Abstract
Nuclear factor E2-related factor 2 (Nrf2) is a transcription factor known to activate cytoprotective genes which may be useful in the treatment of neurodegenerative disease. In order to better understand the structure activity relationship of phenolic diterpenes from Salvia officinalis L., we isolated carnosic acid, carnosol, epirosmanol, rosmanol, 12-methoxy-carnosic acid, sageone, and carnosaldehyde using polyamide column, centrifugal partition chromatography, and semi-preparative high performance liquid chromatography. Isolated compounds were screened in-vitro for their ability to active the Nrf2 and general cellular toxicity using mouse primary cortical cultures. All compounds except 12-methoxy-carnosic acid were able to activate the antioxidant response element. Furthermore both carnosol and carnoasldehyde were able to induce Nrf2-dependent gene expression as well as protect mouse primary cortical neuronal cultures from H2O2 induced cell death.
Keywords: Nuclear factor E2-related factor 2, antioxidant response element, sage, neuroprotection, phenolic diterpenes, carnosic acid, carnosol
1. Introduction
Salvia officinalis L. and Rosmarinus officinalis L. are plants well known to produce the phenolic diterpenes carnosic acid (1) and carnosol (2).1 Compounds 1 and 2 are strong antioxidants possessing anti-microbial, anti-cancer, anti-inflammatory, and lipid lowering properties.2,3 Recently a number of investigations have demonstrated that these compounds also exhibit biological activity which may be useful against neurodegenerative diseases. Protective effects of 2 on dopaminergic neuronal cell lines against rotenone induced toxicity and sodium nitroprusside toxicity in glial cells have been reported.4,5 Carnosic acid protects against glutamate toxicity in primary rat cortical cultures and against cerebral ischemia in mice by activating the nuclear factor E2-related factor 2 (Nrf2) / Kelch-like ECH-associated protein 1 (Keap1) pathway.6
Nrf2 is a transcription factor known to induce a cis-acting regulatory element called the antioxidant response element (ARE) located in the promoter region of genes that encode various cytoprotective and antioxidant enzymes. Nrf2 localization and degradation is regulated by its cytoplasmic repressor protein Keap1. Under conditions of oxidative stress or disruption by small molecules Nrf2 is freed from Keap1 and enters the nucleus activating the ARE.7 Since many neurodegenerative diseases may be caused or exacerbated by oxidative stress, activation of Nrf2 regulated genes is a potential therapeutic strategy.8,9
Little information is available on the structure activity relationship of phenolic diterpenes for Nrf2/Keap1 activation. Comparisons have been made between 1, 2, and a series of alkyl-ester derivatives at carbon 11 and 12 of 1 in HT22 cell lines.10,11 Therefore in order to gain further insights into the structure activity of phenolic diterpenes for Nrf2/ARE activation we isolated 1, 2, epirosmanol (3), rosmanol (4), 12-methoxy-carnosic acid (5), sageone (6), and carnosaldehyde (7) from S. officinalis (Fig 1). Compounds were screened for Nrf2/ARE activation using primary mouse cortical neuronal cultures derived from the ARE-hPAP (human placental alkaline phosphatase) transgenic reporter mice.15 Potential toxicity of the isolated compounds was tested against the cultures using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl) 2H tetrazoluim inner salt (MTS) assay. Finally, changes in Nrf2-dependent gene expression and neuroprotection was evaluated for the most potent compounds.
Figure 1.
Chemical structures of 1-7
2. Results and Discussion
2.1. Isolation
Phenolic diterpenes 1-7 were isolated from an acetone extract of S. officinalis by first partitioning hexane soluble material over polyamide, followed by centrifugal partition chromatography, and finally with reverse phased semi-preparative HPLC. Isolated compounds were identified by comparing 1H-NMR with literature, confirming with 2D-COSY, and LC-MS.12-14 Compound 7 is reported in S. officinalis for the first time. All compounds were > 95% pure, except 7 (91%) according to HPLC at 230 nm. Detailed structural data is available online as supplementary information.
2.2. Bioassay hPAP and MTS
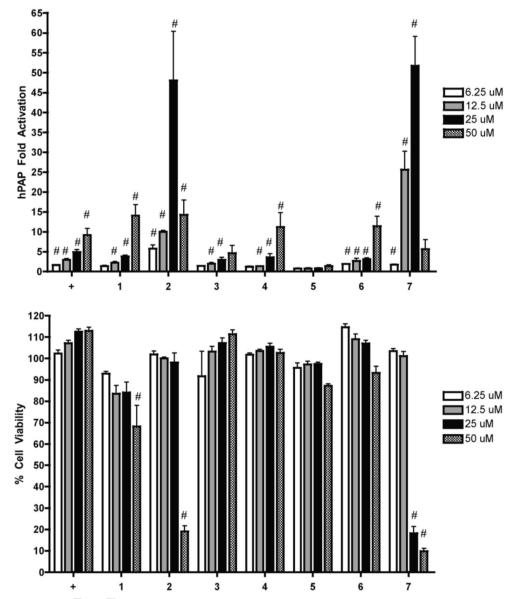
The ARE-hPAP transgenic reporter mice were used for primary cortical neuronal cultures and have been previously validated as a model system for studying activation of the Nrf2/ARE pathway.15 The hPAP reporter is driven by a 51 basepair segment of the rat NQO1 promoter sequence containing the ARE.15 The positive control tert-butylhydroquinone (tBHQ) has previously been demonstrated to show Nrf2/ARE dependent neuroprotective effects in such cultures.16 Results of hPAP assays are expressed as the fold increase in hPAP activity over basal levels (Fig 2). All compounds except 5 were able to dose dependently increase hPAP activity. Previous studies suggested that the mechanism of action of 1 to activate the Nrf2/ARE pathway was due to the catechol moiety initially acting as an antioxidant against reactive oxygen species. Upon oxidation 1 converts into an ortho quinone whose C-14 position can act as an electrophile, reacting with biological thiols such as the cysteine residues of Keap1 thus activating Nrf2.6 Another study demonstrated that ester derivatives at both the C-11 and C-12 eliminated activity of 1.10 In our experiments a methoxy group at position 12 as in 5 eliminated activity in the hPAP assay, confirming the importance of the catechol moiety for Nrf2/ARE pathway activation.
Figure 2.
Nrf2 activation and cytotoxicity of compounds tested.
Primary cortical cultures were prepared and treated with vehicle or compounds at increasing concentrations for 48 hours. A) hPAP activity was measured. All values are standardized to the vehicle treated value and presented as fold change. Mean ± SEM, n = 5, #p < 0.05 vs. vehicle treated cells. B) Cell viability was assessed using the soluble MTS assay. All values are standardized to the vehicle treated value and presented as percent of Mean ± SEM, n = 5, #p < 0.05 vs. vehicle treated cells. + = tBHQ.
Compounds 2 and 7 were the most potent activators in the hPAP assay reaching approximately 50 fold activation at 25 μM (Fig 2). However both compounds displayed a sharp reduction in hPAP activity at 50 μM. This reduction is explained by the significant toxicity observed in the MTS assay at 50 μM for 2 and at 25 μM and 50 μM for 7 (Fig 2). Compounds 1, 4, and 6 had a similar pattern of hPAP activity as the positive control tBHQ with only 1 displaying significant toxicity in the MTS assay at 50 μM (Fig 2). Compound 3 was weaker than 4 in the hPAP assay suggesting that the stereochemistry of the hydroxyl group at C-7 is important is important for the activity of these two compounds.
2.3. Quantitative PCR of cytoprotective and antioxidant genes
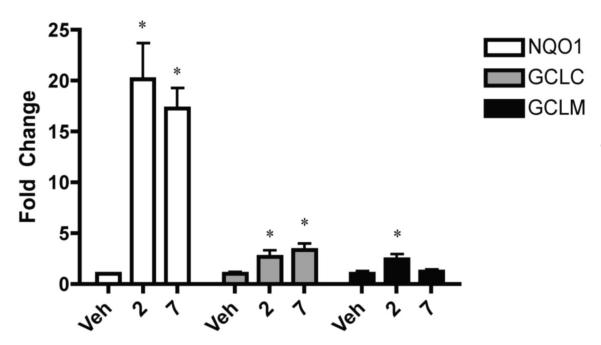
In order to confirm that compounds 2 and 7 can induce ARE mediated genes in the mouse primary cortical neuronal cultures, quantitative PCR (qPCR) was performed. Expression of genes encoding NAD(P)H:quinone oxidoreductase 1 (NQO1), glutamate cysteine ligase catalytic (GCLC) and regulatory (GCLM) subunits were chosen due to previous results demonstrating ARE mediated expression of these genes in mouse primary cortical neuronal cultures.15 Cultures were exposed to 2 at a concentration of 20 μM and 7 at a concentration of 10 μM for 24 hours after which qPCR was performed. The doses were chosen because both compounds displayed significant hPAP activation and no significant toxicity in the MTS assay at these levels. Compound 2 induced significant expression of NQO1, GCLC, and GCLM while compound 7 induced significant expression of NQO1 and GCLC (Fig 3).
Figure 3.
Increased expression of Nrf2-dependent genes.
Primary cortical cultures were prepared and treated with 20 μM of compound 2, 10 μM of compound 7, or vehicle for 24 hours. Cells were harvested and total RNA was used for quantitative PCR. All data was standardized to GAPDH and are presented as fold change relative to the vehicle. Mean ± SEM, n = 4 (Veh), n=8 (compound 2), and n=8 (compound 7) *p < 0.05 vs. vehicle treated cells.
2.4. Protection of primary cortical cultures against oxidative stress
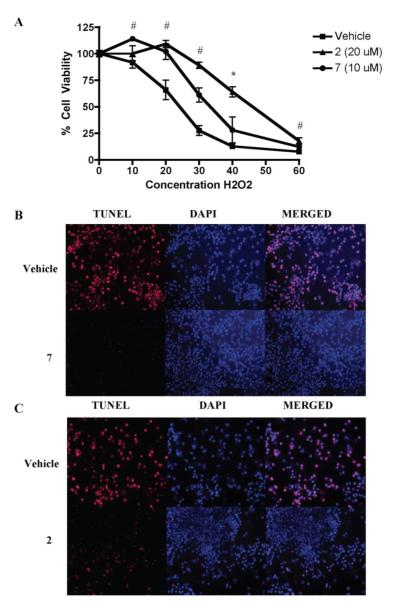
In order to confirm that compounds 2 and 7 were able to confer Nrf2 mediated neuroprotection against oxidative stress, primary cortical neuronal cultures were first treated with either compound at the same doses used in qPCR experiments for 48 hours. Cells were then exposed to H2O2 at various concentrations and after 24 hours cell viability was measured with MTS assay. Both 2 and 7 were able to protect cells against H2O2 induced toxicity compared to vehicle, with 2 generally conferring more protection than 7 at the dose tested (Fig 4A). As another measure of cellular protection, TUNEL staining was used to measure fragmented DNA and DAPI staining to measure intact DNA of the primary cortical cultures. Again cells were exposed to 2 and 7 for 48 hours at the same doses used in qPCR experiments and then exposed to 35 μM H2O2 for 24 hours. Both compounds were able to protect cells from H2O2 mediated oxidative stress induced cell death (Fig 3B and C).
Figure 4.
Nrf2 activation by compound treatment protects cortical neuronal cultures from oxidative stress-induced cell death.
A) Cultures were treated with 20 μM of compound 2, 10 μM of compound 7, or vehicle for 48 hours and then treated with increasing concentrations of H2O2 (μM) for an additional 24 hours. Cell viability was assessed using the soluble MTS assay. Mean ± SEM, (n = 4 for compounds, n = 8 for vehicle), #p < 0.05 vs. vehicle treated cells for both compounds, *p < 0.05 vs. vehicle treated cells for 2 only. B,C) TUNEL staining of cells displaying fragmented DNA (red) overlaid with DAPI (blue) was used to further demonstrate the protection abilities of the compounds from oxidative stress-induced cell death. Cells were treated with B) 10 μM of compound 7, C) 20 μM of compound 2, or vehicle for 48 hours and then treated with 35 μM H2O2 for an additional 24 hours (B, C).
2.5. Conclusions
In conclusion, phenolic diterpenes isolated from S. officinalis can activate the Nrf2/ARE pathway in mouse primary cortical cultures with varied potency. The catechol moiety is essential for this activity. The most potent Nrf2/ARE pathway activators 2 and 7 display neuroprotective activity in mouse primary cortical neuronal cultures against H2O2 induced cell death. Due to lower toxicity of 2 compared to 7 against the primary cortical cultures suggests that 2 is a promising compound to study as an agent which may be useful in treating neurodegenerative disease.
3. Experimental
3.1. Plant material
Dried aerial parts of Salvia officinalis were purchased from De Groene Luifel BV, Sluis, Netherlands. A voucher specimen was deposited in the economic botany collection of the National Herbarium Nederland in Leiden under the following barcode: LamiaceaeSalviaofficinalisL.L 0991403J. FischedickNo. 192010.
3.2. Extraction
Plant material (100 g) was extracted with 2 L acetone for 2 hours to yield 9 g crude extract. The extract was extracted 2 times with 100 mL warm hexane and hexane solution added to polyamide column (100 g). The column was eluted with additional 300 mL of hexane to give 2 g hexane fraction and further eluted with 650 mL MeOH to give 2 g MeOH fraction.
3.3. CPC
The MeOH and hexane fractions were further purified using a Fast Centrifugal Partition Chromatograph with a 1 L internal volume rotor (Kromaton Technologies, Angers, France). The MeOH fraction was separated by using a two phase solvent system composed of 5 L heptane: acetone: H2O (3: 5: 2). The MeOH fraction was dissolved in 30 mL 1:1 mixture of upper and lower layers of the solvent system. The CPC system was filled with the lower layer and the phase held in place with a rotor speed of 1000 rpm while the upper layer was pumped into the CPC at a flow rate of 10 mL/min in ascending mode. Equilibrium between the layers was reached when the upper layer began to elute from the CPC (lower layer displaced = 230 mL), after which the sample was immediately injected. After 250 mL of the mobile phase eluted 120 fractions (10 mL each) were collected. The system was then rinsed with 1 L of the remaining lower layer and the eluent collected as an additional rinse fraction. Pressure was stable between 63-65 bar throughout the run. The hexane fraction was separated using a 2 phase solvent system composed of 2.5: 6: 1.5 heptane: acetone: H2O. All other CPC conditions were the same as for the MeOH fraction except the volume of the lower layer displaced during equilibration was 260 mL, the pressure 48 bar, and 80 (10 mL) fractions were collected. Fractions were analyzed by silica gel TLC (hexane: ethyl acetate: acetic acid, 7: 3: 0.1, vanillin/sulfuric acid reagent) and combined based on similarity of profile. Combined fractions from CPC experiment on MeOH fraction are referred to as CPC-M Fr# and fractions from CPC on hexane fraction as CPC-H Fr#.
3.4. Semi-preparative HPLC
Final purification was performed on a Shimadzu semi-preparative HPLC. A Luna C18 (2) 100 A 5 micron 250 × 10 mm column was used for separations (Phenomenex, Torrance, California, USA). H2O (A) and MeOH (B) were used as mobile phase. Flow rates were 5 mL/min, 10 mL fractions were collected, and UV detector set to 230 and 280 nm. Samples were injected manually using a Rheodyne injector equipped with a 5 mL injection loop.
CPC-M Fr40-75 (660 mg) was dissolved in 10 mL 70% aqueous MeOH and run twice using gradient from 70% B to 85% B in 60 min. Fractions 5, 8-15, and 18-20 were separately combined and MeOH removed under reduced pressure at 40 °C. Combined fractions were then dissolved in 100 mL H2O and 150 mL EtOAc. The EtOAc layer was collected and solvent removed to yield 12.6 mg 2 from fraction 5, 506.7 mg 1 from fractions 8-15, and 31.4 mg 5 from fractions 18-20. CPC-M rinse fraction (242 mg) was purified using gradient from 50% B to 100% B in 120 min. Solvent was removed from fractions 7, 9, and 24-25 to yield 2.1 mg 3, 2.6 mg 4, and 20.3 mg 2 respectively. CPC-H Fr26-40 (164 mg) was dissolved in 5 mL 70% aqueous MeOH and run with gradient from 70% B to 100% B in 120 min. Fractions 9-10 yielded 11.2 mg 6 and fractions 17-18 yielded 5.8 mg 7. CPC-H Fr41-80 (50 mg) was purified under the same conditions as Fr26-40 to yield an additional 1.4 mg 6.
3.5. Structure elucidation
The structure of isolated compounds was determined by 1H-NMR and 2D-COSY on a Bruker DMX 500 MHz NMR (Karlsruhe, Germany). An Agilent single quadropole mass spectrometer equipped with an atmospheric pressure chemical ionization probe (APCI) was used for mass confirmation. A 150 × 4.6 mm Luna 5 micron C18 (2) 100A column was used for separation (Phenomenex Inc). Solvent system was composed of MeOH and H2O plus 0.1% formic acid. The flow rate was 0.5 mL/min. Gradient of 70% MeOH to 100% MeOH in 20 min with 10 min hold at 100% MeOH was used. Mass spectra were acquired in both positive and negative mode with a mass range of 50-500 amu. APCI spray chamber gas temperature was set to 350 °C, a vaporizer temperature of 325 °C, a drying gas flow rate of 5 L/min, and a nebulizer pressure of 40 psig. The VCap was set to 4000 V for positive scans and negative scans while the corona current was 5 μA for positive scans and 15 μA for negative scans.
3.6. Cell cultures
Primary cortical neuronal cultures were derived from ARE-hPAP reporter mice as previously described.15,16 In brief cortices from E15 mouse pups were pooled in 10 mL ice-cold Ca2+ and Mg2+ free HBSS (Life Technologies, Carlsbad, California, USA). Tissue was then minced, centrifuged, and digested in 0.05% trypsin containing no EDTA in HBSS for 15 min at 37 °C. Cells were rinsed 3 times with HBSS following digestion. Then cells were washed with CEMEM (minimum essential media with Earle’s salts); (Life Technologies) 0.5 mM glutamine, 1% penicillin/streptomycin, 10% heat inactivated fetal bovine serum, and 10% horse serum (Atlanta Biologicals, Inc., Lawrenceville, Georgia, USA). Cells were triturated to a single-cell suspension and strained through a 70 μM cell strainer (BD Biosciences, San Jose, California, USA). Cells were counted, assayed for viability using trypan blue, and plated on poly-D-lysine coated plates at a density of 3 × 105 cell/cm2. Cells were kept in CEMEM for 45 min, after which medium was changed with CEMEM. After two days, medium was changed from CEMEM to NBM (Neurobasal media; Life Technologies), supplemented with B27 with antioxidants, and 0.5 mM glutamine. These mixed cultures (~ 40% astrocytes and 60% neurons) were incubated at 37 °C in a tri-gas incubator with 5% O2, 5% CO2, and 90% N2.
3.7. Bioassay hPAP and MTS
After 6 days in culture, compounds were dissolved in 100% DMSO and administered to cells for 48 hours (final concentration of 0.1% DMSO). After 48 hours, Nrf2 activation was determined by measuring for hPAP activity as previously described.16 In brief, cells were lysed in TMNC lysis buffer (50 mM Tris, 5 mM MgCl2, 100 mM NaCl, 1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS)) and freeze-thawed at −20 °C. To inactivate endogenous alkaline phosphatase activity extracts were incubated with 200 mM diethanolamine (DEA) buffer at 65 °C. The hPAP activity was quantified in 200 mM DEA with 0.8 mM CSPD ((disodium 3-(4-methoxyspiro (1,2-dioxetane-3,2′-(5′-chloro)tricycle(3.3.1.1 3,7)decan)-4-yl)phenyl phosphate) (Life technologies), 2× Emerald, and 5 mM MgCl2). Using one-second integration luminescence was measured on a Berthold Orion microplate luminometer (Berthold Technologies GmbH & Co., Bad Wildbad, Germany). Baseline signals from hPAP negative control culture samples were subtracted from all values. Cell viability was assayed using the MTS assay following the manufacturer’s suggested protocol (Promega, Madison, Wisconsin, USA).
3.8. Quantitative PCR
Total RNA was isolated using Trizol reagent (Invitrogen Corporation). RNA quality was assessed with the 2100 Bioanalyzer (Agilent Technologies, Foster City, CA, USA) and 1 μg of RNA (RIN number ≥ 9.0) was reverse-transcribed with oligo (dT)15 primers using Reverse Transcription System (Promega Corporation, Madison, WI, USA) according to the manufacturer’s protocol. PCR was performed in a 20-μl reaction with 1 × Light-Cycler480 SYBR Green I Master in a LightCycler480 Real-time PCR System (Roche Applied Science, Indianapolis, IN, USA). Specific primers were:
NQO1 forward: 5′-GCGAGAAGAGCCCTGATTGTACTG-3′
NQO1 reverse: 5′-TCTCAAACCAGCCTTTCAGAATGG-3′
GCLM forward: 5′-TGCTCTTCACGATGACCGAGTACC-3′
GCLM reverse: 5′-GCCACCAGATTTGACTGCCTTTG-3′
GCLC forward: 5′-CTCAAGAACATCGCCTCCATTCAG-3′
GCLC reverse: 5′-ACATCTACCACGCAGTCAAGGACC-3′
GAPDH forward: 5′-TGGCAAAGTGGAGATTGTTGCC-3′
GAPDH reverse: 5′-AAGATGGTGATGGGCTTCCCG-3′
3.9. TUNEL staining
Terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) staining was performed using the Cell Death Detection kit (Roche Applied Science) according to the manufacturer’s protocol. Incubation of slides in 4′6-diamidino-2-phenylindole (DAPI) was used to stain nuclei. Images were taken using a Zeiss (Oberkochen, Germany) photomicroscope.
3.10. Data analysis
All hPAP and MTS data are represented as mean ± SEM (n number indicated in figure legend). Statistical analysis was performed using one-way ANOVA followed by Newman-Keuls multiple comparison (GraphPad Prism, version 4). A p < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
We would like to thank the European Union Seventh Framework Program for funding the Terpmed project, grant number 227448. This work was also partially funded by R01ES08089 and R01ES10042 from the National Institute of Environmental Health Sciences (JAJ).
Footnotes
Conflicts of interest Justin Fischedick was employed as a junior researcher at PRISNA BV at the time of this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abreu ME, Müller M, Alegre L, Munné-Bosch S. J. Sci. Food Agric. 2008;88:2648–2653. [Google Scholar]
- 2.Bonito MC, Cicala C, Marcotullio MC, Maione F, Mascolo N. Nat Prod Commun. 2011;6:1205–1215. [PubMed] [Google Scholar]
- 3.Johnson JJ. Cancer Lett. 2011;305:1–7. doi: 10.1016/j.canlet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim S-J, Kim J-S, Cho H-S, Lee HJ, Kim SY, Kim S, Lee S-Y, Chun HS. Neuroreport. 2006;17:1729–1733. doi: 10.1097/01.wnr.0000239951.14954.10. [DOI] [PubMed] [Google Scholar]
- 5.Kim SY, Park E, Park JA, Choi B-S, Kim S, Jeong G, Kim C-S, Kim DK, Kim S-J, Chun HS. J. Agric. Food Chem. 2010;58:1543–1550. doi: 10.1021/jf903294x. [DOI] [PubMed] [Google Scholar]
- 6.Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, Kitajima C, Cui J, Kamins J, Okamoto S, Izumi M, Shirasawa T, Lipton SA. J. Neurochem. 2008;104:1116–1131. doi: 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itoh K, Tong KI, Yamamoto M. Free Radic. Biol. Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 8.de Vries HE, Witte M, Hondius D, Rozemuller AJM, Drukarch B, Hoozemans J, van Horssen J. Free Radic. Biol. Med. 2008;45:1375–1383. doi: 10.1016/j.freeradbiomed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Calkins MJ, Johnson DA, Townsend JA, Vargas MR, Dowell JA, Williamson TP, Kraft AD, Lee J-M, Li J, Johnson JA. Antioxid Redox Signal. 2009;11:497–508. doi: 10.1089/ars.2008.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh T, Izumi M, Inukai Y, Tsutsumi Y, Nakayama N, Kosaka K, Shimojo Y, Kitajima C, Itoh K, Yokoi T, Shirasawa T. Neurosci. Lett. 2008;434:260–265. doi: 10.1016/j.neulet.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 11.Tamaki Y, Tabuchi T, Takahashi T, Kosaka K, Satoh T. Planta Med. 2010;76:683–688. doi: 10.1055/s-0029-1240622. [DOI] [PubMed] [Google Scholar]
- 12.Pukalskas A, van Beek TA, de Waard P. J Chromatogr A. 2005;1074:81–88. doi: 10.1016/j.chroma.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 13.Cuvelier ME, Berset C, Richard H. J. Agric. Food Chem. 1994;42:665–669. [Google Scholar]
- 14.Tada M, Okuno K, Chiba K, Ohnishi E, Yoshii T. Phytochemistry. 1994;35:539–541. [Google Scholar]
- 15.Johnson DA, Andrews GK, Xu W, Johnson JA. J. Neurochem. 2002;81:1233–1241. doi: 10.1046/j.1471-4159.2002.00913.x. [DOI] [PubMed] [Google Scholar]
- 16.Kraft AD, Johnson DA, Johnson JA. J. Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.