Abstract
Purpose
To report, from Cancer and Leukemia Group B Protocol 9082, the impact of high-dose cyclophosphamide, cisplatin, and BCNU (HD-CPB) vs. intermediate-dose CPB (ID-CPB) on the ability to start and complete the planned course of local–regional radiotherapy (RT) for women with breast cancer involving ≥10 axillary nodes.
Methods and Materials
From 1991 to 1998, 785 patients were randomized. The HD-CPB and ID-CPB arms were balanced regarding patient characteristics. The HD-CPB and ID-CPB arms were compared on the probability of RT initiation, interruption, modification, or incompleteness. The impact of clinical variables and interactions between variables were also assessed.
Results
Radiotherapy was initiated in 82% (325 of 394) of HD-CPB vs. 92% (360 of 391) of ID-CPB patients (p = 0.001). On multivariate analyses, RT was less likely given to patients who were randomized to HD treatment (odds ratio [OR] = 0 .38, p < 0.001), older (p = 0.005), African American (p = 0.003), postmastectomy (p = 0.02), or estrogen receptor positive (p = 0.03). High-dose treatment had a higher rate of RT interruption (21% vs. 12%, p = 0.001, OR = 2.05), modification (29% vs. 14%, p = 0.001, OR = 2.46), and early termination of RT (9% vs. 2%, p = 0.0001, OR = 5.35), compared with ID.
Conclusion
Treatment arm significantly related to initiation, interruption, modification, and early termination of RT. Patients randomized to HD-CPB were less likely to initiate RT, and of those who did, they were more likely to have RT interrupted, modified, and terminated earlier than those randomized to ID-CPB. The observed lower incidence of RT usage in African Americans vs. non–African Americans warrants further study.
Keywords: Breast cancer, Radiotherapy, High-dose chemotherapy, Toxicity
Introduction
Most women with breast cancer present with local–regional disease (breast with or without the regional axillary nodes), without evidence of distant systemic disease. The risk of subsequent systemic relapse is related to the extent of axillary disease (1–4). Among patients with ≥10 involved axillary nodes at presentation, approximately 50–90% will relapse systemically within 5 years despite conventional adjuvant chemotherapy (5, 6).
Some studies (7–9), albeit not all (10, 11), suggest that chemotherapy intensity might influence its ability to sterilize subclinical distant metastases. Toward this goal, a series of patients with ≥10 positive axillary nodes were treated on a Phase II study with four cycles of conventional-dose CAF (cyclophosphamide, doxorubicin, and 5-fluorouracil) followed by high-dose cyclophosphamide, cisplatin, and 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) (HD-CPB) with autologous bone marrow support (Cancer and Leukemia Group B [CALGB] Protocol 8782). The 3-year relapse-free survival rate was 72% (12), better than in historical controls.
This observation led to a multi-institution, intergroup, randomized, Phase III study (CALGB 9082, Southwest Oncology Group 9114, National Cancer Institute of Canada MA-13) to test this approach in patients with ≥10 positive axillary nodes. After surgical removal of all gross disease, patients received initial CAF chemotherapy, followed by randomization to either HD-CPB or intermediate doses of the same drugs (ID-CPB). Preliminary analysis, with a median 3-year follow-up, suggested a reduction in breast cancer relapses but an increase in treatment-related mortality in the HD-CPB arm compared with the ID-CPB arm. Overall survival rates were equivalent in the two arms (13, 14).
All patients in this study were prescribed to receive local–regional radiotherapy (RT) after chemotherapy. We herein report the impact of HD-CPB vs. ID-CPB on the ability to start and complete the planned course of local–regional RT. Other clinical variables that might be associated with omission of the planned RT were also considered. This is an important issue because local–regional RT dramatically reduces the risk of locoregional relapse, reduces the risk of systemic relapse, and improves overall survival (15–25). Therefore, modifications in systemic therapy that reduce one's ability to deliver RT might negatively affect patient outcome.
Methods and Materials
Patients
The trial was open to accrual between January 1991 and May 1998; details reported elsewhere (14). Eligibility criteria included (1) age ≥18 years with operable non–locally advanced/inflammatory breast adenocarcinoma involving ≥10 axillary nodes, but without evidence of distant metastases (negative results on bilateral bone marrow biopsies and aspirates, bone scan, and chest, abdomen, pelvis, and brain CT scans); (2) resection of all gross disease by mastectomy (radical or modified radical) or lumpectomy with Level I to II axillary dissection; (3) negative surgical margins (lymphatic and vascular involvement was permitted) (pathologic stage T1–3, N1–2; American Joint Committee on Cancer 1988 criteria); (4) Eastern Cooperative Oncology Group performance score of 0–1; (5) no previous chemotherapy or RT; and (6) not pregnant. Patients with prior or concomitant malignancies, other than curatively treated carcinoma in situ of the cervix or nonmelanotic skin cancer, were not eligible. Patients with serious comorbid diseases that would have negatively affected their tolerance for therapy were not eligible. Patients with synchronous bilateral breast cancers were eligible. Patients provided written, informed consent to participate in the study.
Treatment
Systemic therapy
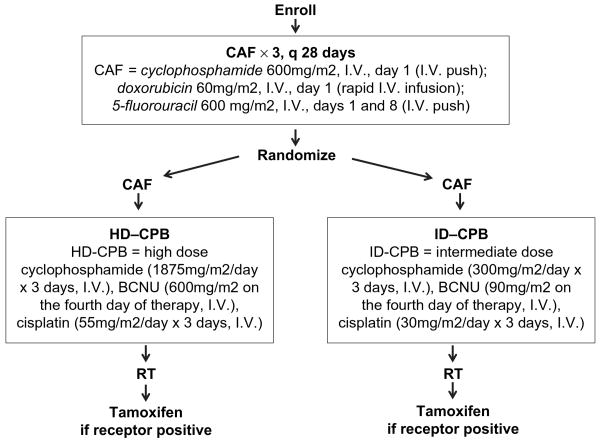
Protocol treatment included three cycles of adjuvant CAF chemotherapy followed by randomization to a fourth cycle of CAF plus HD-CPB or a fourth cycle of CAF plus ID-CPB (Fig. 1) (14). Patients were restaged approximately 6 weeks after completion of all chemotherapy. The protocol-prescribed RT was to be delivered in all patients provided there was no evidence of systemic disease at restaging. Patients with positive/unknown hormone receptor status were to receive tamoxifen for 5 years starting 7 weeks after the initiation of CPB. In many patients, tamoxifen was not started until after RT (Fig. 1).
Fig. 1.
Scheme of Cancer and Leukemia Group B Protocol 9082. RT = radiotherapy.
Radiotherapy
Sites to be irradiated included the ipsilateral breast/chest wall and supraclavicular lymph nodes. Irradiation of the axilla was not recommended but was permitted at the discretion of the treating radiation oncologist. Treatment of the internal mammary nodes (IMN) was strongly recommended, though not required.
Three treatment techniques were given as options: (1) Technique A: tangent breast/chest wall fields with or without IMN. In some cases, partly wide tangent fields were used that included the superior but not the inferior IMN chain, to reduce incidental irradiation to the heart (26). To consider the possible effect of inclusion of IMN within the RT fields on outcome, patients were further subdivided by IMN dose <30 Gy (Technique A1) or ≥30 Gy (Technique A2). The 30-Gy threshold was chosen on the basis of human clinical studies suggesting that doses of ≥25 Gy can be associated with lung injury. (2) Technique B: anterior or anterior-oblique electron/photon IMN field with tangent photon breast/chest wall fields. (3) Technique C: en face chest wall and IMN electron fields (not appropriate for patients undergoing lumpectomy), with bolus as clinically indicated.
For all techniques, CT planning was recommended to assess IMN location and for dosimetry. Cobalt-60 and high-energy photons between 4 and 15 MV were used to treat the supraclavicular and axillary nodes, and the chest wall (and IMN) for patients receiving Techniques A and B. Electron energy was selected at the discretion of the treating physician to provide homogeneous coverage to the target volume. Wedges and bolus were used as necessary. A separate anterior medial oblique supraclavicular field (angled approximately 15° to the contralateral side; i.e., medial-anterior oblique) was used. This angulation was recommended to provide adequate coverage of the medially placed nodes without exiting into the spine. Field borders were as follows: inferior—inferior to the clavicular head and matched to the superior border of the breast/chest wall field; medial—medial to the sternocleidomastoid muscle; superior—at the level of the cricoid cartilage; and lateral—at the medial extent the axillary surgery, or at approximately the coracoid process or medial humeral head. Treatment of the full axilla, by extending the lateral border of this supraclavicular field into the axilla, and the use of posterior axillary boost were not recommended but were permitted.
All sites were to be treated to 50.4 Gy at 1.8 cGy per fraction. The prescription point for the supraclavicular field was 3 cm, or at the depth of the supraclavicular vessels as measured on CT imaging. An additional 10 Gy in 5 fractions with electrons was prescribed to the region of the mastectomy scar or tumor bed. This could be omitted at the physician's discretion for patients who had breast reconstruction.
Submission of patient therapy data
Patient registration and data collection were managed by the CALGB Statistical Center. Demographics and baseline clinicopathologic variables were collected before initiation of protocol treatment. The following data were collected at regular intervals during and after treatment: toxicity, treatment information (dosing, site of delivery, and reason for early termination/noncompliance) for chemotherapy and RT, and disease and survival status. These data were initially reviewed for accuracy and completeness at CALGB Data Operations and subsequently in an independent audit process. Radiotherapy data underwent central review by the Quality Assurance Review Center.
Statistical considerations
The relationship of demographic and baseline clinicopathologic factors to each of four different outcomes was assessed: (1) initiation, (2) interruption, (3) modification, and (4) completion of protocol-prescribed RT. Because the reason for the occurrence of each outcome was not recorded, each outcome was scored as a dichotomous event, that is, occurred or not, regardless of reason. Outcome 1 was measured for all patients randomized to the Treatment Study 9082, whereas Outcomes 2, 3, and 4 were measured only for those patients who began protocol RT.
Candidate explanatory variables used to model the relationship with Outcome 1 included chemotherapeutic treatment arm (HD/ID), age at enrollment (continuous), race (African American [AA]/non-AA), menopausal status (pre/post), tumor-related variables (estrogen receptor [ER] status [positive/negative]), tumor size (≤5/>5 cm), number of positive nodes (10–15/16+), and type of surgery (mastectomy/nonmastectomy). Radiotherapy technique (A1/A2/B/C) was also included as a candidate predictor for Outcomes 2–4.
Forward stepwise logistic regression (with an α = 0.05 to enter the model) was used to study the multivariate association of each outcome with the candidate predictors listed above. Univariate associations were tested (α = 0.05) with the χ2-test or the Mann-Whitney U test, as appropriate. The following interactions, selected a priori, were descriptively examined for associations with outcome variables: arm × technique, arm × race, arm × age, and race × age. On the basis of this examination, only the following interactions were subsequently tested in the multivariate model: (1) race × age on RT initiation, and (2) arm × technique on RT modification, interruption, and completion. In addition, for Outcomes 2–4, the interaction of RT technique with surgery type was examined descriptively only, because it contained a zero cell (i.e., lumpectomy patients cannot undergo RT Technique C). A two-sided α = 0.05 was used for all statistical tests. Cancer and Leukemia Group B statisticians performed all statistical analyses on study data stored in the CALGB database, using SAS software (SAS Institute, Cary, NC).
Results
A total of 785 patients were randomized on CALGB 9082 (Table 1). The two arms were well balanced in terms of patient characteristics. The median survival follow-up, measured from randomization, for the 466 surviving patients was 7.5 years (range, 2.6–11.8 years).
Table 1.
Patient demographics and baseline disease characteristics by treatment arm
| Treatment arm | ||||
|---|---|---|---|---|
| Characteristic | HD-CPB | ID-CPB | ||
| Total randomized | 394 | (100) | 391 | (100) |
| Age at study entry (y) | ||||
| <40 | 115 | (29) | 114 | (29) |
| 40–49 | 170 | (43) | 189 | (49) |
| 50–59 | 100 | (26) | 80 | (20) |
| 60+ | 9 | (2) | 8 | (2) |
| Race/ethnicity | ||||
| Caucasian | 315 | (80) | 317 | (81) |
| Hispanic | 6 | (2) | 5 | (1) |
| African American | 28 | (7) | 22 | (6) |
| Asian | 10 | (2) | 7 | (2) |
| Other | 35 | (9) | 40 | (10) |
| Menopausal status | ||||
| Premenopausal | 268 | (68) | 263 | (67) |
| Peri-/postmenopausal | 126 | (32) | 127 | (32) |
| Missing | 0 | (0) | 1 | (<1) |
| Tumor size (cm) | ||||
| ≤2 | 103 | (26) | 96 | (24) |
| >2 and ≤5 | 193 | (49) | 206 | (53) |
| >5 | 90 | (23) | 75 | (19) |
| Missing | 8 | (2) | 14 | (4) |
| No. of positive nodes | ||||
| 10–12 | 162 | (41) | 160 | (41) |
| 13–15 | 82 | (21) | 92 | (24) |
| 16–20 | 83 | (21) | 79 | (20) |
| ≥21 | 67 | (17) | 60 | (15) |
| Surgery type | ||||
| Mastectomy | 310 | (79) | 301 | (77) |
| Lumpectomy | 81 | (20) | 87 | (22) |
| Other* | 3 | (1) | 1 | (<1) |
| Missing | 0 | (0) | 2 | (<1) |
| Initiated RT | ||||
| No | 69 | (18) | 31 | (8) |
| Yes | 325 | (82) | 360 | (92) |
| If yes, RT technique | ||||
| A | 200 | (62) | 226 | (62) |
| B | 83 | (25) | 85 | (24) |
| C | 26 | (8) | 32 | (9) |
| Other/Unknown | 16 | (5) | 17 | (5) |
Abbreviations: HD-CPB = high-dose cyclophosphamide, 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU), and cisplatin; ID-CPB = intermediate-dose CPB; RT = radiotherapy.
Values are number (percentage).
Includes biopsy alone, and no breast surgery (i.e. only axillary dissection), for patients presenting with axillary disease without a clear primary breast lesion.
The endpoints of interruption, modification, and failure to complete RT were not mutually exclusive endpoints. Seventy-one percent (71%) of patients who began RT successfully completed it without either interruption or modification. One hundred thirty-two (132) patients (19%) experienced only one endpoint (interruption, 8%; modification, 10%; early termination, 1%), whereas 8% experienced two (interruption and modification, 6%; modification and early termination, 3%). Two percent terminated RT early with both interruptions and modifications.
Initiation of protocol radiotherapy
Univariate and multivariate results are shown in Tables 2 and 3, respectively. Of the 785 randomized patients, 87% began protocol RT. A larger proportion of ID vs. HD patients initiated RT (92% vs. 82%; p = 0.001). After adjusting for patient age and race, surgery type, and tumoral ER status in multivariate logistic regression, treatment arm remained a significant predictor of RT (odds ratio [OR] = 0.38, 95% confidence interval [CI] = 0.23–0.61, p < 0.0001). The interaction of race × age was marginally significant in the multivariate model (p = 0.07). Only three of the eight AAs aged ≥50 years (38%) initiated RT, compared with much larger percentages for the 42 AAs aged <50 years (83%), the 546 Caucasians aged <50 years (90%), and the 189 Caucasians aged ≥50 years (82%).
Table 2.
Incidence of RT initiation, interruption, modification, and early termination by treatment arm
| Treatment arm | |||||
|---|---|---|---|---|---|
| Parameter | HD-CPB | ID | p* | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
| Randomized, n (%) | 394 (100) | 391 (100) | |||
| Began RT, n (% of randomized) | 325 (82) | 360 (92) | 0.001 | 0.41 (0.26–0.64) | 0.38 (0.23–0.61) |
| Began RT, n (%) | 325 (100) | 360 (100) | |||
| RT interrupted | 69 (21) | 43 (12) | 0.001 | 2.05 (1.34–3.14) | N/A |
| RT modified | 94 (29) | 51 (14) | 0.001 | 2.46 (1.68–3.61) | N/A |
| RT not completed† | 29 (9) | 8 (2) | 0.0001 | 5.35 (2.17–13.16) | 5.68 (2.29–14.1) |
Abbreviations: OR = odds ratio of HD:ID related to each endpoint; 95% CI = 95% confidence interval around the OR; N/A = treatment arm was the only significant variable, therefore the univariate OR is used. Other abbreviations as in Table 1.
Unadjusted OR is from univariate analysis; adjusted OR is from multivariate logistic regression controlling for other covariates.
p value from comparison of two proportions.
Excludes 27 patients with unknown completion status.
Table 3.
Initiation of RT: Results from multivariate logistic regression (n = 774)
| Effect | More:Less likely to begin RT | OR (95% CI) |
p |
|---|---|---|---|
| Treatment arm | ID:HD | 2.66 (1.65–4.27) |
<0.0001 |
| Age (y) | 40:60 | 2.22 (1.27–3.92) |
0.005 |
| Race* | Non-AA:AA | 2.99 (1.45–6.17) |
0.003 |
| Surgery | Nonmastecomy: mastectomy | 2.10 (1.12–3.94) |
0.02 |
| Tumor ER status | Negative: positive | 1.72 (1.06–2.78) |
0.03 |
| Race × age interaction | 0.07 | ||
| Age <50 y | Non-AA:AA | 1.83 (0.76–4.41) |
|
| Age ≥50 y | Non-AA:AA | 9.43 (2.08–43.48) |
Abbreviations: AA= African American; ER = estrogen receptor. other abbreviations as in Table 1.
Main effects of race and age tested in the absence of the interaction term.
Among those who initiated RT, the median time to start of RT (from the start of CPB) was shorter for the ID arm than for the HD arm (50 vs. 62 days; p < 0.0001).
Interruptions in protocol RT
Of the 685 patients who began RT, 112 (16%) had their RT delivery interrupted, 21% on the HD arm and 12% on the ID arm (p = 0.001, unadjusted OR = 2.05, 95% CI = 1.34–3.14; Table 2). Multivariate analyses indicated that only treatment arm significantly related to RT interruption. There was, however, a suggestion that RT technique as a main effect related to interruption (p = 0.064). Technique C was more likely to result in RT interruption than each of the other techniques (OR [95% CI] of C:A1, A2, and B, respectively, were 2.02 [0.96–4.27], 2.31 [1.10–4.85], and 1.36 [0.65–2.80]).
Table 4 shows the percentage of patients whose RT was interrupted according to RT technique and surgery type. Among nonmastectomy patients, there was no difference among Techniques A1, A2, and B; however, among mastectomized patients, Technique C (26%) had a slightly higher incidence of interruption than Techniques A1, A2, or B (17%, 13%, and 20%, respectively).
Table 4.
Incidence of RT interruption by RT technique and type of surgery
| Mastectomy | Nonmastectomy† | |||
|---|---|---|---|---|
| RT technique | n | % with interruption | n | % with interruption |
| Total patients who began RT* | 525 | 17 | 158 | 15 |
| A: Tangents | ||||
| A1: IMN <30 Gy | 145 | 17 | 40 | 13 |
| A2: IMN ≥30 Gy | 160 | 13 | 79 | 15 |
| B: Separate IMN field | 137 | 20 | 31 | 23 |
| C: Chest wall electrons | 58 | 26 | 0 | — |
| Other/unknown | 25 | 0 | 8 | 0 |
Abbreviation: IMN = internal mammary nodes. Other abbreviations as in Table 1.
Excludes patients with missing data on type of surgery.
Lumpectomy, n = 155; axillary dissection, n = 3.
Modifications to protocol RT
One hundred forty-five (145, 21%) of the patients who initiated RT underwent modifications to the planned RT regimen. Incidence of modification on the HD arm (29%) was more than twice that of the ID arm (14%; p = 0.001, OR = 2.46, 95% CI = 1.68–3.61; Table 2). In multivariate analysis, treatment arm was the only significant predictor of modification. The most frequent reason for RT modification was RT-related toxicity for the ID arm (33%) and chemotherapy-related toxicity for the HD arm (39%) (Table 5).
Table 5.
Reasons for RT modification by treatment arm
| Treatment arm | |||
|---|---|---|---|
| Reason | HD | ID | Total |
| RT modified | 94 (100) | 51 (100) | 145 (100) |
| Reason for modification* | |||
| Toxicity from RT | 15 (16) | 17 (33) | 32 (22) |
| Toxicity from chemotherapy | 37 (40) | 3 (6) | 40 (28) |
| Patient noncompliance | 2 (2) | 0 | 2 (1) |
| Scheduling difficulties/mechanical problems | 6 (6) | 7 (14) | 13 (9) |
| Complicating medical conditions | 20 (21) | 8 (16) | 28 (19) |
| Other | 14 (15) | 16 (31) | 30 (21) |
Abbreviations as in Table 1.
Values are number (percentage).
Taken from data sheets submitted, usually not containing detailed information; thus the categorization may not be specific.
Completion of protocol RT
The noncompletion incidence on the ID arm was only 2%, compared with 9% on HD (p = 0.0001, unadjusted OR = 5.35; Table 2). In multivariate analyses, treatment arm (p = 0.0002) and RT technique (p = 0.023) significantly predicted RT noncompletion. The OR of HD:ID on noncompletion was 5.68 (95% CI = 2.29–14.1). Technique C was more likely to result in noncompletion than each of the other techniques (OR [95% CI] for C:A1, A2, and B, respectively, was 2.89 [0.98–8.55], 2.50 [0.89–7.04], and 6.62 [1.80–24.2]).
Table 6 shows the incidence of noncompletion according to RT technique and surgery type. Failure to complete RT was greatest for mastectomized patients irradiated with Technique C (14%) compared with any of the other subgroups defined by technique and surgery (ranging from 0 to 8%).
Table 6.
Incidence of noncompletion of RT by RT technique and type of surgery
| Mastectomy | Nonmastectomy | |||
|---|---|---|---|---|
| RT technique | n | % terminated early | n | % terminated early |
| Total patients who began RT* | 505 | 6 | 151 | 5 |
| A: Tangents | ||||
| A1: IMN <30 Gy | 140 | 5 | 36 | 8 |
| A2: IMN ≥30 Gy | 152 | 6 | 79 | 5 |
| B: Separate IMN field | 132 | 3 | 29 | 0 |
| C: Chest wall electrons | 56 | 14 | 0 | — |
| Other/unknown | 25 | 8 | 7 | 0 |
Reported RT-associated toxicities
The precise reason for an interruption, modification, or cessation of RT was not always clear (Table 5). Data forms on RT-related toxicity were submitted for 681 patients (Table 7). The most frequently reported side effect was skin related (ID: 34% vs. HD: 24%). The HD vs. ID arms differed most in incidence of Grade ≥2 RT-associated events in the lung (7% vs. <1%), anemia (8% vs. <1%), and thrombocytopenia (9% vs. <1%). Other events were infrequent. Although these events were scored as RT-associated, they likely were also related to the prior chemotherapy.
Table 7.
Incidence of radiation-associated toxicity rates by treatment arm*
| Treatment arm | ||
|---|---|---|
| Toxicity and grade | HD-CPB (n = 322) |
ID-CPB (n = 359) |
| Skin | ||
| Grade 2 | 72 (22) | 98 (27) |
| Grade 3 | 8 (2) | 22 (6) |
| Grade 4 | 0 | 2 (<1) |
| Lung | ||
| Grade 2 | 8 (2) | 1 (<1) |
| Grade 3 | 13 (4) | 1 (<1) |
| Grade 4 | 2 (<1) | 0 |
| Leukopenia | ||
| Grade 2 | 17 (5) | 17 (5) |
| Grade 3 | 6 (2) | 6 (2) |
| Grade 4 | 0 | 0 |
| Anemia | ||
| Grade 2 | 19 (6) | 1 (<1) |
| Grade 3 | 7 (2) | 0 |
| Grade 4 | 1 (<1) | 0 |
| Thrombocytopenia | ||
| Grade 2 | 15 (5) | 1 (<1) |
| Grade 3 | 11 (3) | 0 |
| Grade 4 | 2 (<1) | 1 (<1) |
| Upper gastrointestinal | ||
| Grade 2 | 2 (1) | 0 |
| Grade 3 | 1 (<1) | 0 |
| Grade 4 | 0 | 0 |
Abbreviations as in Table 1.
Values are number (rate [%]).
Grade ≥2 only. Data available in 681 patients. Some patients with more than one toxicity scored. Events scored as “radiation-related,” but certainly may be related to chemotherapy. Other minor toxicities with <1% incidence are not shown (e.g., breast edema).
Discussion
Initial data from CALGB 9082, with modest follow-up, suggests that HD-CPB does not provide an advantage with regard to overall survival compared with ID-CPB (13, 14). Interestingly, there seems to be a small improvement in breast cancer–specific survival in the high-dose arm that was offset by treatment-related mortality. High-dose chemotherapy can be toxic (12, 14, 27–30). With improvements in our ability to prevent and manage these morbidities, high dose may provide some therapeutic advantage. At present the majority of available data do not support the routine use of high-dose chemotherapy for patients with breast cancer (31–33).
The present analysis suggests that HD-CPB hinders the ability to deliver RT, compared with ID-CPB. This difference is not related to any pretreatment factors, and it persists in subgroups defined by race, age, and RT technique. This observation is noteworthy because RT plays an important role in the care of patients with breast cancer. Postlumpectomy RT reduces the risk of in-breast recurrences by approximately 80% (34–36). Postmastectomy RT reduces the risk of local–regional recurrence by approximately 67% and improves overall survival by 5–12% (15–25, 37). Therefore, alterations in the chemotherapy regimen that hinder the ability to deliver radiation might negatively impact on disease-free and overall survival. However, in CALGB 9082 the relapse-free survival in the HD group was higher than in the ID group (14), suggesting that possible detrimental effects on disease control resulting from the limitations on the RT delivery were offset by the possible additional anticancer effects afforded by the HD therapy.
The relevance of the current data set can be questioned, given that high-dose chemotherapy and bone marrow rescue is not typically performed for patients with multi-node-positive breast cancer at present. Nevertheless, this approach may have some utility in certain subsets of patients (e.g., young patients), and the findings from the present project might be applicable if this therapy were to make a resurgence. Furthermore, there is continued interest in intensifying chemotherapy regimens for some subsets of patients, as evidenced by the use of dose-dense chemotherapy regimens (38), and the use of combined anthracycline/taxane regimens (39). Therefore, the potential impact of these more-intensive chemotherapy regimens on the ability to deliver subsequent postchemotherapy radiation may be relevant as well.
Radiation technique
It is not clear why Technique C was associated with a higher rate of treatment interruption and failure to complete treatment (Tables 4 and 6). The use of en face chest wall electrons is a well-accepted technique and was used in the Danish trials demonstrating an improved survival with postmastectomy RT (18, 40–41). Fewer patients were treated with Technique C than the other techniques; additionally, the technique was not used on lumpectomy patients. This made robust comparisons difficult. This finding may reflect physician practice patterns regarding the use of different RT techniques and their tendency to alter an initiated course of RT. A review of RT techniques among participating institutions did not reveal any obvious systematic differences. Nevertheless, this observation may be due to the preferential use of Technique C in patients with a lot of chemotherapy-associated toxicity. The exact cause of an interruption or failure to complete therapy was not systematically recorded and often is multifactorial. Therefore, the degree to which, for example, increased skin toxicity may have been seen in the electron patients is not known.
Among patients treated with Technique A, there were no statistically significant differences in the rates of interruption or completion between those in whom the IMN dose was estimated to be <30 Gy vs. ≥30 Gy. Treatment of the IMN tends to increase irradiated lung volumes, suggesting that lung irradiation was not the major cause for treatment modifications. However, because IMN treatment was not assigned randomly, this may not be a valid conclusion. Furthermore, given interpatient anatomic variability, inclusion of the IMN within the tangent fields does not always translate into a larger volume of incidentally irradiated lung (42).
In this analysis, patients treated with mastectomy and lumpectomy were combined. This is reasonable given that the RT fields in the postlumpectomy and postmastectomy settings are essentially equivalent. One would not therefore expect the rates of, for example, treatment modification and interruption, to be related to the presence/absence of an intact breast. It is nevertheless possible that physicians use a different threshold for interruption/modification/suspension of RT in postlumpectomy vs. postmastectomy settings. Similarly, some of the observed results (HD vs. ID) may be due to the application of more conservative thresholds (e.g., more likely to use a treatment interruption) in patients that have received more aggressive chemotherapy (i.e., with a more troubling toxicity profile).
Postmastectomy and postlumpectomy RT are generally well tolerated. Acute RT-associated morbidity requiring treatment interruption or modification in the planned RT is unusual. Therefore the 12% rate of treatment interruption in the ID-CPB cohort might be higher than would be expected. It is possible that the chemotherapy regimen in the control arm of this study was more aggressive than conventional chemotherapy. The majority of modifications seem to have been treatment related. However, treatment interruptions were liberally scored to include events such as machine breakdown and the development of systemic disease, suggesting that the reported rate may be artificially high.
Prior Phase I–II studies suggest that it is more difficult for patients to complete local–regional RT after high-dose chemotherapy than after conventional-dose chemotherapy. In the Phase I–II pilot study of the HD-CPB regimen (CALGB 8782), radiotherapy was interrupted or discontinued in 9 of 40 irradiated patients (22%) (43)—a rate similar to the 21% interruption rate observed in the HD-CPB arm of the present study. Several other reports of non-randomized patients receiving HD-chemotherapy along with RT do not note similar RT-compliance issues (44–46). However, inter-study differences make comparisons difficult.
Influence of other clinical factors
Radiotherapy was more likely to be initiated in patients who were younger, non-AA, underwent lumpectomy, or had ER-negative tumors. It is logical that younger patients were more likely to start RT because the benefits of RT are often considered to be more relevant in younger women. Furthermore, younger patients may be more motivated, healthy, and interested in receiving the prescribed RT compared with older women. Similarly, the impact of type of surgery on RT initiation is expected. Postlumpectomy RT is more widely accepted as an important component of cancer care than is mastectomy RT (whose value is more often questioned). The increased use of RT in the ER-negative patients (vs. ER-positive) may be due to the fact that these patients will not derive the anticancer benefits afforded by hormonal therapy, and thus the RT may be considered to be relatively more important.
The observation that the rate of delivering RT according to protocol was influenced by race was unexpected. African American patients received RT less frequently than non-AA patients in both the HD-CPB and ID-CPB arms. This finding is independent of the other baseline clinical/pathologic variables, namely, type of surgery, extent of axillary disease, tumor size, age, or menopausal status. Therefore, race does seem to be an independent factor for whether a patient initiated RT according to protocol. Once patients started RT, however, there was no impact of race on the frequency of modification or failure to complete the planned RT.
The impact of race on the receipt of therapy has been considered in several settings. Studies of men with prostate cancer and women with breast cancer suggest that Caucasians and AAs seemed to have received comparable treatments (45–50). However, AAs seem to be less likely than Caucasians to undergo surgery for early-stage lung cancer (51), coronary revascularization procedures (52, 53), breast cancer screening (54–56), and renal transplantation (57). Non–guideline-concordant adjuvant chemotherapy regimens were more frequently used in AA women, which might contribute to less favorable outcomes in these populations (58). Therefore, systematic differences related to race might exist. Most studies addressing this issue rely on epidemiologic information in large databases. Patient candidates for a particular procedure are identified, and the incidence of the procedure is calculated in racial subgroups. Although care is taken to try to compensate for nonracial differences between patients, this might be difficult. In the present study, patients were enrolled onto a prospective clinical trial that prescribed RT to be delivered. This is a fundamentally different type of data set and may suggest racial biases. On the basis of these findings from CALGB 9082, similar analyses in other cooperative group studies should be considered to further address this interesting observation.
This study suggests that the delivery of postoperative local–regional RT can be compromised by the use of high-dose chemotherapy. Because such RT improves both local control and survival, future clinical trials of adjuvant high-dose chemotherapy should carefully consider this issue.
Acknowledgments
The authors thank Jane Hoppenworth and Jessica Hubbs for assistance with manuscript preparation.
Supported by National Cancer Institute (NCI) Grants CA31946 (to Cancer and Leukemia Group B [CALBG], Richard L. Schilsky, Chairman), CA33601 (to the CALGB Statistical Center, Stephen George, Director), CA29511 (to the Quality Assurance Review Center), CA47577 (to J.V. and L.R.P.), CA42777 (to E.J.S.), CA77202 (to M.C.), CA32291 (to P.G.R.), CA35279 (to M.W.S.), CA77651 (to L.N.), CA11789 (to S.S.), CA60138 (to I.C.H.), CA03927 (to D.D.H.), and CA14028 (to W.P.P.). This manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NCI.
Appendix
The following institutions participated in this study.
Dana-Farber Cancer Institute, Boston, MA: Eric P. Winer, M.D., supported by CA32291.
Dartmouth Medical School–Norris Cotton Cancer Center, Lebanon, NH: Marc S. Ernstoff, M.D., supported by CA04326.
Duke University Medical Center, Durham, NC: Jeffrey Crawford, M.D., supported by CA47577.
Long Island Jewish Medical Center, Lake Success, NY: Kanti R. Rai, M.D., supported by CA11028.
Massachusetts General Hospital, Boston, MA: Jeffrey W. Clark, M.D., supported by CA12449.
McGill University, Montreal, QC: Gerald Batist, M.D.
Medical University of South Carolina, Charleston, SC: Mark Green, M.D., supported by CA03927.
Memorial Sloan-Kettering Cancer Center, New York, NY: Clifford A. Hudis, M.D., supported by CA77651.
Mount Sinai Medical Center, Miami, FL: Rogerio C. Li-lenbaum, M.D., supported by CA45564.
North Shore–Long Island Jewish Medical Center, Manhas-set, NY: Daniel R. Budman, M.D., supported by CA35279.
Rhode Island Hospital, Providence, RI: William Sikov, M.D., supported by CA08025.
Roswell Park Cancer Institute, Buffalo, NY: Ellis Levine, M.D., supported by CA02599.
State University of New York Upstate Medical University, Syracuse, NY: Stephen L. Graziano, M.D., supported by CA21060.
University of California at San Francisco, San Francisco, CA: Alan P. Venook, M.D., supported by CA60138.
University of Chicago, Chicago, IL: Gini Fleming, M.D., supported by CA41287.
University of Iowa, Iowa City, IA: Daniel A. Vaena, M.D., supported by CA47642.
University of Maryland Greenebaum Cancer Center, Baltimore, MD: Martin Edelman, M.D., supported by CA31983.
University of Massachusetts Medical School, Worcester, MA: William V. Walsh, M.D., supported by CA37135.
University of Minnesota, Minneapolis, MN: Bruce A Peterson, M.D., supported by CA16450.
University of Missouri/Ellis Fischel Cancer Center, Columbia, MO: Michael C Perry, M.D., supported by CA12046.
University of North Carolina at Chapel Hill, Chapel Hill, NC: Thomas C. Shea, M.D., supported by CA47559.
Wake Forest University School of Medicine, Winston-Salem, NC: David D Hurd, M.D., supported by CA03927.
Walter Reed Army Medical Center, Washington, DC: Thomas Reid, M.D., supported by CA26806.
Washington University School of Medicine, St. Louis, MO: Nancy Bartlett, M.D., supported by CA77440.
Weill Medical College of Cornell University, New York, NY: John Leonard, M.D., supported by CA07968.
Southwest Oncology Group, San Antonio, TX: Charles Coltman, M.D., Chairman, supported by CA32102.
National Cancer Institute of Canada, Toronto, Ontario: Elizabeth Eisenhauer, M.D., President, supported by CA77202.
Footnotes
Conflict of interest: L.B.M. receives honoraria from Varian Medical Systems, and grants from the National Institutes of Health, the Lance Armstrong Foundation, and the U.S. Department of Defense. P.G.R. receives an honorarium from Millennium Pharmaceuticals.
References
- 1.Jones SE, Moon TE, Bonadonna G, et al. Comparison of different trials of adjuvant chemotherapy in stage II breast cancer using a natural history database. Am J Clin Oncol. 1987;10:387–395. doi: 10.1097/00000421-198710000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Hellman S, Harris JR. Natural history of breast cancer. In: Harris JR, Lippman ME, Morrow M, editors. Diseases of the breast. 2nd. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 407–424. [Google Scholar]
- 3.Hoehne F, Chen S, Mabry H, et al. An update on prognosis in breast cancer patients with extensive axillary disease. Breast J. 2008;14:76–80. doi: 10.1111/j.1524-4741.2007.00517.x. [DOI] [PubMed] [Google Scholar]
- 4.Rack B, Janni W, Gerber B, et al. Patients with recurrent breast cancer: Does the primary axillary lymph node status predict more aggressive tumor progression? Breast Cancer Res Treat. 2003;82:83–92. doi: 10.1023/B:BREA.0000003955.73738.9e. [DOI] [PubMed] [Google Scholar]
- 5.Montero AJ, Rouzier R, Lluch A, et al. The natural history of breast carcinoma in patients with ≥10 metastatic axillary lymph nodes before and after the advent of adjuvant therapy: A multi-institutional retrospective study. Cancer. 2005;104:229–235. doi: 10.1002/cncr.21182. [DOI] [PubMed] [Google Scholar]
- 6.Geara FB, Nasr E, Tucker SL, et al. Breast cancer patients with 10 or more involved axillary lymph nodes treated by multimodality therapy: Influence of clinical presentation on outcome. Int J Radiat Oncol Biol Phys. 2007;68:364–369. doi: 10.1016/j.ijrobp.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Frei E, Canellos GP. Dose: A critical factor in cancer chemotherapy. Am J Med. 1980;69:585–594. doi: 10.1016/0002-9343(80)90472-6. [DOI] [PubMed] [Google Scholar]
- 8.Hryniuk W, Bush H. The importance of dose intensity in chemotherapy of metastatic breast cancer. J Clin Oncol. 1984;2:1281–1288. doi: 10.1200/JCO.1984.2.11.1281. [DOI] [PubMed] [Google Scholar]
- 9.Hryniuk W, Levine MN. Analysis of dose intensity for adjuvant chemotherapy trials in stage II breast cancer. J Clin Oncol. 1986;4:1162–1170. doi: 10.1200/JCO.1986.4.8.1162. [DOI] [PubMed] [Google Scholar]
- 10.Eddy DM. High-dose chemotherapy with autologous bone marrow transplantation for the treatment of metastatic breast cancer. J Clin Oncol. 1992;10:657–670. doi: 10.1200/JCO.1992.10.4.657. [DOI] [PubMed] [Google Scholar]
- 11.Fisher B, Anderson S, Wickerham DL, et al. Increased intensification and total dose of cyclophosphamide in a doxorubicin-cyclophosphamide regimen for the treatment of primary breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-22. J Clin Oncol. 1997;15:1858–1869. doi: 10.1200/JCO.1997.15.5.1858. [DOI] [PubMed] [Google Scholar]
- 12.Peters WP, Ross M, Vredenburgh JJ, et al. High-dose chemotherapy and autologous bone marrow support as consolidation after standard-dose adjuvant therapy for high-risk primary breast cancer. J Clin Oncol. 1993;11:1132–1143. doi: 10.1200/JCO.1993.11.6.1132. [DOI] [PubMed] [Google Scholar]
- 13.Peters W, Rosner GL, Vredenburgh JJ, et al. Updated results of a prospective, randomized comparison of two doses of combination alkylating agents (AA) as consolidation after CAF in high-risk primary breast cancer involving ten or more axillary lymph nodes (LN): CALGB 9082/SWOG 9114/NCIC (Abstr.). Presented at the 37th Annual Meeting of the America Society of Clinical Oncology; May 12–15, 2001; San Francisco, CA. [Google Scholar]
- 14.Peters WP, Rosner GL, Vredenburgh JJ, et al. Prospective, randomized comparison of high-dose chemotherapy with stem-cell support versus intermediate-dose chemotherapy after surgery and adjuvant chemotherapy in women with high-risk primary breast cancer: A report of CALGB 9082, SWOG 9114, and NCIC MA-13. J Clin Oncol. 2005;23:2191–2200. doi: 10.1200/JCO.2005.10.202. [DOI] [PubMed] [Google Scholar]
- 15.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 16.Blomqvist C, Tiusanen K, Elomaa I, et al. The combination of radiotherapy, adjuvant chemotherapy (cyclophosphamide-doxorubicin-ftorafur) and tamoxifen in stage II breast cancer. Long-term follow-up results of a randomised trial. Br J Cancer. 1992;66:1171–1176. doi: 10.1038/bjc.1992.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutqvist LE, Cedermark B, Glas U, et al. Radiotherapy, chemotherapy, and tamoxifen as adjuncts to surgery in early breast cancer: A summary of three randomized trials. Int J Radiat Oncol Biol Phys. 1989;16:629–639. doi: 10.1016/0360-3016(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 18.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 19.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 20.Martinez A, Ahmann D, O'Fallow J. An interim analysis of the randomized surgical adjuvant trial for patients with unfavorable breast cancer. Int J Radiat Oncol Biol Phys. 1984;10(Suppl 2):106. [Google Scholar]
- 21.McArdle CS, Crawford D, Dykes EH, et al. Adjuvant radiotherapy and chemotherapy in breast cancer. Br J Surg. 1986;73:264–266. doi: 10.1002/bjs.1800730407. [DOI] [PubMed] [Google Scholar]
- 22.Moss WT, Brand WN, Battifora H. Radiation oncology Rationale, technique, results. St. Louis: C.V. Mosby; 1979. [Google Scholar]
- 23.Griem KL, Henderson IC, Gelman R, et al. The 5-year results of a randomized trial of adjuvant radiation therapy after chemotherapy in breast cancer patients treated with mastectomy. J Clin Oncol. 1987;5:1546–1555. doi: 10.1200/JCO.1987.5.10.1546. [DOI] [PubMed] [Google Scholar]
- 24.Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007;82:247–253. doi: 10.1016/j.radonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen HM, Overgaard M, Grau C, et al. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: Long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol. 2006;24:2268–2275. doi: 10.1200/JCO.2005.02.8738. [DOI] [PubMed] [Google Scholar]
- 26.Marks LB, Hebert ME, Bentel G, et al. To treat or not to treat the internal mammary nodes: A possible compromise. Int J Radiat Oncol Biol Phys. 1994;29:903–909. doi: 10.1016/0360-3016(94)90584-3. [DOI] [PubMed] [Google Scholar]
- 27.Rodenhuis S, Richel DJ, van der Wall E, et al. Randomised trial of high-dose chemotherapy and haemopoietic progenitor-cell support in operable breast cancer with extensive axillary lymph-node involvement. Lancet. 1998;352:515–521. doi: 10.1016/S0140-6736(98)01350-6. [DOI] [PubMed] [Google Scholar]
- 28.Winer EP. Quality-of-life research in patients with breast cancer. Cancer. 1994;74:410–415. doi: 10.1002/cncr.2820741328. [DOI] [PubMed] [Google Scholar]
- 29.Damon LE, Hu WW, Stockerl-Goldstein KE, et al. High-dose chemotherapy and hematopoietic stem cell rescue for breast cancer: Experience in California. Biol Blood Marrow Transplant. 2000;6:496–505. doi: 10.1016/s1083-8791(00)70020-6. [DOI] [PubMed] [Google Scholar]
- 30.de Graaf H, Willemse PH, de Vries EG, et al. Intensive chemotherapy with autologous bone marrow transfusion as primary treatment in women with breast cancer and more than five involved axillary lymph nodes. Eur J Cancer. 1994;30A:150–153. doi: 10.1016/0959-8049(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 31.Roche H, Viens P, Biron P, et al. High-dose chemotherapy for breast cancer: The French PEGASE experience. Cancer Control. 2003;10:42–47. doi: 10.1177/107327480301000105. [DOI] [PubMed] [Google Scholar]
- 32.Farquhar C, Basser R, Marjoribanks J, et al. High dose chemotherapy and autologous bone marrow or stem cell transplantation versus conventional chemotherapy for women with early poor prognosis breast cancer. Cochrane Database Syst Rev. 2003;CD003139 doi: 10.1002/14651858.CD003139. [DOI] [PubMed] [Google Scholar]
- 33.Farquhar C, Basser R, Hetrick S, et al. High dose chemotherapy and autologous bone marrow or stem cell transplantation versus conventional chemotherapy for women with metastatic breast cancer. Cochrane Database Syst Rev. 2003;CD003142 doi: 10.1002/14651858.CD003142. [DOI] [PubMed] [Google Scholar]
- 34.Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. Early Breast Cancer Tria-lists' Collaborative Group. N Engl J Med. 1995;333:1444–1455. doi: 10.1056/NEJM199511303332202. [DOI] [PubMed] [Google Scholar]
- 35.Fisher B, Anderson S, Redmond CK, et al. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1995;333:1456–1461. doi: 10.1056/NEJM199511303332203. [DOI] [PubMed] [Google Scholar]
- 36.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 37.Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–575. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]
- 38.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 39.Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28. 2005;23:3686–3696. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 40.Overgaard M, Christensen JJ, Johansen H, et al. Evaluation of radiotherapy in high-risk breast cancer patients: Report from the Danish Breast Cancer Cooperative Group (DBCG 82) Trial Int J Radiat Oncol Biol Phys. 1990;19:1121–1124. doi: 10.1016/0360-3016(90)90214-5. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen HM, Overgaard J, Grau C, et al. Audit of the radiotherapy in the DBCG 82 b&c trials—A validation study of the 1,538 patients randomised to postmastectomy radiotherapy. Radio-ther Oncol. 2005;76:285–292. doi: 10.1016/j.radonc.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Bentel G, Marks LB, Hardenbergh P, et al. Variability of the location of internal mammary vessels and glandular breast tissue in breast cancer patients undergoing routine CT-based treatment planning. Int J Radiat Oncol Biol Phys. 1999;44:1017–1025. doi: 10.1016/s0360-3016(99)00123-6. [DOI] [PubMed] [Google Scholar]
- 43.Marks LB, Halperin EC, Prosnitz LR, et al. Post-mastectomy radiotherapy following adjuvant chemotherapy and autologous bone marrow transplantation for breast cancer patients with greater than or equal to 10 positive axillary lymph nodes. Cancer and Leukemia Group B. Int J Radiat Oncol Biol Phys. 1992;23:1021–1026. doi: 10.1016/0360-3016(92)90908-z. [DOI] [PubMed] [Google Scholar]
- 44.Moreno M, Azinovic I, Lopez-Picazo JM, et al. Radiation therapy after high-dose chemotherapy with peripheral blood stem cell support for high-risk breast cancer. Am J Clin Oncol. 2002;25:347–353. doi: 10.1097/00000421-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Buchholz TA, Tucker SL, Moore RA, et al. Importance of radiation therapy for breast cancer patients treated with high-dose chemotherapy and stem cell transplant. Int J Radiant Oncol Biol Phys. 2000;46:337–343. doi: 10.1016/s0360-3016(99)00429-0. [DOI] [PubMed] [Google Scholar]
- 46.Moore HC, Mick R, Solin LJ, et al. Autologous stem-cell transplant after conventional dose adjuvant chemotherapy for high-risk breast cancer: impact on the delivery of local-regional radiation therapy. Ann Oncol. 1999;10:929–936. doi: 10.1023/a:1008393204854. [DOI] [PubMed] [Google Scholar]
- 47.Optenberg SA, Thompson IM, Friedrichs P, et al. Race, treatment, and long-term survival from prostate cancer in an equal-access medical care delivery system. JAMA. 1995;274:1599–1605. [PubMed] [Google Scholar]
- 48.Demark-Wahnefried W, Schildkraut JM, Iselin CE, et al. Treatment options, selection, and satisfaction among African American and white men with prostate carcinoma in North Carolina. Cancer. 1998;83:320–330. doi: 10.1002/(sici)1097-0142(19980715)83:2<320::aid-cncr16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 49.Banerjee M, George J, Yee C, et al. Disentangling the effects of race on breast cancer treatment. Cancer. 2007;110:2169–2177. doi: 10.1002/cncr.23026. [DOI] [PubMed] [Google Scholar]
- 50.Shen Y, Dong W, Esteva FJ, et al. Are there racial differences in breast cancer treatments and clinical outcomes for women treated at M.D. Anderson Cancer Center? Breast Cancer Res Treat. 2007;102:347–356. doi: 10.1007/s10549-006-9337-2. [DOI] [PubMed] [Google Scholar]
- 51.Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 52.Canto JG, Allison JJ, Kiefe CI, et al. Relation of race and sex to the use of reperfusion therapy in Medicare beneficiaries with acute myocardial infarction. N Engl J Med. 2000;342:1094–1100. doi: 10.1056/NEJM200004133421505. [DOI] [PubMed] [Google Scholar]
- 53.Peterson ED, Shaw LK, DeLong ER, et al. Racial variation in the use of coronary-revascularization procedures. Are the differences real? Do they matter? N Engl J Med. 1997;336:480–486. doi: 10.1056/NEJM199702133360706. [DOI] [PubMed] [Google Scholar]
- 54.Bickell NA. Race, ethnicity, and disparities in breast cancer: Victories and challenges. Womens Health Issues. 2002;12:238–251. doi: 10.1016/s1049-3867(02)00145-7. [DOI] [PubMed] [Google Scholar]
- 55.Hahn KM, Bondy ML, Selvan M, et al. Factors associated with advanced disease stage at diagnosis in a population-based study of patients with newly diagnosed breast cancer. Am J Epidemiol. 2007;166:1035–1044. doi: 10.1093/aje/kwm177. [DOI] [PubMed] [Google Scholar]
- 56.Gorey KM, Luginaah IN, Schwartz KL, et al. Increased racial differences on breast cancer care and survival in America: Historical evidence consistent with a health insurance hypothesis, 1975-2001. Breast Cancer Res Treat. 2009;113:595–600. doi: 10.1007/s10549-008-9960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ayanian JZ, Cleary PD, Weissman JS, et al. The effect of patients' preferences on racial differences in access to renal transplantation. N Engl J Med. 1999;341:1661–1669. doi: 10.1056/NEJM199911253412206. [DOI] [PubMed] [Google Scholar]
- 58.Griggs JJ, Culakova E, Sorbero ME, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007;25:2522–2527. doi: 10.1200/JCO.2006.10.2749. [DOI] [PubMed] [Google Scholar]