Abstract
Frank Burr Mallory’s landmark observation in 1911 on the histopathology of alcoholic liver disease (ALD) was the first identification of a link between an inflammation and ALD. In this review, we summarize recent advances regarding the origins and roles of various inflammatory components in ALD. Metabolism of ethanol generates a number of metabolites, including acetate, reactive oxygen species, acetaldehyde, and epigenetic changes, that can induce inflammatory responses. Alcohol and its metabolites can also initiate and aggravate inflammatory conditions by promoting gut leakiness of microbial products, by sensitizing immune cells to stimulation and by activating innate immune pathways, such as complement. Chronic alcohol consumption also sensitizes non-immune cells, e.g., hepatocytes, to inflammatory signals and impairs their ability to respond to protective signals. Based on these advances, a number of inflammatory targets have been identified with potential for therapeutic intervention in ALD, presenting new opportunities and challenges for translational research.
Keywords: Ethanol, liver injury, hepatic macrophage, cytokines, complement, inflammasome
INTRODUCTION
Acute inflammation is a critical homeostatic mechanism for protecting a host against noxious conditions, including infection and tissue injury. Such inflammation is characterized by innate immune cell infiltration into target tissues. These effector cells and their secreted mediators orchestrate the killing and removal of infected/damaged cells and promotes tissue repair and regeneration. Chronic inflammation occurs when an acute response becomes dysregulated or fails to resolve because of the continued presence of an inducer of inflammation. In addition to innate immune cells, adaptive immune cells are often involved in chronic inflammatory responses. The excessive (acute) or persistent (chronic) presence of cytotoxic immune cells and their mediators in tissues have been recognized to play important roles in the pathogenesis of human diseases.
Last year (2011) marks the centennial of Frank Burr Mallory’s landmark report that described for the first time a large number of infiltrating neutrophils in cirrhotic livers from alcoholic patients (69). These liver-associated inflammatory activities were initially viewed as a host protective mechanism to remove damaged hepatocytes. This simplistic view was challenged and amended following a series of studies from the late Dr. Thurman’s laboratory in the 1980–1990s which demonstrated that gut microflora-derived lipopolysaccharides (LPS) and the pro-inflammatory cytokine TNF-α play an important role in ALD development in alcohol-fed animal models, suggesting that alcohol-related inflammation contributes to the ALD development. Furthermore, elevated plasma LPS (endotoxemia) is commonly detected in patients with alcoholic steatohepatitis and cirrhosis, implicating a pathological role for LPS-induced inflammation in the pathogenesis of human ALD (28).
In the past decade, we have seen a significant expansion of research aimed to search for the key pro-inflammatory mediators that contribute to the ALD development (33). LPS was the first identified and best understood inflammatory inducer in the pathogenesis of ALD. In addition, many other alcohol-related mediators have also been identified to contribute to liver inflammation (Table 1). These mediators and LPS often co-exist and contribute, additively or synergistically, to ALD development. This review focuses on significant findings from recent studies, in particular: 1) the identification of the key role of microbial products and their host receptors (e.g., LPS/TLR4) in the development of ALD; 2) the identification of multiple inflammatory inducers in the pathogenesis of ALD, including alcohol, its metabolites, and alcohol-induced metabolic and cellular changes; and 3) functional study of inflammatory mediators, pro-inflammatory cytokines, anti-inflammatory cytokines, and hepatoprotective cytokines in the pathogenesis of ALD. These advances allow us to evaluate inflammation-based approaches for effective ALD intervention.
Table 1.
Inflammation inducers associated with ALD.
| Sensors/early mediators | Reference | |
|---|---|---|
| LPS | TLR4 | (131, 140) |
| Alcohol & metabolite | TLR4; (alcohol metabolites): SIRT; HDAC and Acetyl-coA synthetase | (8, 9, 49, 114) |
| Saturated fatty acids | TLR4 , Inflammasome? | (19, 61, 135) |
| Unsaturated fatty acids | ROS | (112) |
| ROS | NF-κB; Sensitization of existing response | (146) |
| Apoptotic cells | C1q | (16) and reference within |
| Necrotic cells | Inflammasome NLD3 via ATP | (45, 63) |
| Hypoxia | HIF-1α via VHL (von Hippel-Lindau tumor suppressor gene product) | (90, 92) |
MICROBIAL PRODUCTS: THE LPS/TLR4 PARADIGM
The best known inflammation inducers include products of microbes and components of dead host cells. Their presence is detected by host-encoded receptors expressed predominantly on tissue specific and circulating innate immune cells, e.g., macrophages/monocytes and dendritic cells. Ligand binding to these receptors triggers a cascade of signaling events that induce the production of secreted mediators responsible for orchestrating an acute inflammatory response. The mammalian genome encodes a large number of pattern-recognition receptors that respond to diverse threats from pathogens and dead cells. The best characterized pattern-recognition receptor is Toll-like receptor (TLR) family, which has up to 13 members, each recognizing a unique set of microbial and host products. TLR4, for example, is the receptor for LPS.
As a cell wall component of Gram-negative bacteria, LPS is the best known for its role in bacteria-induced inflammation and multi-organ failure in sepsis. The ability of LPS to produce inflammation is mediated by the MD2 protein and the membrane receptor TLR4. In innate immune cells, such as tissue macrophages and their progenitor monocytes, the LPS-MD2/TLR4 complex triggers MyD88-dependent and MyD-independent (TRIF-dependent) signaling cascades, resulting in production of many inflammatory mediators, including TNF-α, IL-1β, and IL-6. The key findings linking LPS and ALD are summarized below.
Multiple mechanisms contribute to alcohol-related endotoxemia. In animal studies, continuous intragastric feeding of alcohol results in intestinal bacterial overgrowth and enteric dysbiosis, which is due, in part, to an alcohol-induced downregulation of expression of several intestinal antimicrobial molecules (139). In addition, accumulating evidence suggests that alcohol feeding increases acute LPS translocation due to an increase in gut epithelial membrane permeability and impairment of tight junctions between epithelial cells (107). Moreover, chronic alcohol causes structural changes in the GI tract which might contribute to LPS translocation (107, 134). Following translocation across the intestinal epithelium, LPS enters the circulation by the lymphatic system and/or the portal vein-liver route; the relative contribution of these two dissemination routes in the presence of alcohol use is still not defined. Regardless of the route, the majority of circulating LPS is removed by a healthy liver, primarily through the abundant Kupffer cells and hepatocytes (134). Consequently, a healthy liver bears the brunt of inflammation induced by LPS. When the liver is damaged, the bioavailability of LPS in the circulation increases, which explains, in part, the prevalence of endotoxemia in cirrhotic patients (93).
LPS clearly plays a pathological role in end-stage liver diseases and complications such as sepsis and the associated multi-organ failure. Reports in the last two decades have also built a strong case for a pathological role of LPS in the early phase of ALD. Pretreatment with non-absorbable antibiotics in alcohol-fed rats reduces alcohol-induced steatosis, liver injury, and inflammatory markers (2). Similarly, probiotic treatment mitigates the effect of alcohol on liver injury, reduces LPS in the circulation, and reduces inflammatory markers in alcohol-fed animals (88). Consistent with this, in a preliminary study of human alcoholic patients, probiotic use improved liver functions (52). In addition, administration of LPS to alcohol-fed rodents promotes pathogenesis from simple steatosis to steatohepatitis (105). Lastly, the disruption of the TLR4 gene or CD14 gene (a gene that encodes for the LPS-binding receptor) reduces liver pathology and inflammation in a murine model of alcoholic liver injury (41, 131, 140). The important role of LPS in inducing liver inflammation in ALD is likely mediated by binding to TLR4 on Kupffer cells/macrophages, which then produce a variety of pro-inflammatory cytokines and subsequently promote infiltration of inflammatory cells including neutrophils. Although neutrophil infiltration is always low to mild in rodents fed alcohol, when exogenous LPS is given to alcohol-fed animals, a significant number of neutrophils is observed in the liver (106). Such data support the idea that sufficient LPS (and consequently, the LPS-induced cytokine milieu) is required for neutrophil infiltration in alcoholic steatohepatitis. Comparative analysis of the circulating and liver cytokine milieu in alcohol and LPS-treated animals, and in alcoholic hepatitis patients might help identify the important mechanisms how LPS promotes liver inflammation in ALD.
The role of LPS in inducing inflammation can be augmented by alcohol and its metabolites. Prolonged alcohol exposure in vitro and in vivo potentiates LPS-induced cytokine production in monocytes and Kupffer cells, respectively (3, 29). Acetaldehyde, a metabolic product of alcohol, interacts with malondialdehyde, a product of lipid peroxidation, to form malondialdehyde-acetaldehyde adduct (MAA), another example of an LPS sensitizing agent (24). This suggests that alcohol consumption not only elevates LPS levels but also increases the sensitivity of Kupffer cells/macrophages to LPS stimulation, contributing to liver inflammation in ALD.
LPS is not the only gut microbial product that enters the circulation due to alcohol use. For example, a significant increase in circulating peptidoglycan, which is a bacterial wall product and TLR2 ligand, was observed in acute and chronic alcohol-fed rodents (18, 122). A few studies have examined the roles of these microbial products and their cellular sensors, other than LPS-TLR4, in the inflammatory state observed in ALD patients. Acute alcohol exposure augments TNF-α production in human monocytes when both TLR2 and TLR4 ligands are present (95). Increased expression of TLR2, 4, and 9 is associated with neutrophil dysfunction and endotoxemia; inhibition of these TLRs prevents the increase in oxidative burst and chemokine production, but has no effect on phagocytic activity in these neutrophils (118). In addition, deletion of TLR2 in mice has no significant impact on the development of the early phase of ALD, such as the development of fatty liver and the elevation of liver enzymes (41, 118). Further studies are required to clarify the roles of TLR2 and TLR9 in the pathogenesis of ALD.
The mechanisms by which TLR4 induces early alcoholic liver injury have been extensively explored in last decade. LPS-MD2/TLR4 intracellular signaling is controlled by two adaptor molecules, MyD88 and TRIF(48), which induce activation of three key signaling transducer/regulators, NF-κB, MAPK, and IRF3, and subsequently control the expression a large array of inflammatory mediators. IRF3 is activated only by the TRIF dependent pathway whereas NF-κB and MAPK are activated by both the MyD88 and TRIF pathways. In addition, LPS/TLR4 receptor complex also activates NADPH oxidase to produce ROS, which then plays a supporting role in the activation of NF-κB and MAPK. Among these signaling molecules, NADPH oxidase and NADPH oxidase derived ROS, members of MAPK (p38, ERK1/2) and its downstream target (Egr1) have been found to play a causal role in developing ALD in animal models (53, 54, 56, 102, 125). Moreover, recent studies suggest that the role of LPS/TLR4 in the pathogenesis of ALD is mediated via activation of the MyD88-independent and TRIF/IRF3-dependent pathway, as IRF3-knockout mice, but not MyD88-knockout mice, are resistant to liver injury induced by an ethanol diet (41, 145). Further studies using bone marrow chimeric mice suggest that IRF3 in parenchymal cells is protective against ALD via the upregulation of type I IFNs and IL-10, whereas IRF3 in nonparenchymal cells is detrimental via the upregulation of TNF-α (98). Together, these results demonstrated the complex functions of the TLR4 downstream pathway in immune cells and hepatocytes that not only afford the liver to respond to an inflammatory challenge but also provide it with a certain level of protection.
Although an important role of LPS in the pathogenesis of ALD is well documented, it is worth noting that, at least in some animal models and a small percent of ALD patients, an increase in LPS level is not always apparent (31, 109). This suggests that LPS is not a sole factor to induce inflammation in ALD. Indeed, many other factors, in addition to LPS, have also found to play important roles in promoting liver inflammation in ALD (see below).
A DIRECT ROLE OF ALCOHOL AND ITS METABOLITES IN INDUCING INFLAMMATORY RESPONSES
Besides their ability to alter or enhance immune cell response to LPS, alcohol and its metabolites also play a direct role in activating an inflammatory program in immune cells. For example, recent studies from Guerri’s group suggest that ethanol can directly induce activation of NF-κB and downstream Cox-1 and iNOs expression in cultured astrocytes and glial cells via the induction of the translocation of TLR4 into lipid rafts (4, 8, 9), contributing to ethanol-induced neuroinflamamtion. However, it is not clear whether ethanol can also directly activate TLR4 signaling in liver parenchymal and nonparenchymal cells, thereby promoting liver inflammation.
Alcohol metabolites, both acetaldehyde and acetate, have been shown not only to directly induce an inflammatory response but also to potentiate LPS-mediated induction of pro-inflammatory cytokines in Kupffer cells/macrophages. It was reported that acetaldehyde and acetate exposure of rodent macrophage cells caused activation of NF-κB signaling and production of TNF-α (114). This NF-κB activation is, in part, mediated via the down regulation of SIRT1, an NF-κB antagonist; such effect appears to be limited to alcohol metabolites, while alcohol itself has no effect (114). Additionally, acetate exposure also augments cytokine production in human macophages in response to LPS (49); this sensitizing effect of acetate appears to be dependent on the conversion of acetate to acetyl-CoA, which is the substrate for histone acetylation necessary for the enhanced transcription of cytokine genes (49). Recent studies from Min You’s group (You, M.: Adiponectin reverses impairment of Sirt1-TNF-α axis by LPS or acetate through stimulating AMPK signaling in cultured rat Kupffer cells. Alcoholism: Experimental and Clinical Research, volume 35, supplement S1, 302A) suggest alcohol and alcohol metabolites induce liver inflammation via the alteration of energy homeostasis. The end product of alcohol metabolism, acetate, is a highly usable substrate for energy production in the mitochondria via the TCA cycle. Metabolism of alcohol and acetaldehyde leads to an increase in the NADH/NAD+ ratio, which when reoxidized causes the mitochondrial electron transport chain to produce reactive oxygen species (ROS) as byproducts. Such observation is analogous to the finding that high glucose induces inflammatory response via ROS production (113). As discussed in the section below, ROS can activate inflammatory responses via activation of NF-κB and its downstream inflammatory events.
ALCOHOL-INDUCED METABOLIC AND CELLULAR CHANGES CONTRIBUTING TO INFLAMMATION
Free fatty acids
Compared with a control cohort, liver biopsy samples from patients with ALD have a greater than 10 fold increase in free fatty acids (FFAs), consisted of saturated fatty acids (SFAs) and unsaturated fatty acids (UFAs)(77). Accumulating evidence suggests that these SFAs have the potential to directly or indirectly induce inflammatory responses in the liver (Table 1). First, SFAs promote inflammation by activating TLR4 on macrophages, contributing to liver inflammation. It was reported that free SFAs, but not UFAs, induce TLR4-mediated NF-κB activation and Cox-2 expression in macrophages in vitro (61). The role of this SFA-TLR4 link in liver inflammation was also confirmed in vivo by the fact that TLR4 knockout mice were partially resistant to a high fat infusion-induced liver TNF-α expression (116). Second, SFAs (e.g., palmitic acid) directly activate the inflammasome in hepatocytes or stimulate hepatocytes to produce danger signals that activate the inflammasome in immune cells (19). Inflammasome is an intracellular complex that controls the release of pro-inflammatory cytokines IL-1β and IL-18, and its activation promotes liver inflammation in nonalcoholic steatohepatitis and possibly in alcoholic steatohepatitis (19). Third, free SFAs can also directly promote hepatocytes to produce chemokines. Exposure of palmitic acid to rat or human hepatocytes promotes the NFκB-dependent production of pro-inflammatory chemokines CINC-2/MIP-2 and IL-8 (47). Finally, the liver might be particularly vulnerable to SFAs because, unlike those in circulation, liver FFAs are populated predominantly by SFAs (57, 77).
In light of these recent advances, it will be important in the near future to determine experimentally whether free SFAs also play a significant role in alcohol-related liver inflammatory conditions. Interestingly, early studies have shown that diets rich in esterified SFAs (beef tallow, cocoa butter, or medium-chain triglycerides) are protective against alcohol-induced liver pathology including inflammation (87, 110), an outcome believed to be attributable to an increased adiponectin production and/or increased resistance of membrane lipid to oxidation. However, whether or not consumption of such esterified SFAs modulates the levels of free SFAs in the liver is not known.
Free UFAs at a high level are also capable of inducing an inflammatory response. High fat diets consisting of free UFAs or esterified UFAs promote early ALD development in animal models through increasing lipid peroxidation and oxidative stress, which in turn induce inflammation by activating NFκB (86). Though free UFAs can inhibit SFA-induced inflammatory response from cultured macrophages (ref 62), a recent study showed that they can act synergistically with alcohol in inducing oxidative stress and activating TNF-α production in Kupffer cells (20).
ROS
ROS are important signaling mediators for receptor-mediated activation of NFκB and the associated downstream cytokine production. Exogenous ROS can substitute for cytokine signaling to induce inflammatory responses. For example, H2O2 exposure of endothelial cells induces NF-κB-dependent ICAM-1 expression, which, as a ligand for integrin CD18/11A on leukocytes, is critical for neutrophil transmigration (59). ROS also mediate glucose-induced production of TNF-α by microglial cells and monocytes (103, 104).
It has been well documented that alcohol increases ROS production via multiple mechanisms, including the mitochondrial electron transport chain and CYP2E1-mediated alcohol metabolism (11, 143). Mechanistically, alcohol-induced ROS may contribute to inflammation by direct induction of the inflammatory response and/or amplification of inflammatory signaling. A close functional relationship between alcohol-induced ROS and liver inflammation is supported by the ability of N-acetylcysteine, an antioxidant, to reduce the level of inflammatory markers in alcohol-fed mice (146).
Additionally, ROS produced via respiratory burst during phagocytosis process by phagocytic cells is well established for its capacity of killing pathogens or pathogen-infected cells. Alcohol metabolism-induced ROS is also found to be sufficient in promoting cell injury, including necrosis (80). In turn, necrotic cells can activate the inflammatory response. Thus, alcohol-induced ROS may function as a catalyst to initiate a vicious cycle of cell killing, inflammatory responses, and further ROS production, thereby promoting liver inflammation.
Damaged cells
Necrosis and apoptosis, the two most common forms of cell death, are prominent features of human alcoholic steatohepatitis (69, 91, 129). Many factors have been shown to induce hepatocyte necrosis in ALD. These include activated neutrophils (6, 46), alcohol-related oxidative stress (80), pro-inflammatory cytokine TNF-α, and Fas ligand (17, 91). Necrotic cells, but not apoptotic cells, have the potential of inducing inflammatory responses; such induction requires the activation of the inflammasome, an intracellular sensor complex, in necrotic cells, which further leads to the release of IL-1α and, to some extent, IL-1β (45). These necrotic cell-derived IL-1α and IL-1β cytokines then activate macrophages to generate a full range of inflammatory responses including neutrophil infiltration (25, 55). Finally, RNA and DNA released from cells undergoing necrosis are capable of inducing inflammation via TLR7/9 (43). The important roles of these endogenous molecules are well documented in rodent models of nonalcoholic steatohepatitis (83) and drug-induced liver injury (43), but have not been explored in ALD-related liver inflammation.
Complement
When activated, key components of the complement system play several important functions in inflammation. For example, C3a and C5a are chemotactic factors and pathogen-associated molecular pattern sensors that can activate leukocytes to produce inflammatory cytokines. The level of complement is increased in alcoholics with withdrawal symptoms but is reduced in alcoholics with cirrhosis (67). Hepatic C1q and C3 deposition are markedly elevated in mice after 2-4 days of alcohol feeding. Further studies suggest that ethanol activates the classical complement pathway via C1q binding to apoptotic cells in the liver. Deletion of C1q abolishes ethanol-induced complement activation, prevents the upregulation of TNFα and IL-6 expression in the liver, and reduces ethanol-induced liver injury (16). These findings indicate that the activation of complement components also contributes to inflammatory responses in ALD (Tables 1 and 2).
Table 2.
Genetic and functional studies of inflammatory components associated with ALD
| ALD Association*1 | Knockout/antagonist phenotype | References | |
|---|---|---|---|
| Pathogen/Danger Sensors | |||
| TLR4 | Increased expression on the neutrophils and in the liver in patients with alcoholic cirrhosis | TLR4KO (whole body or bone-marrow derived cells): reduced inflammation and injury in mice; | (44, 131, 140) |
| TLR2 | Increased liver expression in alcohol-fed mice and in neutrophils of alcoholic cirrhotic patients | TLR2KO: No appreciable effect on inflammation and liver injury in alcohol-fed mice | (35, 41) |
| Cytokines/chemokines | |||
| TNFα | Elevated in circulation and liver in alcoholics with ALD; elevated in alcohol-fed rodents | TNF-αKO and antagonists: reduced liver fat and injury in rodents; TNF-α antagonists: increased infections in AH patients | (10, 42) |
| TNFR1 | Receptor for TNFα | TNFR1KO: reduced liver fat, inflammation, and hepatocyte necrosis | (141) |
| IL-6 | Elevated in circulation and liver in alcoholics with or without liver disease; | IL-6KO: increased liver fat and injury; increased mitochondrial DNA damage; | (26, 144) |
| IL10 | Reduced in chronic alcohol fed mice; Moderately to highly elevated in alcoholics with liver diseases | IL-10KO: [alcohol only] reduced liver fat but increased inflammation w/o increased injury; [alcohol+LPS]: increased liver | (36, 81, 117) |
| IL-8, IL-17 | Elevated in alcoholics with liver disease | N/D | (62, 115) |
| HIF-1α | Induced in alcohol-fed animal model in response to hypoxia | Hepatocyte-specific HIF-1αKO: reduced liver fat, inflammation, and hepatocyte necrosis | (90) |
| Inflammatory Signaling pathways | |||
| IRF3 | (One of two major signal transducers for TLR4) | Global IRF3KO: reduced liver fat, inflammation and injury | (98, 145) |
| IRF3 deficiency in parenchymal cells: aggravated liver fat, inflammation and injury | (98, 145) | ||
| IRF3 deficiency in myeloid cells: reduced inflammation, but no change in liver injury | (98, 145) | ||
| STAT3 | (downstream target for IL-6, 10, and 22) Activation at early phase in animal; low activation in AH patients | STAT3 deficiency in hepatocyte: increased steatosis & lipogenic gene expression | (40) |
| STAT3 deficiency in macrophage/neutrophil or endothelial cells: increased inflammation and liver injury | (40, 82) | ||
| Immune cells | |||
| Kupffer cells (macrophage) | (acute alcohol) Increased tolerance to LPS; (Chronic alcohol) Increased sensitization to LPS. | Depletion: reduced liver injury | (1) |
| Neutrophils | Increased liver infiltration in AH with high resting respiratory burst | Anti-PMN antibody attenuated alcohol- induced liver injury in rat | (6, 84) |
| monocytes | Increased spontaneous and LPS induced cytokine production | Knockout of monocyte chemoattractant protein-1 (MCP-1) attenuates alcohol- induced liver injury, steatosis and oxidative stress in mice | (73, 79) |
| Plasma components | |||
| complement | Increases C1q, C3b liver deposition in alcohol fed mice; Low in alcoholics with cirrhosis | C1q KO: blocked an early inflammation and attenuated liver injury | (16) |
| C5KO: reduced liver inflammation and injury with no effect on liver fat accumulation | (101) | ||
| C3, C3R, or C5RKO: reduced an early phase of inflammation and injury | (111) | ||
Adducts and immune complexes
Adducts generated by the reaction of lipid peroxide products or the alcohol metabolite acetaldehyde with proteins or lipids are commonly observed in alcohol-fed animals (130). Interestingly, adducts alone or in combination with LPS can stimulate the inflammatory response in rat sinusoidal endothelial cells and Kupffer cells (24). It was proposed that this inflammation-stimulating effect is mediated through the scavenger receptor (SR-A) on these cells. As neo-antigens, these adducts can also elicit adaptive immune responses, including antibody production and/or cytotoxic T cell activation. Adduct-specific antibodies (IgG and IgA) have been found in patients with ALD, and their titers correlate well with disease severity and TNFα levels (108, 132). Conceptually, antibody-adduct complexes formed on the cell surface can also activate the complement system, which in turn activates inflammatory responses.
Hypoxia
Hypoxia is a well established feature of alcoholic liver injury, resulting from increased consumption of oxygen by alcohol metabolism (5). HIF-1α is a transcription factor whose stability is negatively regulated by oxygen. Under hypoxic conditions, HIF1α is stabilized and cooperates with NF-κB to induce the production of pro-inflammatory and angiogenic chemokines and receptors, including CXCL12, CXCR4, and VEGF (92). Hepatocyte-specific deletion of HIF1α diminishes alcohol-induced liver fat accumulation, injury, and inflammation (90) (see table 1), suggesting that alcohol-induced hypoxia contributes to the inflammatory response in the liver.
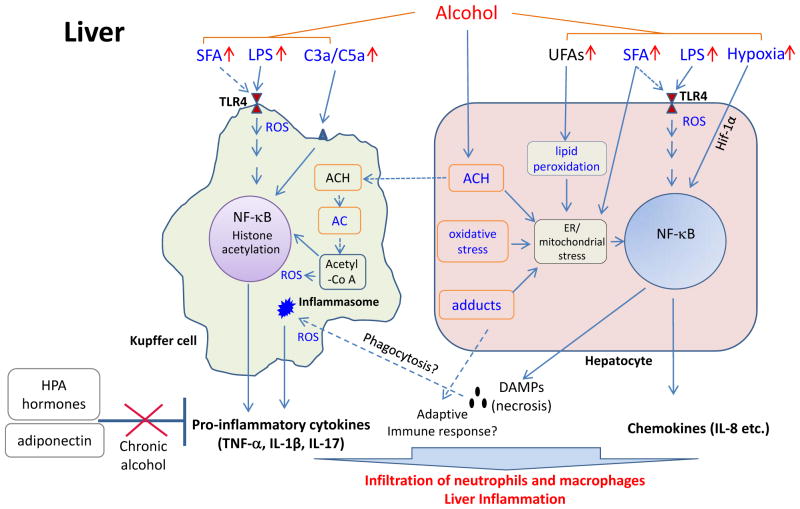
In conclusion, the list of recognized alcohol-related inflammatory inducers has grown significantly in the last decade (Table 1 and depicted in Figure 1). Because many of these factors are closely linked to alcohol and its metabolism and are present at significant levels during chronic alcohol abuse, their roles in alcohol-related inflammation are of great interest. Nevertheless, many of the connections we made between these inducers and ALD pathogenesis are based on circumstantial evidence and still require vigorous examination.
Fig. 1. Alcohol–induced inflammation in the liver.
Emphasis is given to the contribution from hepatocytes and macrophages (Kupffer cells and likely infiltrating monocytes) in inducing proinflammatory mediators required for attracting immune cells (neutrophils, monocytes and lymphocytes) to the liver. Solid lines indicate data with strong evidences and dotted lines indicate data still needs confirmation, including adaptive immune cells. Contributions from stellate cells and endothelial cells to inflammation are not discussed. Terms and items in bright blue indicate immediate inducers for inflammatory response. Abbreviations: ACH, acetaldehyde; AC, acetate; DAMPs, damage associated molecular pattern molecules; HPA, Hypothalamic–pituitary–adrenal; Hif–1α, hypoxia–inducible factor 1 alpha; ROS, reactive oxygen species; C3/C5, complement factors 3a and 5a; SFA, free saturated fatty acid; UFA, unsaturated free fatty acids.
PATHOLOGICAL VERSUS PROTECTIVE ROLE OF INFLAMMATORY MEDIATORS (CELLS, MEDIATORS, AND SIGNALING PATHWAYS) IN ALD
Infiltration of neutrophils and monocytes in the liver of alcoholics or alcohol-fed animals has received significant attention in the past. Neutrophil infiltration is the prominent feature of alcoholic steatohepatitis. Compared to neutrophils, monocyte infiltrates are often lower and are widely distributed in all stages of ALD, including fatty liver, the convalescent stage of steatohepatitis, and cirrhosis. In animal models, a pattern of neutrophil infiltration followed by monocytes infiltration is observed (7). There is also a significant lymphocyte infiltrate in ALD, yet the functions of these cells are poorly understood. The alterations in the activity of liver-specific resident immune cells, including Kupffer cells, NK cells, and NKT cells, have also been observed and reviewed recently (34).
Neutrophils
Intralobular neutrophil infiltration, secondary to hepatocyte degeneration, is a defining feature of alcoholic steatohepatitis. Accumulating evidence suggests that both IL-8 and IL-17 play an important role in inducing neutrophil infiltration in ALD. The elevation of these chemokines in the circulation and the liver has been consistently observed in alcoholic patients (62, 78). The grade of neutrophil infiltrate, e.g., density of these cells and frequency of their presence, positively coincides with the peak of hepatocyte injury (ballooning and necrosis) and other diagnostic features of liver diseases such as serum bilirubin, Maddrey DF and Childs score (27, 99). With alcohol abstinence, the grade of neutrophil infiltrate in most patients declines, followed by an increase in monocyte infiltration and eventual resolution of inflammation (21, 27). Nevertheless, the inflammatory condition can last for days to months before resolution. With continuous alcohol use, such acute conditions can become prolonged and recurring events that are superimposed over the development of fibrosis and cirrhosis.
As suggested as early as 100 years ago by Dr. Frank Burr Mallory, liver neutrophil/monocyte infiltrates in alcoholic hepatitis patients may play a protective role for the host by killing and removing alcohol-damaged hepatocytes (69). However, the question remains whether this inflammatory response is excessive and contributes to liver injury, and thus pathological; conceptually, this hypothesis is highly plausible because the studies from other liver injury models in rodents suggest that activation of neutrophils contributes to the pathogenesis of hepatocellular damage by releasing reactive oxygen species and proteases (106).
Alterations in neutrophil function in alcoholics have been described in the literature. Neutrophils isolated from patients with alcoholic hepatitis, for example, have a lower baseline phagocytic capacity (117). Removal of LPS from serum restores neutrophil phagocytic function in a subset of patients whose neutrophils have a high frequency of resting oxidative burst, and probiotic treatment restores partially the phagocytic function of circulating neutrophils in patients with alcoholic cirrhosis, suggesting that the compromised neutrophil function in patients with alcoholic steatohepatitis is largely due to endotoxemia (84, 117). Interestingly, neutrophils from a small percentage of patients in this study had a low resting oxidative burst and a low phagocytic activity that was not restored by LPS removal, implicating that other mechanisms may also contribute to the compromised neutrophil functions in these patients (117). In addition, blockade of TLR2, TLR4 and TLR9 with neutralizing antibodies in these neutrophils prevented the increase in oxidative burst and chemokine production, but not phagocytic activity, suggesting that microbial ligands for TLR2, TLR4 and TLR9 play a role in neutrophil dysfunction (118). In patients with severe alcoholic hepatitis, one major cause of mortality is infection; thus, restoration of neutrophil function is expected to play a protective role against infectious agents. However, whether restoration of neutrophil functions is protective against or harmful to alcohol-induced liver injury is not known.
Monocytes and hepatic macrophages
A series of studies from Dr. Thurman’s laboratory have provided strong evidence that liver-resident macrophages, Kupffer cells, play a pathological role in the early phase of ALD (127). Furthermore, they showed that chronic alcohol feeding in animals potentiates the ability of Kupffer cells to produce inflammatory cytokines in response to LPS.
Besides resident macrophages, infiltration of circulating monocytes, which become macrophages once in the tissue, is common at different stages of ALD, including fatty liver, steatohepatitis, and cirrhosis. Alcoholics with fatty liver often have macrophage aggregates (lipogranulomas) with fat droplets in the center that can be found in the majority of alcoholics without cirrhosis (15). In the intragastric infusion rat model, alcohol intoxication causes mild necrosis and infiltration of immune cells in the liver that are pericentrally localized and have a mostly mononuclear morphology (89, 128). A genetic study shows that mice deficient in monocyte chemoattrant protein-1 (MCP-1) attenuate alcohol-induced liver injury, supporting a pathogenic role for infiltrating monocytes (73). A separate study showed that feeding rats with alcohol plus a high-fat diet promotes the development of infiltrated monocytes into macrophages with a pro-inflammatory phenotype (138), whereas another study has shown that voluntary feeding with an alcohol diet alone upregulates both hepatic classical (M1) and alternative (M2) markers (65). In a rat ALD model, chronic ethanol feeding itself is sufficient to cause a shift of Kupffer cells to an M1 macrophage polarization and upon LPS stimulation these cells had increased TNFα expression (71). Together, these pro-inflammatory M1 macrophages likely play an important role in inducing inflammation during ALD pathogenesis.
Like neutrophils, monocytes/macrophages also have a host-protective role because of their ability to remove damaged cells, combat infectious agents, produce hepatoprotective cytokines (such as IL-6, discussed below), and promote liver regeneration; such a host protective role has to be considered when these cells are targeted for ALD intervention. In addition to contributing to the development of liver fibrosis, macrophages/monocytes also play an important role in fibrosis resolution during the recovery phases of inflammatory fibrosis (23). These findings suggest that macrophages/monocytes play a complex role in the pathogenesis of ALD.
Lymphocytes and NK cells
Lymphocyte infiltrates in the liver are also commonly observed in patients with alcoholic steatohepatitis. Unlike neutrophils (which usually undergo rapid apoptosis after activation), the number of lymphocytes in the liver did not change significantly with alcohol abstinence and recovery (27). Very little is known about the functions of these lymphocytes except for a recent report on IL-17-secreting T cells in the circulation and the liver of alcoholics with ALD (see below). As discussed in the previous section, adducts between reactive MAA (malondialdehyde-acetaldehyde) and proteins, commonly observed in alcohol-fed animals and human alcoholics, have the potential to generate adaptive immune responses. A recent study has shown that immunization of mice with MAA-modified cytosolic proteins induces autoimmune-like hepatitis with elevated TNFα, IL-1β, and IFN-γ. Lymphocytes isolated from these mice, upon adoptive transfer, can also cause liver injury (126). These results provided proof of principle evidence that such adducts have liver disease-causing potential. However, whether endogenous adducts are sufficient to induce a disease-causing immune response in ALD remains to be demonstrated.
Liver-resident NK cells have a host protective role in blocking the activation of hepatic stellate cells (HSCs) and fibrogenesis; chronic alcohol intake impairs this protective function in these cells. These observations have been reviewed in detail elsewhere (34).
PRO-INFLAMMATORY CYTOKINES AND THEIR SIGNALING PATHWAYS
Elevated levels of circulating pro-inflammatory cytokines, specifically TNFα, IL-1β, and IL-8, have been consistently observed in ALD patients (78). These cytokines are, at least in part, produced by circulating monocytes in response to endotoxemia (increased circulating LPS). Later studies showed that an increased level of LPS, although not as high as in alcoholic hepatitis, is also a common feature in alcoholics without significant clinical liver disease (31). Interestingly, increased levels of IL-6 and IL-10, but not TNFα, are characteristics of this cohort (60). An elevated level of the newly identified proinflammatory cytokine IL-17 is also found in the circulation and liver of ALD patients (62).
TNFα
TNFα was one of first pro-inflammatory cytokines linked to ALD. A high level of circulating TNFα was found in patients with alcoholic hepatitis, but not in alcoholics without liver diseases. TNFα production is induced early in alcohol-fed animals and is closely coupled with liver injury (111, 146). A pathological role for TNFα in ALD was demonstrated by studies showing that knockout of the TNFα receptor or administration of TNFα neutralizing antibody reduced liver injury induced by alcohol feeding (42, 141); Table 2). Other studies also showed that hepatocytes isolated from alcohol-fed animals are more susceptible to TNFα-induced cell death (97).
Based on these observations, TNFα antagonists have been used for treating patients with alcoholic steatohepatitis, but the results from these studies have been mixed (Table 3). On the one hand, pentoxifylline, a non-specific inhibitor of TNFα synthesis, improved the short-term survival of patients with alcoholic hepatitis (66). On the other hand, numerous trials with anti-TNFα antibody failed to demonstrate an improvement in patient survival, with one trial aborted because of increased mortality primarily owing to infections (10).
Table 3.
ALD interventions by targeting inflammation
| Target | Outcome in animal models | Outcome in human patients | |
|
| |||
| General inflammation | N/A | Corticosteriods (inhibitor of cytokine production) improved the short term survival of severe AH patients; but not recommended for less severe AH patients due to increased infection; | (74, 75) |
|
| |||
| Pentoxifylline (inhibitor of TNFα synthesis and has vasoactivity) improved the short term survival of AH patients; | (66) and references within | ||
|
| |||
| TNFα | Anti-TNFα antibody reduced liver pathology in mice | Anti-TNFα antibody increased AH patients’ short term mortality by infections | (10) |
|
| |||
| ROS | Antioxidant N-acetal cysteine (NAC) reduced liver cell injury and inflammatory conditions | Antioxidant cocktail (alone or with corticosteroid) showed no benefit; | (22, 146) |
| S-adenosyl-L-methionine (restoring alcohol depleted anti-oxidant gluthione) in alcohol-fed baboons attenuate liver injury | S-adenosyl-L-methionine supplement improved mortality in AH patients (after excluding patients with the most severe class Child C) | (64, 76) | |
|
| |||
| Microflora product | Probiotic (Lactobacillus) significantly reduced liver pathology and circulating LPS; Lactobacillus GG ameliorates alcohol-induced gut leakiness and liver injury; Non-absorbable antibiotics reduced liver injury |
Probiotics: 1) reduced liver injury in alcoholics with pycholoysis; 2) improved neutrophil phagocytic capacity and ex vivo LPS stimulated cytokine production from whole blood from patients with compensated alcoholic cirrhosis. Non-absorbable antibiotics moderately improve ALD patients with encephalopathy |
(2, 52, 88, 117) |
|
| |||
| STAT3 | Exogenous IL22 reduced liver fat accumulation and injury | N/D | (51) |
|
| |||
| Adiponectin | Exogenous adiponection reduced liver fat, inflammation, and injury | N/D | (137) |
IL-8
IL-8 is a critical pro-inflammatory chemokine involved in many steps of neutrophil mobilization from bone marrow to tissue infiltration and activation. As discussed earlier, production of IL-8 is also induced by TNFα and by ligands for TLRs via the activation of NF-κB. Circulating IL-8 is highly elevated in patients with alcoholic hepatitis and is closely linked to neutrophil infiltration (30, 115). In comparison, IL-8 is only moderately elevated in alcoholic cirrhosis patients and alcoholics undergoing alcohol withdrawal.
IL-17
IL-17 is a pro-inflammatory cytokine with a relatively weak TNFα-like function. However, this cytokine can act synergistically with other cytokines, i.e., TNFα, to activate NF-κB and induce IL-8 production. A recent study found that elevated IL-17 is also a characteristic of alcoholic patients with liver disease (62). The cellular origins of IL-17 in this study were monocytes and T cells in the circulation and infiltrating neutrophils and T cells in the liver. Interestingly, expression of the IL-17 receptor was mainly localized to hepatic stellate cells in the liver biopsies of ALD patients. Exposure of hepatic stellate cells to IL-17 in vitro induced production of IL-8 and GROα, both of which are important for neutrophil recruitment. Finally, a correlation between liver IL-17-secreting cell infiltrates and fibrosis score was found (62). This suggests that IL-17 plays a role in promoting both liver inflammation and fibrogenesis of ALD.
ANTI-INFLAMMATORY AND HEPATOPROTECTIVE CYTOKINES
IL-6, IL-10, IL-22 and their downstream signaling molecule signal transducer and activator of transcription 3 (STAT3)
IL-6, IL-22, and IL-10 share a common downstream signal transducer, STAT3, which regulates expression of genes of diverse functions in immune defense and cell proliferation, survival, and differentiation. These cytokines, though all activating STAT3, sometimes have different and even opposing functions. For example, IL-6 has both pro- and anti-inflammatory roles, the latter of which is largely due to its hepatoprotective function, which ameliorates hepatocyte necrosis-associated inflammation (133). In contrast, the pro-inflammatory effect of IL-6 is mediated via the upregulation of pro-inflammatory cytokines in macrophages. IL-10 has a strong anti-inflammatory role by blocking the production of TNFα, IL-1β and IL-6 in macrophages.
Elevated IL-6 is found in chronically alcohol-fed animals and in alcoholics, even those without apparent liver disease (60). IL-6 knockout mice fed alcohol, surprisingly, had increased liver fat accumulation, lipid peroxidation, mitochondrial DNA damage, and sensitization of hepatocytes to TNFα-induced apoptosis, which was preventable by administration of recombinant IL-6 (26, 37, 144) (Table 2). The findings suggest that IL-6 has a host protective role at the early phase of ALD. Similarly, IL-22 administration to alcohol-fed mice also prevented liver steatosis and liver injury through activation of hepatic STAT3 (51, 136).
Similar to the IL-6 knockout mice, alcohol-fed mice with a hepatocyte STAT3 deletion had enhanced liver fat accumulation, but reduced inflammation, suggesting that hepatic STAT3 has a protective role against steatosis, but a stimulatory role in inflammation in hepatocytes (40). In contrast, alcohol-fed mice with a conditional STAT3 deletion in myeloid-derived cells or endothelial cells had an increased inflammatory response, consistent with a role for STAT3 in controlling inflammation in these immune cells, possibly via IL-10 (40). Indeed, alcohol-fed IL-10 knockout mice have increased hepatic and systemic inflammatory conditions compared to wild-type mice (81). Surprisingly, though, despite the increased inflammation, IL-10 knockout mice have a reduced fatty liver and lower serum ALT and AST levels after ethanol feeding compared with wild-type mice. This may be because IL-10 knockout mice have elevated IL-6 and STAT3 activation in the liver, which ameliorates steatosis and hepatocellular damage (81). Together, at the early stage of ALD, inflammation involving IL-6/STAT3 has a host protective role against alcoholic steatosis and liver injury.
Finally, hepatic STAT3 activation is impaired in alcoholics with ALD, suggesting the uncoupling of IL-6 from STAT3 activation (119); such an outcome can in part be explained by the fact that chronic alcohol exposure inhibits IL-6-induced STAT3 activation in hepatocytes and sinusoidal endothelial cells (12, 32). Thus, it is plausible that inflammation-associated activation of IL-6/STAT3 plays a compensatory role in ameliorating steatosis and liver injury at the early stage of ALD, whereas chronic alcohol consumption diminishes IL-6/STAT3 activation and its hepatoprotective function in the liver, leading to severe forms of ALD.
Adiponectin
While increased expression of inflammatory mediators during chronic ethanol exposure, associated with both increased exposure and sensitivity of Kupffer cells to LPS (85), has been thought to be primarily a localized response within the liver, ethanol also has systemic effects that can impact the imbalance of pro-inflammatory cytokine production. In particular, recent studies have implicated ethanol-induced changes in the expression of adipokines in mediating the pathophysiological effects of ethanol. While some data suggest that ethanol can affect the release of the adipokines leptin and resistin (100), a considerable body of evidence now consistently demonstrates that chronic, heavy ethanol exposure decreases serum adiponectin concentrations in mice (137, 142) and rats (124). In rats, serum adiponectin concentrations decrease as early as 3 days on the Lieber-DeCarli ethanol diet, prior to the onset of liver injury (14). Adiponectin mediated responses can be regulated both by changes in adiponectin concentration and changes in the expression and functional activity of adiponectin receptors. Very little is known about the role of adiponectin and adiponectin receptors in mediating ALD in humans.
Adiponectin has broad effects on innate and adaptive immunity, with important anti-inflammatory activity. Kupffer cells isolated from ethanol-fed rats are very sensitive to the anti-inflammatory effects of adiponectin (124). Both globular and full-length adiponectin suppress LPS-stimulated cytokine expression, by decreasing TLR4-mediated signaling and shifting macrophages to an M2 phenotype (70-72).
TARGETING INFLAMMATION FOR ALD TREATMENT
ALD accounts for about 37,000 deaths per year in the USA. ALD in alcoholics begins with hepatic steatosis and progresses into more severe alcoholic hepatitis and/or cirrhosis. In the clinical setting, only alcoholics with alcoholic hepatitis and/or cirrhosis have identifiable symptoms and receive treatment. Clinical manifestations, diagnosis, and treatment of alcoholic hepatitis has been thoroughly reviewed [for the most recent updates, see (33, 66, 94, 123)]. Severe alcoholic hepatitis patients, in particular, have a very high mortality rate of about 50%, and those who survive have a 70% probability of developing liver cirrhosis. Severe ALD is also the 2nd leading cause for liver transplant.
The cornerstone of the current treatment for alcoholic hepatitis is alcohol abstinence (33, 94, 123). Although alcohol abstinence is absolutely necessary, it is often insufficient or ineffective for some patients. Nutritional supplementation is also often recommended for alcoholic hepatitis patients because of the prevalence of malnutrition. Only two pharmacological agents, corticosteroids and pentoxifylline, are currently recommended for treating alcoholic hepatitis; significantly, both of these agents are aimed at reducing inflammatory conditions. Corticosteroids reduce cytokine production through transcriptional regulation whereas pentoxifylline has a similar effect through inhibition of phosphodiesterase.
The use of either corticosteroids or pentoxifylline is recommended only for individuals with severe alcoholic hepatitis because these pharmaceuticals do not provide a benefit for patients with milder conditions. In addition, the improvement in short-term survival of alcoholic hepatitis patients as a result of these drugs is modest. A high percentage of patients do not respond to either treatment. Furthermore, a significant side effect of corticosteroids is an increased susceptibility to infections, resulting from immune suppression.
Given that the current medication is limited to treating the severe stage of ALD and has modest efficacy, new and better pharmacological options are needed for treating and minimizing complications in patients with severe alcoholic hepatitis and cirrhosis.
TNFα antagonist
TNFα antagonists were thought to be promising for treating alcoholic hepatitis patients for a number of reasons. Elevated TNFα has long been described as a predictor of alcoholic hepatitis disease severity. Animal studies have shown that hepatocytes from alcohol-fed animals are more susceptible to TNFα-induced cell death. Administration of anti-TNFα antibodies in alcohol-fed animals also reduces liver injury. However, trials with TNFα antibody have had mixed results. Mechanistically, TNFα antagonist treatment causes systemic TNFα deficiency and subsequent infections (10). It is debatable whether a lower dose of a TNFα antagonist might be beneficial in treating severe or mild alcoholic hepatitis. Given the similarity in side effects of TNFα antagonists to corticosteroids, which induce a worse outcome, it will be difficult to justify additional human trials with TNFα antagonists for the treatment of ALD.
Antioxidants
Many antioxidants, such as N-acetylcysteine, are effective in treating alcohol-fed animals against liver fat accumulation, inflammation, and injury (146). Besides a study showing a limited improvement of AH patient survival by S-adenosylmethionine for restoring cellular anti-oxidant glutathione (76), however, studies using such agents have not been consistently successful in treating ALD patients (120). Because ALD in animal models is mostly mild in comparison to human ALD in the clinic, it is possible oxidative stress plays a minor role in developing severe forms of ALD. Laboratory studies with antioxidant post-treatment of severe forms of liver injury in animal models should be performed to confirm the effectiveness of these supplements in reducing liver oxidative stress.
Microflora products
Probiotics have been successfully used in treating mild liver diseases in both animal models (88) and alcoholic patients (52). In addition, the use of probiotics improves neutrophil function in patients with severe liver disease, although mortality is not improved (117). The advantage, and also a challenge, with probiotics is the availability of a wide variety of supplements. Analysis of the microflora in human alcoholics with or without different types of probiotics may provide useful information when developing treatments. The use of probiotics has a number of advantages, including likely affordability and low adverse side effects. However, whether probiotics are beneficial in treating mild to severe alcoholic hepatitis remains to be examined.
The factors that activate STAT3 in hepatocytes
As demonstrated in alcohol-fed animal models, STAT3 activation in hepatocytes induced by the administration of IL-6 or IL-22 reduces liver fat accumulation, ameliorates hepatocellular damage, and promotes liver regeneration (38, 51, 144). However, the clinical application of IL-6 therapy in ALD may be halted because of the many side effects of IL-6 treatment, owing to the ubiquitous expression of IL-6 receptors and their gp130 signaling chain in a wide variety of cell types. In contrast, IL-22 receptor expression is restricted to epithelial cells, including hepatocytes; thus, IL-22 treatment may have limited side effects and may be a promising option for treating ALD because of its antioxidant, antiapoptotic, antisteatotic, proliferative, and antimicrobial effects (51).
A separate application for IL-6 and IL-22 is in treating donor livers ex vivo for transplantation, as has been suggested by a earlier study (121). Liver transplantation is by far the most effective last resort for ALD patients who fail to respond to existing treatments. Steatosis in donor livers, which is prevalent in many countries as a result of obesity, alcohol and inactivity, is an important risk factor for transplant failure. Pretreatment of donor livers in vitro with IL-6 or IL-22 might significantly improve the success rate of transplantation.
The factors that activate STAT3 in myeloid cells
Conditional deletion of STAT3 in myeloid cells markedly increased liver inflammation in a variety of liver injury models, including Con A-induced T cell hepatitis (58), CCl4-induced liver injury (39), and ethanol-induced liver injury (40), suggesting that activation of STAT3 in myeloid cells inhibits liver inflammation. Any factors that activate STAT3 in myeloid cells, such as IL-10, may have beneficial effects in inhibiting liver inflammation in ALD.
Adiponectin
Previous studies have established that adipocyte-derived adiponectin has an inflammation-suppression effect on many cell types, including monocytes and endothelial cells (96). In addition, this chemokine stimulates fatty acid oxidation. These two properties of adiponectin have generated a broad interest of its application in combating atherogenesis and obesity-induced diabetes. Chronic alcohol intake inhibits adiponectin secretion by subcutaneous adipocytes (13). Administration of adiponectin in alcohol-fed mice significantly reduces liver fat, inflammation, and injury (137). A recent study showed that the effect of adiponectin is mediated by a heme oxygenase-1-dependent induction of the anti-inflammatory cytokine IL-10 (70). In summary, adiponectin treatment may have multiple advantages for treating ALD, including ameliorating steatosis and inflammation. Because very high levels of adiponectin are found in the serum of ALD patients, the administration of adiponectin may not be effective in these patients. Current challenges involve finding a pharmacologically suitable drug to mimic adiponectin and/or its down-stream effectors.
IL-17 and IL-8
The failure of TNFα antagonists raises the possibility that antagonists for pro-inflammatory cytokines/chemokines that are more specific and less pleiotropic in global immune defense may prove to be more effective for treating alcoholic hepatitis. IL-17 and IL-8 fit such a description because of their roles in inducing neutrophil infiltration and in synergizing TNFα function. These targets should be tested in animal models and in humans with alcoholic hepatitis in the near future.
In summary, existing therapies for alcoholic hepatitis are limited by their modest efficacy and undesirable side effects on immune suppression. Mitigating the pathology of ALD by targeting aspects of the inflammatory machinery has resulted in success in animal models, but not in patients. In fact, global blocking of inflammatory activity as a treatment option has a clear drawback in compromising immune defense. Interventions targeting narrower and more specific activity, such as IL-8/IL-17/neutrophil infiltration, remain to be explored. Recent findings on the hepatoprotective cytokine IL-22 and its downstream signaling STAT3 also provide an opportunity for interventions that target ALD.
CONCLUSIONS AND FUTURE PERSPECTIVES
Acute and chronic inflammatory conditions have been closely linked to the pathogenesis of ALD. In the past decade, significant advances have been made in identifying the inducers of inflammation and in determining the specific role of inflammatory components in ALD development. These advances have a number of important implications for future research directions and for ALD intervention development.
First, the causes of alcohol-related liver inflammation are complex. Besides the well known gut flora derived LPS, multiple factors have been identified with the capability of inducing liver inflammatory responses. These include, among others, alcohol metabolites, enriched FFAs, necrotic cell products, and complements. Evidence thus far supports that these agents are highly interactive via common downstream intermediates such as ROS. Significantly, many of these inflammatory inducing agents and their downstream intermediates, in addition to their important roles in inflammatory response, are also known to be common causes of cell/tissue injury. Examples include FFA- (68) and ROS-induced cell death. These close links between alcohol-related inflammation and tissue injury strongly support the effort of targeting inflammatory process for ALD intervention.
Second, inflammation and its key components often have dual roles, i.e., pathological and protective functions. The importance of this reflection is underscored by the many cases discussed in this review and the observed side effect of therapies based on corticosteroids and TNFα antagonists. Therefore, when developing effective anti-inflammatory interventions, the challenge is to identify the components and pathways that are primarily pathogenic and/or to develop a strategy that can mitigate the pathogenic effect without significantly compromising the protective functions of these components.
Lastly, we still need to gain a better understanding of the cellular and molecular changes that occur during the development of ALD and, in particular, alcoholic hepatitis. With the significant expansion of knowledge/technology on new types of immune cells and mediators, a renewed study of the cellular and molecular changes that occur during disease development is critically needed; an example is the recently identified cytokine IL-17, which is known for its key role in autoimmunity. Increased IL-17 and IL-17-secreting cells are now known to be unique characteristics of ALD, among liver diseases of different etiologies (62). The classification of patients based on an expanded cytokine profile, for example, could be instrumental in selecting patients with a clear need for treatment. For example, studies in the 1990s indicated that the rise and fall of circulating IL-6 and IL-8 over time in alcoholic hepatitis patients is closely associated with non-survival (death) and recovery, respectively (30, 50, 115). Discoveries made from such renewed efforts will stimulate mechanistic studies and translational research for more effective interventions for ALD.
Acknowledgments
The authors wish to thank Dr. Kathy Jung for critical reading of the manuscript. This work was supported in part by the intramural program of NIAAA, NIH (B. Gao) and grants P20 AA017837, R01 AA016399, R37 AA011876 and R01 AA011975 (L. Nagy).
Footnotes
DISLOSURE TREATMENT
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
Contributor Information
H. Joe Wang, Email: wangh4@mail.nih.gov.
Bin Gao, Email: bgao@mail.nih.gov.
Laura E. Nagy, Email: nagyL3@ccf.org.
References
- 1.Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol–induced liver injury. Hepatology. 1994;20:453–60. [PubMed] [Google Scholar]
- 2.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long–term exposure to ethanol. Gastroenterology. 1995;108:218–24. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 3.Aldred A, Nagy LE. Ethanol dissociates hormone–stimulated cAMP production from inhibition of TNF–alpha production in rat Kupffer cells. Am J Physiol. 1999;276:G98–G106. doi: 10.1152/ajpgi.1999.276.1.G98. [DOI] [PubMed] [Google Scholar]
- 4.Alfonso–Loeches S, Pascual–Lucas M, Blanco AM, Sanchez–Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol–induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–95. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arteel GE, Raleigh JA, Bradford BU, Thurman RG. Acute alcohol produces hypoxia directly in rat liver tissue in vivo: role of Kupffer cells. Am J Physiol. 1996;271:G494–500. doi: 10.1152/ajpgi.1996.271.3.G494. [DOI] [PubMed] [Google Scholar]
- 6.Bautista AP. Chronic alcohol intoxication induces hepatic injury through enhanced macrophage inflammatory protein–2 production and intercellular adhesion molecule–1 expression in the liver. Hepatology. 1997;25:335–42. doi: 10.1002/hep.510250214. [DOI] [PubMed] [Google Scholar]
- 7.Bautista AP. Neutrophilic infiltration in alcoholic hepatitis. Alcohol. 2002;27:17–21. doi: 10.1016/s0741-8329(02)00206-9. [DOI] [PubMed] [Google Scholar]
- 8.Blanco AM, Perez–Arago A, Fernandez–Lizarbe S, Guerri C. Ethanol mimics ligand–mediated activation and endocytosis of IL–1RI/TLR4 receptors via lipid rafts caveolae in astroglial cells. J Neurochem. 2008;106:625–39. doi: 10.1111/j.1471-4159.2008.05425.x. [DOI] [PubMed] [Google Scholar]
- 9.Blanco AM, Valles SL, Pascual M, Guerri C. Involvement of TLR4/type I IL–1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J Immunol. 2005;175:6893–9. doi: 10.4049/jimmunol.175.10.6893. [DOI] [PubMed] [Google Scholar]
- 10.Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, et al. A randomized, double–blinded, placebo–controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953–60. doi: 10.1053/j.gastro.2008.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol–induced liver injury. Arch Toxicol. 2009;83:519–48. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Bao H, Sawyer S, Kunos G, Gao B. Effects of short and long term ethanol on the activation of signal transducer and activator transcription factor 3 in normal and regenerating liver. Biochem Biophys Res Commun. 1997;239:666–9. doi: 10.1006/bbrc.1997.7531. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Sebastian BM, Nagy LE. Chronic ethanol feeding to rats decreases adiponectin secretion by subcutaneous adipocytes. Am J Physiol Endocrinol Metab. 2007;292:E621–8. doi: 10.1152/ajpendo.00387.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Sebastian BM, Tang H, McMullen MM, Axhemi A, et al. Taurine supplementation prevents ethanol–induced decrease in serum adiponectin and reduces hepatic steatosis in rats. Hepatology. 2009;49:1554–62. doi: 10.1002/hep.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christoffersen P, Braendstrup O, Juhl E, Poulsen H. Lipogranulomas in human liver biopsies with fatty change. A morphological, biochemical and clinical investigation. Acta Pathol Microbiol Scand A. 1971;79:150–8. doi: 10.1111/j.1699-0463.1971.tb03323.x. [DOI] [PubMed] [Google Scholar]
- 16.Cohen JI, Roychowdhury S, McMullen MR, Stavitsky AB, Nagy LE. Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol–induced liver injury in mice. Gastroenterology. 2010;139:664–74. 74 e1. doi: 10.1053/j.gastro.2010.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colell A, Garcia–Ruiz C, Miranda M, Ardite E, Mari M, et al. Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology. 1998;115:1541–51. doi: 10.1016/s0016-5085(98)70034-4. [DOI] [PubMed] [Google Scholar]
- 18.Cook RT, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, et al. Thymocytes, pre–B cells, and organ changes in a mouse model of chronic ethanol ingestion––absence of subset–specific glucocorticoid–induced immune cell loss. Alcohol Clin Exp Res. 2007;31:1746–58. doi: 10.1111/j.1530-0277.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133–44. doi: 10.1002/hep.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cubero FJ, Nieto N. Ethanol and arachidonic acid synergize to activate Kupffer cells and modulate the fibrogenic response via tumor necrosis factor alpha, reduced glutathione, and transforming growth factor beta–dependent mechanisms. Hepatology. 2008;48:2027–39. doi: 10.1002/hep.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis WD, Jr, Culpepper WS. Cirrhosis of the liver associated with alcoholism; report of acute exacerbation with serial liver biopsies. Ann Intern Med. 1948;29:942–58. doi: 10.7326/0003-4819-29-5-942. [DOI] [PubMed] [Google Scholar]
- 22.Day CP. Treatment of alcoholic liver disease. Liver Transpl. 2007;13:S69–75. doi: 10.1002/lt.21336. [DOI] [PubMed] [Google Scholar]
- 23.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duryee MJ, Klassen LW, Freeman TL, Willis MS, Tuma DJ, Thiele GM. Lipopolysaccharide is a cofactor for malondialdehyde–acetaldehyde adduct–mediated cytokine/chemokine release by rat sinusoidal liver endothelial and Kupffer cells. Alcohol Clin Exp Res. 2004;28:1931–8. doi: 10.1097/01.alc.0000148115.90045.c5. [DOI] [PubMed] [Google Scholar]
- 25.Eigenbrod T, Park JH, Harder J, Iwakura Y, Nunez G. Cutting edge: critical role for mesothelial cells in necrosis–induced inflammation through the recognition of IL–1 alpha released from dying cells. J Immunol. 2008;181:8194–8. doi: 10.4049/jimmunol.181.12.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El–Assal O, Hong F, Kim WH, Radaeva S, Gao B. IL–6–deficient mice are susceptible to ethanol–induced hepatic steatosis: IL–6 protects against ethanol–induced oxidative stress and mitochondrial permeability transition in the liver. Cell Mol Immunol. 2004;1:205–11. [PubMed] [Google Scholar]
- 27.Elphick DA, Dube AK, McFarlane E, Jones J, Gleeson D. Spectrum of liver histology in presumed decompensated alcoholic liver disease. Am J Gastroenterol. 2007;102:780–8. doi: 10.1111/j.1572-0241.2006.01034.x. [DOI] [PubMed] [Google Scholar]
- 28.Enomoto N, Ikejima K, Bradford BU, Rivera CA, Kono H, et al. Role of Kupffer cells and gut–derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15(Suppl):D20–5. doi: 10.1046/j.1440-1746.2000.02179.x. [DOI] [PubMed] [Google Scholar]
- 29.Enomoto N, Schemmer P, Ikejima K, Takei Y, Sato N, et al. Long–term alcohol exposure changes sensitivity of rat Kupffer cells to lipopolysaccharide. Alcohol Clin Exp Res. 2001;25:1360–7. [PubMed] [Google Scholar]
- 30.Fujimoto M, Uemura M, Nakatani Y, Tsujita S, Hoppo K, et al. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res. 2000;24:48S–54S. [PubMed] [Google Scholar]
- 31.Fukui H. Relation of endotoxin, endotoxin binding proteins and macrophages to severe alcoholic liver injury and multiple organ failure. Alcohol Clin Exp Res. 2005;29:172S–9S. doi: 10.1097/01.alc.0000189278.30237.e9. [DOI] [PubMed] [Google Scholar]
- 32.Gao B. Therapeutic potential of interleukin–6 in preventing obesity– and alcohol–associated fatty liver transplant failure. Alcohol. 2004;34:59–65. doi: 10.1016/j.alcohol.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–85. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao B, Seki E, Brenner DA, Friedman S, Cohen JI, et al. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011;300:G516–25. doi: 10.1152/ajpgi.00537.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, et al. Differential liver sensitization to toll–like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43:989–1000. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- 36.Hill DB, D'Souza NB, Lee EY, Burikhanov R, Deaciuc IV, de Villiers WJ. A role for interleukin–10 in alcohol–induced liver sensitization to bacterial lipopolysaccharide. Alcohol Clin Exp Res. 2002;26:74–82. [PubMed] [Google Scholar]
- 37.Hong F, Kim WH, Tian Z, Jaruga B, Ishac E, et al. Elevated interleukin–6 during ethanol consumption acts as a potential endogenous protective cytokine against ethanol–induced apoptosis in the liver: involvement of induction of Bcl–2 and Bcl–x(L) proteins. Oncogene. 2002;21:32–43. doi: 10.1038/sj.onc.1205016. [DOI] [PubMed] [Google Scholar]
- 38.Hong F, Radaeva S, Pan HN, Tian Z, Veech R, Gao B. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology. 2004;40:933–41. doi: 10.1002/hep.20400. [DOI] [PubMed] [Google Scholar]
- 39.Horiguchi N, Lafdil F, Miller AM, Park O, Wang H, et al. Dissociation between liver inflammation and hepatocellular damage induced by carbon tetrachloride in myeloid cell–specific signal transducer and activator of transcription 3 gene knockout mice. Hepatology. 2010;51:1724–34. doi: 10.1002/hep.23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horiguchi N, Wang L, Mukhopadhyay P, Park O, Jeong WI, et al. Cell type–dependent pro– and anti–inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134:1148–58. doi: 10.1053/j.gastro.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, et al. The critical role of toll–like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–31. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–7. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- 43.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, et al. Acetaminophen–induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–14. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E. Toll–Like Receptor 4 Mediates Alcohol–Induced Steatohepatitis Through Bone Marrow–Derived and Endogenous Liver Cells in Mice. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:20388–93. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaeschke H, Smith CW. Mechanisms of neutrophil–induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–53. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 47.Joshi–Barve S, Barve SS, Amancherla K, Gobejishvili L, Hill D, et al. Palmitic acid induces production of proinflammatory cytokine interleukin–8 from hepatocytes. Hepatology. 2007;46:823–30. doi: 10.1002/hep.21752. [DOI] [PubMed] [Google Scholar]
- 48.Kawai T, Akira S. The role of pattern–recognition receptors in innate immunity: update on Toll–like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 49.Kendrick SF, O'Boyle G, Mann J, Zeybel M, Palmer J, et al. Acetate, the key modulator of inflammatory responses in acute alcoholic hepatitis. Hepatology. 2010;51:1988–97. doi: 10.1002/hep.23572. [DOI] [PubMed] [Google Scholar]
- 50.Khoruts A, Stahnke L, McClain CJ, Logan G, Allen JI. Circulating tumor necrosis factor, interleukin–1 and interleukin–6 concentrations in chronic alcoholic patients. Hepatology. 1991;13:267–76. [PubMed] [Google Scholar]
- 51.Ki SH, Park O, Zheng M, Morales–Ibanez O, Kolls JK, et al. Interleukin–22 treatment ameliorates alcoholic liver injury in a murine model of chronic–binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol–induced liver injury: a pilot study. Alcohol. 2008;42:675–82. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kishore R, Hill JR, McMullen MR, Frenkel J, Nagy LE. ERK1/2 and Egr–1 contribute to increased TNF–alpha production in rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2002;282:G6–15. doi: 10.1152/ajpgi.00328.2001. [DOI] [PubMed] [Google Scholar]
- 54.Kishore R, McMullen MR, Nagy LE. Stabilization of tumor necrosis factor alpha mRNA by chronic ethanol: role of A + U–rich elements and p38 mitogen–activated protein kinase signaling pathway. J Biol Chem. 2001;276:41930–7. doi: 10.1074/jbc.M107181200. [DOI] [PubMed] [Google Scholar]
- 55.Kono H, Karmarkar D, Iwakura Y, Rock KL. Identification of the cellular sensor that stimulates the inflammatory response to sterile cell death. J Immunol. 2010;184:4470–8. doi: 10.4049/jimmunol.0902485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, et al. NADPH oxidase–derived free radicals are key oxidants in alcohol–induced liver disease. J Clin Invest. 2000;106:867–72. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kotronen A, Seppanen–Laakso T, Westerbacka J, Kiviluoto T, Arola J, et al. Comparison of lipid and fatty acid composition of the liver, subcutaneous and intra–abdominal adipose tissue, and serum. Obesity (Silver Spring) 2010;18:937–44. doi: 10.1038/oby.2009.326. [DOI] [PubMed] [Google Scholar]
- 58.Lafdil F, Wang H, Park O, Zhang W, Moritoki Y, et al. Myeloid STAT3 inhibits T cell–mediated hepatitis by regulating T helper 1 cytokine and interleukin–17 production. Gastroenterology. 2009;137:2125–35. e1–2. doi: 10.1053/j.gastro.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lakshminarayanan V, Beno DW, Costa RH, Roebuck KA. Differential regulation of interleukin–8 and intercellular adhesion molecule–1 by H2O2 and tumor necrosis factor–alpha in endothelial and epithelial cells. J Biol Chem. 1997;272:32910–8. doi: 10.1074/jbc.272.52.32910. [DOI] [PubMed] [Google Scholar]
- 60.Latvala J, Hietala J, Koivisto H, Jarvi K, Anttila P, Niemela O. Immune Responses to Ethanol Metabolites and Cytokine Profiles Differentiate Alcoholics with or without Liver Disease. Am J Gastroenterol. 2005;100:1303–10. doi: 10.1111/j.1572-0241.2005.41509.x. [DOI] [PubMed] [Google Scholar]
- 61.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase–2 mediated through Toll–like receptor 4. J Biol Chem. 2001;276:16683–9. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 62.Lemmers A, Moreno C, Gustot T, Marechal R, Degre D, et al. The interleukin–17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646–57. doi: 10.1002/hep.22680. [DOI] [PubMed] [Google Scholar]
- 63.Li H, Ambade A, Re F. Cutting edge: Necrosis activates the NLRP3 inflammasome. J Immunol. 2009;183:1528–32. doi: 10.4049/jimmunol.0901080. [DOI] [PubMed] [Google Scholar]
- 64.Lieber CS, Casini A, DeCarli LM, Kim CI, Lowe N, et al. S–adenosyl–L–methionine attenuates alcohol–induced liver injury in the baboon. Hepatology. 1990;11:165–72. doi: 10.1002/hep.1840110203. [DOI] [PubMed] [Google Scholar]
- 65.Louvet A, Teixeira–Clerc F, Chobert MN, Deveaux V, Pavoine C, et al. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating kupffer cell polarization in mice. Hepatology. 2011 doi: 10.1002/hep.24524. [DOI] [PubMed] [Google Scholar]
- 66.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–69. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 67.MacGregor RR, Gluckman SJ, Senior JR. Granulocyte function and levels of immunoglobulins and complement in patients admitted for withdrawal from alcohol. J Infect Dis. 1978;138:747–55. doi: 10.1093/infdis/138.6.747. [DOI] [PubMed] [Google Scholar]
- 68.Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:360–9. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mallory FB. Bulletin of the Johns Hopkins Hospital. 1911. Cirrhosis of the liver; p. 22. [Google Scholar]
- 70.Mandal P, Park PH, McMullen MR, Pratt BT, Nagy LE. The anti–inflammatory effects of adiponectin are mediated via a heme oxygenase–1–dependent pathway in rat Kupffer cells. Hepatology. 2010;51:1420–9. doi: 10.1002/hep.23427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE. Molecular mechanism for adiponectin–dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full–length adiponectin. J Biol Chem. 2011;286:13460–9. doi: 10.1074/jbc.M110.204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mandal P, Roychowdhury S, Park PH, Pratt BT, Roger T, Nagy LE. Adiponectin and heme oxygenase–1 suppress TLR4/MyD88–independent signaling in rat Kupffer cells and in mice after chronic ethanol exposure. J Immunol. 2010;185:4928–37. doi: 10.4049/jimmunol.1002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mandrekar P, Ambade A, Lim A, Szabo G, Catalano D. An essential role for monocyte chemoattractant protein–1 in alcoholic liver injury: Regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology. 2011 doi: 10.1002/hep.24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mathurin P, Mendenhall CL, Carithers RL, Jr, Ramond MJ, Maddrey WC, et al. Corticosteroids improve short–term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol. 2002;36:480–7. doi: 10.1016/s0168-8278(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 75.Mathurin P, O'Grady J, Carithers RL, Phillips M, Louvet A, et al. Corticosteroids improve short–term survival in patients with severe alcoholic hepatitis: meta–analysis of individual patient data. Gut. 2011;60:255–60. doi: 10.1136/gut.2010.224097. [DOI] [PubMed] [Google Scholar]
- 76.Mato JM, Camara J, Fernandez de Paz J, Caballeria L, Coll S, et al. S–adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo–controlled, double–blind, multicenter clinical trial. J Hepatol. 1999;30:1081–9. doi: 10.1016/s0168-8278(99)80263-3. [DOI] [PubMed] [Google Scholar]
- 77.Mavrelis PG, Ammon HV, Gleysteen JJ, Komorowski RA, Charaf UK. Hepatic free fatty acids in alcoholic liver disease and morbid obesity. Hepatology. 1983;3:226–31. doi: 10.1002/hep.1840030215. [DOI] [PubMed] [Google Scholar]
- 78.McClain CJ, Barve S, Deaciuc I, Kugelmas M, Hill D. Cytokines in alcoholic liver disease. Semin Liver Dis. 1999;19:205–19. doi: 10.1055/s-2007-1007110. [DOI] [PubMed] [Google Scholar]
- 79.McClain CJ, Hill DB, Song Z, Deaciuc I, Barve S. Monocyte activation in alcoholic liver disease. Alcohol. 2002;27:53–61. doi: 10.1016/s0741-8329(02)00212-4. [DOI] [PubMed] [Google Scholar]
- 80.McVicker BL, Tuma PL, Kharbanda KK, Lee SM, Tuma DJ. Relationship between oxidative stress and hepatic glutathione levels in ethanol–mediated apoptosis of polarized hepatic cells. World J Gastroenterol. 2009;15:2609–16. doi: 10.3748/wjg.15.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miller AM, Wang H, Bertola A, Park O, Horiguchi N, et al. Inflammation–associated IL–6/STAT3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in IL–10 deficient mice. Hepatology. 2011 doi: 10.1002/hep.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller AM, Wang H, Park O, Horiguchi N, Lafdil F, et al. Anti–inflammatory and anti–apoptotic roles of endothelial cell STAT3 in alcoholic liver injury. Alcohol Clin Exp Res. 2010;34:719–25. doi: 10.1111/j.1530-0277.2009.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, et al. Toll–like receptor 9 promotes steatohepatitis by induction of interleukin–1beta in mice. Gastroenterology. 2010;139:323–34. e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mookerjee RP, Stadlbauer V, Lidder S, Wright GA, Hodges SJ, et al. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology. 2007;46:831–40. doi: 10.1002/hep.21737. [DOI] [PubMed] [Google Scholar]
- 85.Nagy LE. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp Biol Med (Maywood) 2003;228:882–90. doi: 10.1177/153537020322800803. [DOI] [PubMed] [Google Scholar]
- 86.Nanji AA, French SW. Dietary linoleic acid is required for development of experimentally induced alcoholic liver injury. Life Sci. 1989;44:223–7. doi: 10.1016/0024-3205(89)90599-7. [DOI] [PubMed] [Google Scholar]
- 87.Nanji AA, Jokelainen K, Tipoe GL, Rahemtulla A, Dannenberg AJ. Dietary saturated fatty acids reverse inflammatory and fibrotic changes in rat liver despite continued ethanol administration. J Pharmacol Exp Ther. 2001;299:638–44. [PubMed] [Google Scholar]
- 88.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease) Proc Soc Exp Biol Med. 1994;205:243–7. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- 89.Nanji AA, Tsukamoto H, French SW. Relationship between fatty liver and subsequent development of necrosis, inflammation and fibrosis in experimental alcoholic liver disease. Exp Mol Pathol. 1989;51:141–8. doi: 10.1016/0014-4800(89)90014-2. [DOI] [PubMed] [Google Scholar]
- 90.Nath B, Levin I, Csak T, Petrasek J, Mueller C, et al. Hepatocyte–specific hypoxia–inducible factor–1alpha is a determinant of lipid accumulation and liver injury in alcohol–induced steatosis in mice. Hepatology. 2011;53:1526–37. doi: 10.1002/hep.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Natori S, Rust C, Stadheim LM, Srinivasan A, Burgart LJ, Gores GJ. Hepatocyte apoptosis is a pathologic feature of human alcoholic hepatitis. J Hepatol. 2001;34:248–53. doi: 10.1016/s0168-8278(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 92.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–17. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nolan JP. The role of intestinal endotoxin in liver injury: a long and evolving history. Hepatology. 2010;52:1829–35. doi: 10.1002/hep.23917. [DOI] [PubMed] [Google Scholar]
- 94.O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–28. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 95.Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. TLR2– and TLR4–mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J Immunol. 2006;176:7628–35. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- 96.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, et al. Adiponectin, an adipocyte–derived plasma protein, inhibits endothelial NF–kappaB signaling through a cAMP–dependent pathway. Circulation. 2000;102:1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 97.Pastorino JG, Hoek JB. Ethanol potentiates tumor necrosis factor–alpha cytotoxicity in hepatoma cells and primary rat hepatocytes by promoting induction of the mitochondrial permeability transition. Hepatology. 2000;31:1141–52. doi: 10.1053/he.2000.7013. [DOI] [PubMed] [Google Scholar]
- 98.Petrasek J, Dolganiuc A, Csak T, Nath B, Hritz I, et al. Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells. Hepatology. 2011;53:649–60. doi: 10.1002/hep.24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Popper H, Thung SN, Gerber MA. Pathology of alcoholic liver diseases. Semin Liver Dis. 1981;1:203–16. doi: 10.1055/s-2008-1041748. [DOI] [PubMed] [Google Scholar]
- 100.Pravdova E, Fickova M. Alcohol intake modulates hormonal activity of adipose tissue. Endocr Regul. 2006;40:91–104. [PubMed] [Google Scholar]
- 101.Pritchard MT, McMullen MR, Stavitsky AB, Cohen JI, Lin F, et al. Differential contributions of C3, C5, and decay–accelerating factor to ethanol–induced fatty liver in mice. Gastroenterology. 2007;132:1117–26. doi: 10.1053/j.gastro.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pritchard MT, Nagy LE. Ethanol–induced liver injury: potential roles for egr–1. Alcohol Clin Exp Res. 2005;29:146S–50S. doi: 10.1097/01.alc.0000189286.81943.51. [DOI] [PubMed] [Google Scholar]
- 103.Quan Y, Du J, Wang X. High glucose stimulates GRO secretion from rat microglia via ROS, PKC, and NF–kappaB pathways. J Neurosci Res. 2007;85:3150–9. doi: 10.1002/jnr.21421. [DOI] [PubMed] [Google Scholar]
- 104.Quan Y, Jiang CT, Xue B, Zhu SG, Wang X. High glucose stimulates TNFalpha and MCP–1 expression in rat microglia via ROS and NF–kappaB pathways. Acta Pharmacol Sin. 2011;32:188–93. doi: 10.1038/aps.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramaiah S, Rivera C, Arteel G. Early–phase alcoholic liver disease: an update on animal models, pathology, and pathogenesis. Int J Toxicol. 2004;23:217–31. doi: 10.1080/10915810490502069. [DOI] [PubMed] [Google Scholar]
- 106.Ramaiah SK, Jaeschke H. Hepatic neutrophil infiltration in the pathogenesis of alcohol–induced liver injury. Toxicol Mech Methods. 2007;17:431–40. doi: 10.1080/00952990701407702. [DOI] [PubMed] [Google Scholar]
- 107.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–44. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rolla R, Vay D, Mottaran E, Parodi M, Traverso N, et al. Detection of circulating antibodies against malondialdehyde–acetaldehyde adducts in patients with alcohol–induced liver disease. Hepatology. 2000;31:878–84. doi: 10.1053/he.2000.5373. [DOI] [PubMed] [Google Scholar]
- 109.Ronis MJ, Hakkak R, Korourian S, Albano E, Yoon S, et al. Alcoholic liver disease in rats fed ethanol as part of oral or intragastric low–carbohydrate liquid diets. Exp Biol Med (Maywood) 2004;229:351–60. doi: 10.1177/153537020422900410. [DOI] [PubMed] [Google Scholar]
- 110.Ronis MJ, Korourian S, Zipperman M, Hakkak R, Badger TM. Dietary saturated fat reduces alcoholic hepatotoxicity in rats by altering fatty acid metabolism and membrane composition. J Nutr. 2004;134:904–12. doi: 10.1093/jn/134.4.904. [DOI] [PubMed] [Google Scholar]
- 111.Roychowdhury S, McMullen MR, Pritchard MT, Hise AG, van Rooijen N, et al. An early complement–dependent and TLR–4–independent phase in the pathogenesis of ethanol–induced liver injury in mice. Hepatology. 2009;49:1326–34. doi: 10.1002/hep.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rusyn I, Bradham CA, Cohn L, Schoonhoven R, Swenberg JA, et al. Corn oil rapidly activates nuclear factor–kappaB in hepatic Kupffer cells by oxidant–dependent mechanisms. Carcinogenesis. 1999;20:2095–100. doi: 10.1093/carcin/20.11.2095. [DOI] [PubMed] [Google Scholar]
- 113.Shanmugam N, Reddy MA, Guha M, Natarajan R. High glucose–induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes. 2003;52:1256–64. doi: 10.2337/diabetes.52.5.1256. [DOI] [PubMed] [Google Scholar]
- 114.Shen Z, Ajmo JM, Rogers CQ, Liang X, Le L, et al. Role of SIRT1 in regulation of LPS– or two ethanol metabolites–induced TNF–alpha production in cultured macrophage cell lines. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1047–53. doi: 10.1152/ajpgi.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sheron N, Bird G, Koskinas J, Portmann B, Ceska M, et al. Circulating and tissue levels of the neutrophil chemotaxin interleukin–8 are elevated in severe acute alcoholic hepatitis, and tissue levels correlate with neutrophil infiltration. Hepatology. 1993;18:41–6. [PubMed] [Google Scholar]
- 116.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid–induced insulin resistance. J Clin Invest. 2006;116:3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2008;48:945–51. doi: 10.1016/j.jhep.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 118.Stadlbauer V, Mookerjee RP, Wright GA, Davies NA, Jurgens G, et al. Role of Toll–like receptors 2, 4, and 9 in mediating neutrophil dysfunction in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G15–22. doi: 10.1152/ajpgi.90512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Starkel P, De Saeger C, Leclercq I, Strain A, Horsmans Y. Deficient Stat3 DNA–binding is associated with high Pias3 expression and a positive anti–apoptotic balance in human end–stage alcoholic and hepatitis C cirrhosis. J Hepatol. 2005;43:687–95. doi: 10.1016/j.jhep.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 120.Stewart S, Prince M, Bassendine M, Hudson M, James O, et al. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J Hepatol. 2007;47:277–83. doi: 10.1016/j.jhep.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 121.Sun Z, Klein AS, Radaeva S, Hong F, El–Assal O, et al. In vitro interleukin–6 treatment prevents mortality associated with fatty liver transplants in rats. Gastroenterology. 2003;125:202–15. doi: 10.1016/s0016-5085(03)00696-6. [DOI] [PubMed] [Google Scholar]
- 122.Tabata T, Tani T, Endo Y, Hanasawa K. Bacterial translocation and peptidoglycan translocation by acute ethanol administration. J Gastroenterol. 2002;37:726–31. doi: 10.1007/s005350200118. [DOI] [PubMed] [Google Scholar]
- 123.Tan HH, Virmani S, Martin P. Controversies in the management of alcoholic liver disease. Mt Sinai J Med. 2009;76:484–98. doi: 10.1002/msj.20135. [DOI] [PubMed] [Google Scholar]
- 124.Thakur V, Pritchard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS–stimulated TNF–alpha production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2006;290:G998–1007. doi: 10.1152/ajpgi.00553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS–stimulated ERK1/2 activation and TNF–alpha production. J Leukoc Biol. 2006;79:1348–56. doi: 10.1189/jlb.1005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thiele GM, Duryee MJ, Willis MS, Tuma DJ, Radio SJ, et al. Autoimmune hepatitis induced by syngeneic liver cytosolic proteins biotransformed by alcohol metabolites. Alcohol Clin Exp Res. 2010;34:2126–36. doi: 10.1111/j.1530-0277.2010.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thurman RG, Bradford BU, Iimuro Y, Frankenberg MV, Knecht KT, et al. Mechanisms of alcohol–induced hepatotoxicity: studies in rats. Front Biosci. 1999;4:e42–6. doi: 10.2741/A478. [DOI] [PubMed] [Google Scholar]
- 128.Tsukamoto H, French SW, Benson N, Delgado G, Rao GA, et al. Severe and progressive steatosis and focal necrosis in rat liver induced by continuous intragastric infusion of ethanol and low fat diet. Hepatology. 1985;5:224–32. doi: 10.1002/hep.1840050212. [DOI] [PubMed] [Google Scholar]
- 129.Tsukamoto H, Gaal K, French SW. Insights into the pathogenesis of alcoholic liver necrosis and fibrosis: status report. Hepatology. 1990;12:599–608. doi: 10.1002/hep.1840120325. [DOI] [PubMed] [Google Scholar]
- 130.Tuma DJ, Thiele GM, Xu D, Klassen LW, Sorrell MF. Acetaldehyde and malondialdehyde react together to generate distinct protein adducts in the liver during long–term ethanol administration. Hepatology. 1996;23:872–80. doi: 10.1002/hep.510230431. [DOI] [PubMed] [Google Scholar]
- 131.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll–like receptor 4 is involved in the mechanism of early alcohol–induced liver injury in mice. Hepatology. 2001;34:101–8. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 132.Viitala K, Israel Y, Blake JE, Niemela O. Serum IgA, IgG, and IgM antibodies directed against acetaldehyde–derived epitopes: relationship to liver disease severity and alcohol consumption. Hepatology. 1997;25:1418–24. doi: 10.1002/hep.510250619. [DOI] [PubMed] [Google Scholar]
- 133.Wang H, Lafdil F, Kong X, Gao B. Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. Int J Biol Sci. 2011;7:536–50. doi: 10.7150/ijbs.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut–liver–brain interactions in tissue damage and disease development. World J Gastroenterol. 2010;16:1304–13. doi: 10.3748/wjg.v16.i11.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wen H, Gris D, Lei Y, Jha S, Zhang L, et al. Fatty acid–induced NLRP3–ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–15. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xing WW, Zou MJ, Liu S, Xu T, Wang JX, Xu DG. Interleukin–22 Protects against Acute Alcohol–Induced Hepatotoxicity in Mice. Biosci Biotechnol Biochem. 2011;75:1290–4. doi: 10.1271/bbb.110061. [DOI] [PubMed] [Google Scholar]
- 137.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat–derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu J, Lai KK, Verlinsky A, Lugea A, French SW, et al. Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p–AMPK. J Hepatol. 2011 doi: 10.1016/j.jhep.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, et al. Reduced early alcohol–induced liver injury in CD14–deficient mice. J Immunol. 2001;166:4737–42. doi: 10.4049/jimmunol.166.7.4737. [DOI] [PubMed] [Google Scholar]
- 141.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, et al. Essential role of tumor necrosis factor alpha in alcohol–induced liver injury in mice. Gastroenterology. 1999;117:942–52. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 142.You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568–77. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29:245–54. [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang X, Tachibana S, Wang H, Hisada M, Williams GM, et al. Interleukin–6 is an important mediator for mitochondrial DNA repair after alcoholic liver injury in mice. Hepatology. 2010;52:2137–47. doi: 10.1002/hep.23909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhao XJ, Dong Q, Bindas J, Piganelli JD, Magill A, et al. TRIF and IRF–3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol. 2008;181:3049–56. doi: 10.4049/jimmunol.181.5.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhou Z, Wang L, Song Z, Lambert JC, McClain CJ, Kang YJ. A critical involvement of oxidative stress in acute alcohol–induced hepatic TNF–alpha production. Am J Pathol. 2003;163:1137–46. doi: 10.1016/s0002-9440(10)63473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]