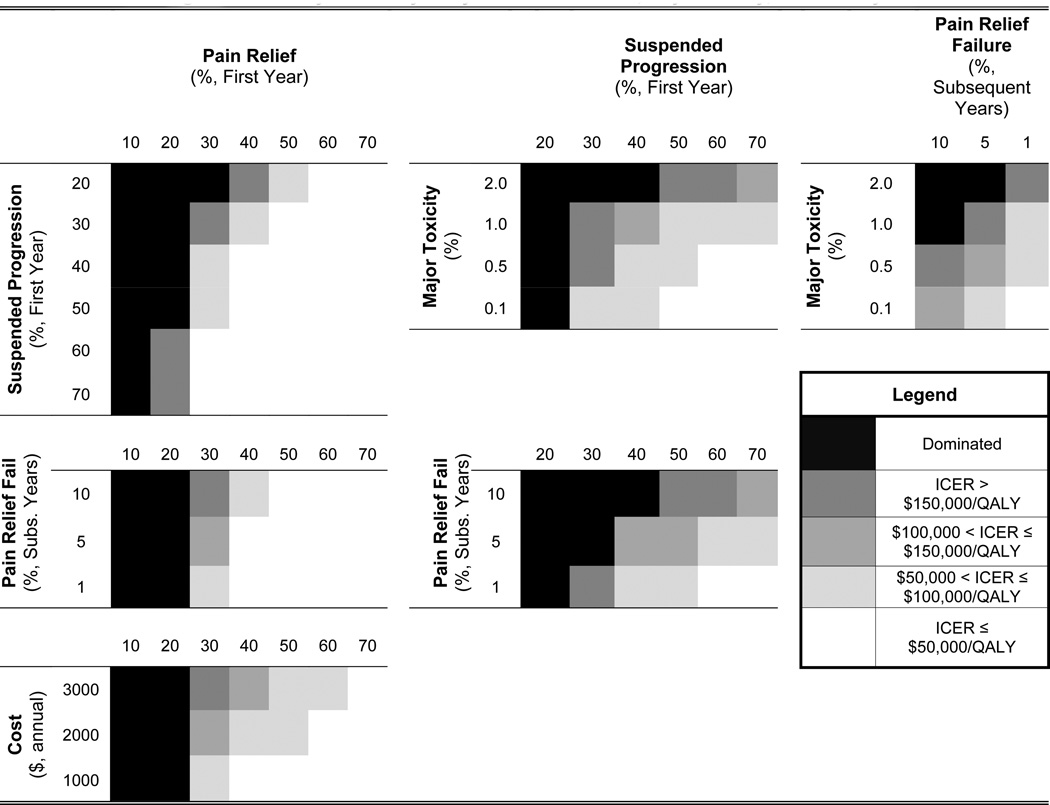
Select, two-way sensitivity analyses are presented in Figure 4 and Tables 2 and 3. Additional two-way sensitivity analyses are presented in the Technical Appendix. The timing of DMOAD administration (anywhere in the sequence prior to TKR) did not have a meaningful impact on the cost-effectiveness of DMOAD therapy (results not shown).
Figure 4. Two-way sensitivity analysis of DMOAD cost, major toxicity, and efficacy.
Each box in Figure 3 represents a single two-way analysis – for instance varying cost ($1000 – $3000) and toxicity (0.1% – 2%) of DMOADs. The shade of each block in the quadrant represents the level of cost-effectiveness for that particular DMOAD in comparison to the standard of care. The darkest shades are the lowest levels of cost-effectiveness, and the lightest shades represent the highest levels of cost-effectiveness. Blocks are shaded black if the particular DMOAD decreased QALE relative to the standard of care, and thus were dominated. The quadrants are organized such that the most beneficial combination of DMOAD parameters appears in the bottom right-hand corner of each square (for example, lowest cost, $1,000, and highest level of pain relief, 70%), and the least beneficial combination of DMOAD parameters appears in the top left-hand corner of each square (for example, highest cost, $3000, and lowest pain relief, 10%).
Table 3.
Two-way sensitivity analysis of DMOAD cost and pain relief, suspended progression, or major toxicity
| $1,000 | $2,000 | $3,000 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment Strategy |
Avg. QALE |
Avg. Cost |
ICER |
Avg. Cost |
ICER |
Avg. Cost |
ICER | ||
| Pain Relief |
30% (base case) |
Standard of Care* | 14.21 | $115,800 | $115,800 | $115,800 | |||
| SoC + DMOADs** | 14.25 | $118,100 | $57,500 | $121,600 | $145,000 | $125,200 | $235,000 | ||
| 40% | Standard of Care | 14.21 | $115,800 | $115,800 | $115,800 | ||||
| SoC + DMOADs | 14.28 | $118,000 | $31,400 | $121,600 | $82,900 | $125,100 | $132,900 | ||
| 50% | Standard of Care | 14.21 | $115,800 | $115,800 | $115,800 | ||||
| SoC + DMOADs | 14.32 | $118,000 | $20,000 | $121,500 | $51,800 | $125,100 | $84,500 | ||
| Suspended Progression |
50% (base case) |
Standard of Care | 14.21 | $115,800 | $115,800 | $115,800 | |||
| SoC + DMOADs | 14.25 | $118,100 | $57,500 | $121,600 | $145,000 | $125,200 | $235,000 | ||
| 60% | Standard of Care | 14.21 | $115,800 | $115,800 | $115,800 | ||||
| SoC + DMOADs | 14.26 | $118,400 | $52,000 | $122,500 | $134,000 | $126,600 | $216,000 | ||
| 70% | Standard of Care | 14.21 | $115,800 | $115,800 | $115,800 | ||||
| SoC + DMOADs | 14.28 | $118,600 | $40,000 | $123,300 | $107,100 | $128,000 | $174,300 | ||
| Major Toxicity | 1% | Standard of Care | 14.21 | $115,800 | $115,800 | $115,800 | |||
| SoC + DMOADs | 14.24 | $118,100 | $76,700 | $121,700 | $196,700 | $125,100 | $310,000 | ||
|
0.5% (base case) |
Standard of Care | 14.21 | $115,800 | $115,800 | $115,800 | ||||
| SoC + DMOADs | 14.25 | $118,100 | $57,500 | $121,600 | $145,000 | $125,200 | $235,000 | ||
| 0.1% | Standard of Care | 14.21 | $115,800 | $115,800 | $115,800 | ||||
| SoC + DMOADs | 14.26 | $118,000 | $44,000 | $121,600 | $118,000 | $125,300 | $190,000 | ||
Standard of care sequence includes: conservative pain management (NSAIDs, acetaminophen, physical therapy), corticosteroid injections, primary TKR, revision TKR
Standard of care + DMOADs sequence includes: conservative pain management, DMOADs, corticosteroid injections, primary TKR, revision TKR
Abbreviations: DMOAD - disease modifying osteoarthritis drug; QALE - quality-adjusted life expectancy; ICER - incremental cost-effectiveness ratio; SoC – standard of care
Table 2 presents results of two-way sensitivity analyses that varied the degree of suspended progression and pain relief within clinically plausible ranges (50–70% for suspended progression and 30–50% for pain relief). When DMOADs were priced at $1,000/year with major toxicity risks at 0.5%/year, DMOADs were likely to have cost-effectiveness ratios below $100,000 compared to the standard of care (no DMOADs). The proportion of the cohort receiving TKR depended on the likelihood that DMOADs suspended progression; base case DMOADs as the second-line regimen (50% suspended progression, 30% concomitant pain relief) resulted in 44.35% lifetime risk for TKR. Increasing suspended progression to 70% decreased lifetime risk of TKR to 41.31%. Figure 4 (upper left box) portrays cost-effectiveness ratios of DMOADs-based strategies for expanded ranges of suspended progression and pain relief. Results of these two-way sensitivity analyses suggest that pain relief 10% or lower led to a lower QALE in patients receiving DMOADs compared to those who did not have a DMOADs-based regimen as a part of their treatment strategy. Pain relief levels of 20% or lower resulted in either lower QALE (in scenarios where suspended OA progression was < 60%) or ICERs greater than $150,000 if suspended progression rates ranged from 60–70%.
Figure 4 also suggests that the cost-effectiveness of DMOADs was very sensitive to the degree of initial pain relief, as well as loss of pain relief benefits in subsequent years, if initial pain relief was between 30–50%. Major toxicity rates played an important role, especially if levels of suspended progression were modest (20–50%).
Table 3 presents results of two-way sensitivity analyses examining the impact of DMOAD cost, efficacy, and toxicity. Improved pain relief (50%) achieved concurrently with suspended progression of 50% led to very favorable cost-effectiveness ratios (<$50,000/QALY); however, ICERs increased over $50,000/QALY when DMOADs were priced at $2,000 or $3,000 annually. Priced at $1,000/year, DMOADs had favorable ICERs across a wide range of plausible values for pain relief, toxicity, and likelihood of suspended progression.
ICERs for DMOADs did not vary substantially when we varied the order of the regimens. When corticosteroid injections (Regimen 2) were received before Regimen 1 in the treatment sequence, DMOADs still carried an ICER of $75,000/QALY. If corticosteroid injections were removed from the treatment sequence altogether, DMOADs carried an ICER of $31,000/QALY.
Altering K-L grade distribution at the time of knee OA diagnosis did not lead to qualitative changes in ICERs. The DMOAD ICERs for cohorts who were 100% K-L grade 1 at the time of diagnosis were $38,000/QALY. The ICER for the 50% K-L grade 1 and 50% K-L grade 2 cohort was $43,000/QALY.
Results of the sensitivity analyses modeling doxycycline as a potential DMOAD showed that doxycycline was a dominated strategy as it did not lead to meaningful improvements in quality of life.
DISCUSSION
Using the OAPol Model, a validated computer simulation of the epidemiology and management of knee OA, we have demonstrated that cost, efficacy, and pain relief are the key drivers of value in DMOADs. We have shown how these drivers trade off with one another. In addition, we have described the many plausible combinations of these drivers which could reduce the need for TKR and satisfy commonly cited cost-effectiveness criteria. There is no general agreement about what defines “cost-effective.” In the United States, maximum willingness-to-pay thresholds ranging from $50,000/QALY to $150,000/QALY and beyond are widely cited44–46.
The cost-effectiveness of DMOADs was highly sensitive to variations in those parameters with direct effects on quality of life, particularly pain relief. Variations in the level of pain relief revealed a distinct threshold of 20%, below which DMOADs would not offer clinical benefits relative to standard care. DMOADs with no intrinsic pain-relieving capacity could only improve quality of life if slowing down progression ultimately reduced pain. Our results validate the importance of targeting pathways which will both reduce progression and offer pain relief.
Since improvements in quality of life are anchored in pain relief, the cost-effectiveness of DMOADs ultimately depends on the level of overall symptom relief achieved by suspended structural progression. Greater rates of suspended OA progression were associated with a lower proportion of the cohort receiving TKR; however, the reduced TKR rates did not translate to greater cost-effectiveness unless DMOADs also offered pain relief because while TKR is costly, it consistently provides pain relief. Thus, in order to justify prolonged DMOAD use before TKR, even in cases of suspended progression, DMOADs must offer pain relief.
Several important limitations of our analyses should be noted. We used the K-L grade as a means of OA progression47,48. While an MRI-based definition of OA and its progression is receiving growing attention, the validation of MRI-based markers is ongoing49. In order to address this limitation and maintain conservative estimates of pain relief, we did not model pain relief as automatically occurring in cases of suspended progression; rather, in the base case, only 30% of subjects experiencing suspended progression also experienced pain relief. Moreover, in the model, the efficacy of DMOADs was expressed in terms of slowing or ‘suspending’ progression based on K-L grade. However, K-L grade is a relatively unresponsive marker of radiographic change and its use may lead to increased time until DMOAD failure detection50. Since conventional radiographs are a current standard of care, our analysis is consistent with clinical practice.
We assumed that failure of DMOADs is detected in the year it occurs. While this assumption biases the results in favor of DMOADs, it seems reasonable since monitoring for failure is triggered by continuous or newly occurring pain.
We chose not to model indirect costs because, at present, there are no data available on the impact of DMOADs on disability or absenteeism. As more data become available, this will be a rich area for future research.
NHIS instruments did not allow for separation between OA occurring at the knee and OA occurring at other sites. The NHIS also did not distinguish OA from gout, RA, lupus, or fibromyalgia. These ambiguities may distort the distributions of sex, BMI and race assigned to persons with knee OA.
This analysis did not consider high-tibial osteotomy (a treatment option for subjects with uni-compartmental disease) as part of the standard treatment sequence because these procedures are performed infrequently in the US51. To ensure that results are generalizable to the overall population with knee OA, we chose to simulate the most common OA treatments.
The cost-effectiveness thresholds will vary from country to country. The results presented in this paper are based on cost and quality of life data measured in the US. This paper offers methodology that could be used to assess cost-effectiveness of DMOADs in other countries, using country-specific data on OA natural history, progression, treatment costs, and potentially alternative thresholds for economic value.
Although we only modeled the use of one DMOAD as part of the OA treatment sequence, it is likely that multiple DMOADs will ultimately become available to patients. It is also possible that DMOADs are more likely to offer pain relief for subjects at earlier stages of OA. However, we did not model varying levels of pain relief based on current K-L grade. In this case, it would be critical to offer DMOADs early in the treatment sequence, thus catching patients before they progress to more severe OA.
The results of our analyses showed that in the absence of DMOADs, the lifetime risk of TKR among those with symptomatic knee OA approached 50%. These results suggest higher TKR rates than estimated in data derived from large cohort studies such as the Osteoarthritis Initiative (OAI)52. There are several reasons for the difference between our model-based estimates and OAI data: 1) persons intending to undergo TKR within 18 months were excluded from OAI, and 2) OAI-based estimates, which indicate a 1%/year conversion to TKR, include data from both incident and prevalent cohorts, with a substantial number of persons at K-L grade 1. In contrast, our model-based estimates used incidence of TKR data derived from the Multicenter Osteoarthritis Study (MOST), which assumes that only subjects with K-L grade 3 or greater were eligible for TKR. Among subjects in the OAI with K-L grade 3 or 4 OA, the conversion to TKR was estimated at about 10%/year52,53. Furthermore, this rate of conversion to TKR among those at K-L grade 3 or 4 was consistent with nationwide estimates of the number of TKRs performed in the US54.
To the best of our knowledge, the results of the analyses documented here comprise the first pre-evaluation of the effectiveness, costs, and cost-effectiveness of DMOAD therapy for knee OA. We have examined the sensitivity of DMOAD value to variations in a wide spectrum of characteristics, most notably efficacy, toxicity, and costs. Our findings may provide critical insights for clinical trial planning and ensure that drug manufacturers focus the development of new regimens on parameters that will affect quality of life, in particular, pain relief. These analyses also offer a new approach in which simulation modeling can be efficiently used to evaluate new treatment strategies under development before the implementation of costly clinical trials.
Supplementary Material
Acknowledgments
ROLE OF THE FUNDING SOURCE
Supported by: NIH/NIAMS R01 AR053112, K24 AR057827, VA Connecticut Healthcare System (Dr. Suter), and Centers for Medicare & Medicaid Services, an Agency of the U.S. Department of Health and Human Services HHSM-500-2008-0025I/HHSM-500-T0001 (Dr. Suter).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Conception and design: Losina, Paltiel, Katz
Analysis and interpretation of the data: Losina, Daigle, Reichmann, Suter, Hunter, Solomon, Walensky, Jordan, Burbine, Paltiel, Katz
Drafting of the article: Losina, Daigle, Katz
Critical revision of the article for important intellectual content: Losina, Daigle, Reichmann, Suter, Hunter, Solomon, Walensky, Jordan, Burbine, Paltiel, Katz
Final approval of the article: Losina, Daigle, Reichmann, Suter, Hunter, Solomon, Walensky, Jordan, Burbine, Paltiel, Katz
Provision of study materials or patients: Losina
Statistical expertise: Losina, Reichmann
Obtaining of funding: Losina
Collection and assembly of data: Losina, Daigle
CONFLICT OF INTEREST
The authors do not have any conflict of interest with respect to the context of this paper.
Contributor Information
Elena Losina, Email: elosina@partners.org.
Meghan E. Daigle, Email: medaigle@partners.org.
William M. Reichmann, Email: breich315@gmail.com.
Lisa G. Suter, Email: lisa.suter@yale.edu.
David J. Hunter, Email: david.hunter@sydney.edu.au.
Daniel H. Solomon, Email: dsolomon@partners.org.
Rochelle P. Walensky, Email: rwalensky@partners.org.
Joanne M. Jordan, Email: joanne_jordan@med.unc.edu.
Sara A. Burbine, Email: sara.burbine@gmail.com.
A. David Paltiel, Email: david.paltiel@yale.edu.
Jeffrey N. Katz, Email: jnkatz@partners.org.
REFERENCES
- 1.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000 Oct 17;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008 Jan;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niu J, Zhang YQ, Torner J, Nevitt M, Lewis CE, Aliabadi P, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009 Mar 15;61(3):329–335. doi: 10.1002/art.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum. 2004 Sep;50(9):2811–2819. doi: 10.1002/art.20489. [DOI] [PubMed] [Google Scholar]
- 5.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004 Mar;63(3):269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. [Accessed August 4, 2012];National Electronic Injury Surveillance System (NEISS) 2000–2009 https://www.cpsc.gov/cgibin/NEISSQuery/home.aspx.
- 7.Zhang Y, Jordan JM. Epidemiology of Osteoarthritis. Clinics in Geriatric Medicine. 2010;26(3):355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64(4):465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008 Feb;16(2):137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Richmond J, Hunter D, Irrgang J, Jones MH, Snyder-Mackler L, Van Durme D, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on The Treatment of Osteoarthritis (OA) of the Knee. J Bone Joint Surg Am. 2010 Apr 1;92(4):990–993. doi: 10.2106/JBJS.I.00982. 2010. [DOI] [PubMed] [Google Scholar]
- 11.Solomon SD, McMurray JJV, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular Risk Associated with Celecoxib in a Clinical Trial for Colorectal Adenoma Prevention. New England Journal of Medicine. 2005;352(11):1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 12.Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. JAMA. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 13.Abramson SB, Attur M, Yazici Y. Prospects for disease modification in osteoarthritis. Nat Clin Pract Rheum. 2006;2(6):304–312. doi: 10.1038/ncprheum0193. [DOI] [PubMed] [Google Scholar]
- 14.Reginster JY, Badurski J, Bellamy N, Bensen W, Chapurlat R, Chevalier X, et al. Efficacy and safety of strontium ranelate in the treatment of knee osteoarthritis: results of a double-blind, randomised placebo-controlled trial. Ann Rheum Dis. Nov 9; doi: 10.1136/annrheumdis-2012-202231. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Efficacy and safety of oral salmon calcitonin in patients with knee osteoarthritis. ClinicalTrials.gov identifier: NCT00486434. 2010 http://clinicaltrials.gov/ct2/show/NCT00486434.
- 16.A long-term, placebo-controlled X-ray study investigating the safety and efficacy of SD-6010 in subjects with osteoarthritis of the knee (ITIC). ClinicalTrials.gov identifier: NCT00565812. 2010 http://clinicaltrials.gov/ct2/show/NCT00565812.
- 17.Hunter DJ. Pharmacologic therapy for osteoarthritis--the era of disease modification. Nat Rev Rheumatol. 2010 Jan;7(1):13–22. doi: 10.1038/nrrheum.2010.178. [DOI] [PubMed] [Google Scholar]
- 18.Losina E, Walensky RP, Reichmann WM, Holt HL, Gerlovin H, Solomon DH, et al. Impact of Obesity and Knee Osteoarthritis on Morbidity and Mortality in Older Americans. Annals of Internal Medicine. 2011 Feb 15;154(4):217–226. doi: 10.1059/0003-4819-154-4-201102150-00001. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holt HL, Katz JN, Reichmann WM, Gerlovin H, Wright EA, Hunter DJ, et al. Forecasting the burden of advanced knee osteoarthritis over a 10-year period in a cohort of 60–64 year-old US adults. Osteoarthritis Cartilage. 2011 Jan;19(1):44–50. doi: 10.1016/j.joca.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for Reporting Cost-effectiveness Analyses. JAMA. 1996 Oct 23;276(16):1339–1341. doi: 10.1001/jama.276.16.1339. 1996. [DOI] [PubMed] [Google Scholar]
- 21.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007 Jan;34(1):172–180. [PubMed] [Google Scholar]
- 22.2005–2006 National Health and Nutrition Examination Survey (NHANES) Data. [Accessed August 4, 2012];National Center for Health Statistics (NCHS), U.S. Department of Health and Human Services. 2006 http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/nhanes05_06.htm.
- 23.2007–2008 National Health and Nutrition Examination Survey (NHANES) Data. [Accessed August 4, 2012];National Center for Health Statistics (NCHS), U.S. Department of Health and Human Services. 2008 http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/nhanes07_08.htm.
- 24. [Accessed August 4, 2012];National Health Interview Survey (NHIS) 2007 http://www.cdc.gov/nchs/nhis.htm.
- 25.Arias E. National Vital Statistics Reports. Vol 58. Hyattsville, Maryland 20782-2003, USA: National Center for Health Statistics; 2010. United States Life Tables, 2006. [PubMed] [Google Scholar]
- 26.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-Mass Index and Mortality among 1.46 Million White Adults. New England Journal of Medicine. 2010;363(23):2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention, National Center for Health Statistics. Chronic conditions, ages 18+ US: 1997–2008. [Accessed August 4, 2012]. Health Data Interactive. http://www.cdc.gov/nchs/hdi.htm. [Google Scholar]
- 28.Brazier J, Usherwood T, Harper R, Thomas K. Deriving a Preference-Based Single Index from the UK SF-36 Health Survey. Journal of Clinical Epidemiology. 1998;51(11):1115–1128. doi: 10.1016/s0895-4356(98)00103-6. [DOI] [PubMed] [Google Scholar]
- 29.Lee DW, Meyer JW, Clouse J. Implications of controlling for comorbid conditions in cost-of-illness estimates: a case study of osteoarthritis from a managed care system perspective. Value Health. 2001 Jul-Aug;4(4):329–334. doi: 10.1046/j.1524-4733.2001.44012.x. [DOI] [PubMed] [Google Scholar]
- 30.Red Book: Pharmacy's Fundamental Reference: 2010 Edition. Montvale, NJ: Thomson Healthcare; 2010. [Google Scholar]
- 31.Tan-Torres Edejer T. Making choices in health : WHO guide to cost-effectiveness analysis. Geneva: World Health Organization; 2003. [Google Scholar]
- 32. [Accessed August 4, 2012];Physician Fee Schedule Search. Center for Medicare and Medicaid Services (CMS) 2010 http://www.cms.gov/pfslookup/02_PFSearch.asp.
- 33.Goldstein JL, Silverstein FE, Agrawal NM, Hubbard RC, Kaiser J, Maurath CJ, et al. Reduced risk of upper gastrointestinal ulcer complications with celecoxib, a novel COX-2 inhibitor. Am J Gastroenterol. 2000;95(7):1681–1690. doi: 10.1111/j.1572-0241.2000.02194.x. [DOI] [PubMed] [Google Scholar]
- 34.Scott DL, Berry H, Capell H, Coppock J, Daymond T, Doyle DV, et al. The long-term effects of non-steroidal anti-inflammatory drugs in osteoarthritis of the knee: a randomized placebo-controlled trial. Rheumatology. 2000 Oct 1;39(10):1095–1101. doi: 10.1093/rheumatology/39.10.1095. 2000. [DOI] [PubMed] [Google Scholar]
- 35.Bensen WG, Fiechtner JJ, McMillen JI, Zhao WW, Yu SS, Woods EM, et al. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trial. Mayo Clinic Proceedings. 1999 Nov 1;74(11):1095–1105. doi: 10.4065/74.11.1095. 1999. [DOI] [PubMed] [Google Scholar]
- 36.Losina E, Weinstein AM, Reichmann WM, Burbine SA, Solomon DH, Daigle ME, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arthritis Care & Research. 2012 doi: 10.1001/archinternmed.2009.136. In Press (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pope GC, Kautter J, Ellis RP, Ash AS, Ayanian JZ, Lezzoni LI, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health care financing review. 2004 Summer;25(4):119–141. [PMC free article] [PubMed] [Google Scholar]
- 38. [Accessed August 4, 2012];Consumer Price Index Inflation Calculator. 2010 http://www.bls.gov/data/inflation_calculator.htm.
- 39.Medicare Current Beneficiary Survey. Centers for Medicare & Medicaid Services; 2006. [Google Scholar]
- 40.Reichmann WM, Katz JN, Kessler CL, Jordan JM, Losina E. Determinants of self-reported health status in a population-based sample of persons with radiographic knee osteoarthritis. Arthritis Rheum. 2009 Aug 15;61(8):1046–1053. doi: 10.1002/art.24839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diehr P, Patrick DL, Spertus J, Kiefe CI, McDonell M, Fihn SD. Transforming self-rated health and the SF-36 scales to include death and improve interpretability. Med Care. 2001 Jul;39(7):670–680. doi: 10.1097/00005650-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Torrance GW, Feeny DH, Furlong WJ, Barr RD, Zhang Y, Wang Q. Multiattribute utility function for a comprehensive health status classification system. Health Utilities Index Mark 2. Med Care. 1996 Jul;34(7):702–722. doi: 10.1097/00005650-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Losina E, Walensky RP, Kessler CL, Emrani PS, Reichmann WM, Wright EA, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009 Jun 22;169(12):1113–1121. doi: 10.1001/archinternmed.2009.136. discussion 1121-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008 Apr;46(4):349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 45.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn't it increase at the rate of inflation? Arch Intern Med. 2003 Jul 28;163(14):1637–1641. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 46.Hidden Costs, Value Lost: Uninsurance in America. Institute of Medicine of the National Academies; 2003. [PubMed] [Google Scholar]
- 47.Neogi T, Felson D, Niu J, Nevitt M, Lewis CE, Aliabadi P, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ (Clinical research ed) 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001 Apr 3;134(7):541–549. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 49.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston–Leeds Osteoarthritis Knee Score) Annals of the Rheumatic Diseases. 2008 Feb 1;67(2):206–211. doi: 10.1136/ard.2006.066183. 2008. [DOI] [PubMed] [Google Scholar]
- 50.Reichmann WM, Maillefert JF, Hunter DJ, Katz JN, Conaghan PG, Losina E. Responsiveness to change and reliability of measurement of radiographic joint space width in osteoarthritis of the knee: a systematic review. Osteoarthritis Cartilage. 2011 May;19(5):550–556. doi: 10.1016/j.joca.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. [Accessed August 4, 2012];Healthcare Cost and Utilization Project (HCUP). Nationwide Inpatient Sample (NIS) 2009 http://hcupnet.ahrq.gov/.
- 52. [Accessed August 4, 2012];Osteoarthritis Initiative (OAI) 2010 http://oai.epi-ucsf.org/datarelease/default.asp.
- 53.Wise BL, Felson DT, Clancy M, Niu J, Neogi T, Lane NE, et al. Consistency of Knee Pain and Risk of Knee Replacement: The Multicenter Osteoarthritis Study. The Journal of Rheumatology. 2011 Apr 15;38(7):1390–1395. doi: 10.3899/jrheum.100743. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. [Accessed August 4, 2012];Healthcare Cost and Utilization Project (HCUP). Nationwide Inpatient Sample (NIS) 1999–2009 http://hcupnet.ahrq.gov/.
- 55.Silverstein FE, Graham DY, Senior JR, Davies HW, Struthers BJ, Bittman RM, et al. Misoprostol Reduces Serious Gastrointestinal Complications in Patients with Rheumatoid Arthritis Receiving Nonsteroidal Anti-Inflammatory Drugs. Annals of Internal Medicine. 1995 Aug 15;123(4):241–249. doi: 10.7326/0003-4819-123-4-199508150-00001. 1995. [DOI] [PubMed] [Google Scholar]
- 56. [Accessed August 4, 2012];Medicare Fee Schedules. Centers for Medicare & Medicaid Services. 2010 http://www.cms.gov/home/medicare.asp.
- 57. [Accessed August 4, 2012];Medicare Hospital Outpatient Prospective Payment System. Centers for Medicare & Medicaid Services. 2010 http://www.cms.gov/HospitalOutpatientPPS/.
- 58. [Accessed August 4, 2012];Medicare Hospital Inpatient Prospective Payment System. Centers for Medicare & Medicaid Services. 2010 http://www.cms.gov/MedicareFeeforSvcPartsAB/03_MEDPAR.asp.
- 59.Van der Esch M, Heijmans M, Dekker J. Factors contributing to possession and use of walking aids among persons with rheumatoid arthritis and osteoarthritis. Arthritis Care & Research. 2003;49(6):838–842. doi: 10.1002/art.11463. [DOI] [PubMed] [Google Scholar]
- 60.Grindrod KA, Marra CA, Colley L, Cibere J, Tsuyuki RT, Esdaile JM, et al. After patients are diagnosed with knee osteoarthritis, what do they do? Arthritis Care & Research. 2010;62(4):510–515. doi: 10.1002/acr.20170. [DOI] [PubMed] [Google Scholar]
- 61.Raynauld JP, Buckland-Wright C, Ward R, Choquette D, Haraoui B, Martel-Pelletier J, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003 Feb;48(2):370–377. doi: 10.1002/art.10777. [DOI] [PubMed] [Google Scholar]
- 62.Ayral X. Injections in the treatment of osteoarthritis. Best Pract Res Clin Rheumatol. 2001;15(4):609–626. doi: 10.1053/berh.2001.0177. [DOI] [PubMed] [Google Scholar]
- 63.Katz JN, Mahomed NN, Baron JA, Barrett JA, Fossel AH, Creel AH, et al. Association of hospital and surgeon procedure volume with patient-centered outcomes of total knee replacement in a population-based cohort of patients age 65 years and older. Arthritis Rheum. 2007 Feb;56(2):568–574. doi: 10.1002/art.22333. [DOI] [PubMed] [Google Scholar]
- 64.Paxton EW, Namba RS, Maletis GB, Khatod M, Yue EJ, Davies M, et al. A prospective study of 80,000 total joint and 5000 anterior cruciate ligament reconstruction procedures in a community-based registry in the United States. J Bone Joint Surg Am. 2010 Dec;92(Suppl 2):117–132. doi: 10.2106/JBJS.J.00807. [DOI] [PubMed] [Google Scholar]
- 65.Katz JN, Barrett J, Mahomed NN, Baron JA, Wright RJ, Losina E. Association between hospital and surgeon procedure volume and the outcomes of total knee replacement. J Bone Joint Surg Am. 2004 Sep;86-A(9):1909–1916. doi: 10.2106/00004623-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 66.Buntin MB, Garten AD, Paddock S, Saliba D, Totten M, Escarce JJ. How much is postacute care use affected by its availability? Health Serv Res. 2005 Apr;40(2):413–434. doi: 10.1111/j.1475-6773.2005.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Teeny SM, York SC, Mesko JW, Rea RE. Long-term follow-up care recommendations after total hip and knee arthroplasty: Results of the american association of hip and knee surgeons' member survey. The Journal of Arthroplasty. 2003;18(8):954–962. doi: 10.1016/j.arth.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 68. [Accessed August 4, 2012];Healthcare Cost and Utilization Project (HCUP). Nationwide Inpatient Sample (NIS). Agency for Healthcare Research and Quality. 2008 http://hcupnet.ahrq.gov/. [PubMed]
- 69.Sullivan PW, Ghushchyan V. Preference-Based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006 Jul-Aug;26(4):410–420. doi: 10.1177/0272989X06290495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jansen JP, Pellissier J, Choy EH, Ostor A, Nash JT, Bacon P, et al. Economic evaluation of etoricoxib versus non-selective NSAIDs in the treatment of ankylosing spondylitis in the UK. Curr Med Res Opin. 2007 Dec;23(12):3069–3078. doi: 10.1185/030079907X242575. [DOI] [PubMed] [Google Scholar]
- 71.Kamath CC, Kremers HM, Vanness DJ, O'Fallon WM, Cabanela RL, Gabriel SE. The Cost-Effectiveness of Acetaminophen, NSAIDs, and Selective COX-2 Inhibitors in the Treatment of Symptomatic Knee Osteoarthritis. Value Health. 2003;6(2):144–157. doi: 10.1046/j.1524-4733.2003.00215.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.