Abstract
While the benefits of continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP) for patients with obstructive sleep apnea are well described, reports in the literature of complications from its use are rare. A patient who received postoperative BiPAP after undergoing transsphenoidal craniopharyngioma resection developed severe pneumocephalus and unplanned intensive care unit admission. Although the pneumocephalus resolved with conservative management over two weeks, we propose caution in the use of CPAP postoperatively in patients undergoing procedures of the head and neck.
Keywords: Bilevel positive airway pressure, Head and neck surgery, Pneumocephalus, Transsphenoidal craniopharyngioma surgery
1. Introduction
Continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP) are beneficial in the postoperative management of patients with obstructive sleep apnea (OSA). A patient who developed pneumocephalus after BiPAP following transsphenoidal resection of a craniopharyngioma is presented.
2. Case report
A 51 year old, ASA physical status 3, nonsmoking man with a history of sellar and suprasellar craniopharyngioma, presented to our institution for evaluation of bitemporal hemianopsia caused by tumor recurrence. The primary tumor had been resected 7 years earlier. In the intervening time, his body mass index (BMI) increased from 34.8 to 50.1 kg/m2, and he developed OSA syndrome. His preoperative BiPAP settings were 20/16 cm H20 based on a titration polysomnogram showing a combined sleep disturbance index of 3.6 events of central apnea or hypopnea per hour with BiPAP, with the lowest oxygen saturation recorded as 84%.
The patient was scheduled for an endoscopic endonasal posterior septectomy and bilateral sphenoidotomy as an approach for mass resection with abdominal fat graft closure. Based on obesity, history of OSA, and endocrine abnormalities, the patient was deemed to be at increased risk for perioperative complications at his perioperative anesthesia clinic visit. In line with the current ASA practice guidelines [1], the patient was instructed to bring his home nasal BiPAP machine to the hospital for use postoperatively (Table 1).
Table 1.
Timeline of pertinent events
| POD 0 | Revision endoscopic pituitary tumor excision |
| POD 1 | Postoperative MRI (Fig. 1 A, B) |
| POD 3 | BiPAP applied over night |
| POD 4 | Respiratory failure, confusion, and transfer to ICU with intubation for sepsis workup and CT scan of the head (Fig. 1C, D) |
| POD 6 | Transfer to the neurosurgery floor |
| POD 8 | Lumbar drain placement, BiPAP administration over night |
| POD 9 | Acute mental status changes, repeat head CT scan and plain radiography films (Fig. 1 E, F, G, H), admission to the ICU |
| POD 48 | Discharge to home |
POD=postoperative day, MRI=magnetic resonance imaging, BiPAP=bilevel positive airway pressure, ICU=intensive care unit, CT=computed tomography.
At the time of surgery, the anesthesia record reflected a Cormack-Lehane Grade II laryngeal view with uneventful intubation using a GlideScope. There were no immediate postoperative complications or desaturations. Although he was neurologically intact, from postoperative day (POD) 1 to 3 the patient suffered repeated episodes of fever, dyspnea, and tachypnea. Initial workup ruled out several possible causes, including pulmonary embolism. On POD 4, morning rounds showed that the patient had received nasal BiPAP overnight. The nurse on duty had inquired of the patient’s family whether he routinely used BiPAP and asked them to bring the machine from the car into the hospital room. The patient subsequently developed acute respiratory failure and confusion. With particular concern for meningitis and presumed sepsis, he was re-intubated and transferred to the intensive care unit (ICU). An urgent computed tomographic (CT) scan of the brain showed an interval decrease in postoperative pneumocephalus (Fig. 1 C, D) as compared with the routine postoperative magnetic resonance imaging (MRI; Fig. 1 A, B); analysis of cerebrospinal fluid (CSF) obtained via lumbar puncture was within normal limits. With reassuring imaging and no other source of infection identified, the patient was weaned from ventilatory support, extubated, and discharged from the ICU to the neurosurgery floor. After this event, the nurses were instructed verbally and in the chart not to apply the patient’s BiPAP machine.
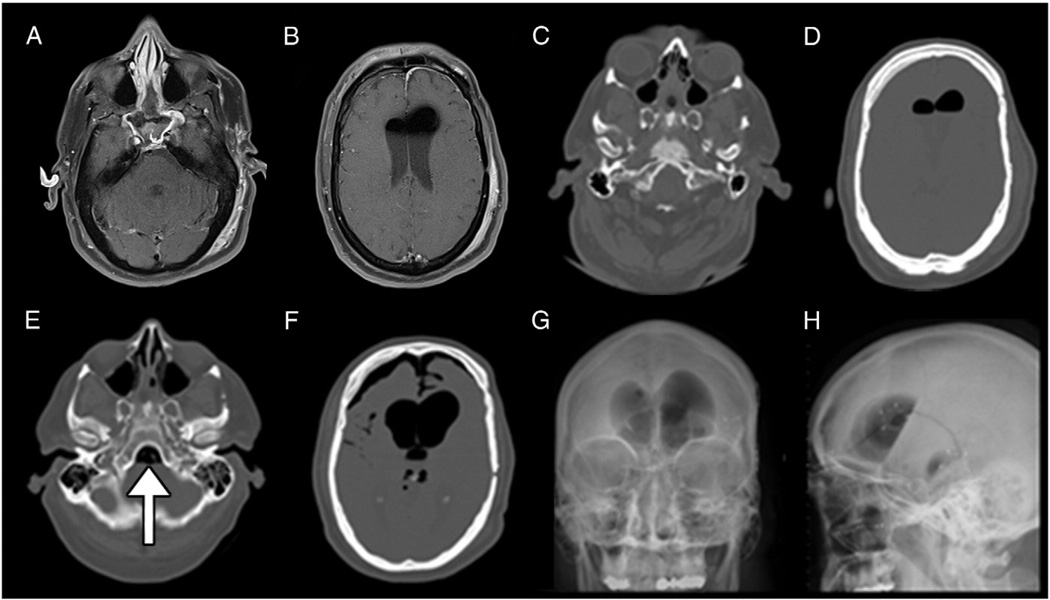
Fig. 1.
Postoperative pneumocephalus after bilevel positive airway pressure (BiPAP) use. A. and B. Axial cuts of T1 magnetic resonance imaging (MRI) of the head on postoperative (POD)1 showing normal postoperative pneumocephalus and fluid collection in the lateral ventricles. C. and D. Axial cuts of a computed tomographic (CT) scan of the head on POD 4 showing an interval decrease in pneumocephalus after receiving BiPAP, compared with postoperative MRI. E. and F. Axial cuts of a CT scan of the head after receiving BiPAP again on POD 9 with placement of a lumbar drain earlier the same day, with expanded pneumocephalus at the skull base (arrow in E.) and in the lateral ventricles and anteriorly in F. G. Plain anteroposterior and H. lateral views of radiographic films of the head showing a large amount of pneumocephalus with a large air-fluid interface.
On POD 8, the patient had CSF rhinorrhea and a lumbar drain was placed for decompression. Later that same evening (after a witnessed apneic event), he again received nasal BiPAP for three hours. With concomitant worsening of mental status, an urgent CT scan of the brain showed a large increase in intraventricular pneumocephalus (Fig. 1 E, F). After this complication was identified, the patient was managed with close observation, avoidance of BiPAP, emphasis on ambulation and pulmonary toilet, and definitive removal of the BiPAP machine from the patient’s room. Serial imaging showed slow resolution of the pneumocephalus over a period of two weeks.
The patient’s patent skull base defect and persistent CSF rhinorrhea, however, necessitated placement of a ventricular drain, repeat endonasal surgery for fat graft closure of the CSF leak site prior to discharge, and ultimately a ventriculoperitoneal shunt. At discharge, the patient was counseled to avoid bearing down, heavy lifting, or using BiPAP until agreed upon by both the attending neurosurgeon and otolaryngologist. To date, the patient continues to struggle with obesity and memory problems, but is disease-free one year from discharge. He has resumed his use of BiPAP during sleep without complications.
In multidisciplinary review of this case (Table 1), a systemic problem was identified in the recommendation, made at the time of his pre-anesthesia clinic visit, that the patient bring his BiPAP to the hospital after surgery. Our anesthesia preoperative evaluation clinic is in the process of instituting an automatic warning in the electronic health record system for patients with CPAP or BiPAP who are scheduled to undergo surgery of the head and/or neck in order to facilitate closer evaluation and coordination of postoperative care. In addition, otolaryngology clinic nurses now instruct preoperative patients to avoid the use of CPAP or BiPAP after surgery until cleared by the operative surgeon.
3. Discussion
The prevalence of OSA in patients undergoing surgery is estimated to be from 3.2% in noncardiac general surgery cases [2] to 70% in bariatric surgery patients [3], and is likely to increase. The surgical patient with confirmed or suspected OSA requires a number of specialized services from anesthesiologists, including thorough preoperative evaluation, intubation and extubation protocols, and careful postoperative monitoring [1,4]. Retrospective series have found that patients with OSA are roughly twice as likely to experience a postoperative complication as those without OSA [5,6]. The most frequent complication is oxygen desaturation to less than 90% secondary to apnea or hypopnea [7]. In a case-controlled series of orthopedic surgery patients with untreated OSA, the risk of serious complications requiring unplanned ICU admission after surgery was increased by 15% and all complications increased by 21% [5]. The danger of hypoxemia increases early in the postoperative recovery period when soft-tissue obstruction may be exacerbated by atelectasis, reduced pulmonary volumes, and decreased respiratory drive. This danger extends as late as POD 4 to 5 when a rebound in rapid-eye movement (REM) sleep typically occurs, leading to increased frequency of apnea-hypopnea events during sleep.
The 2006 ASA guidelines state that OSA patients who use CPAP prior to surgery or who suffer frequent apneic events should have CPAP applied as soon as is feasible after surgery unless contraindicated by the surgical procedure [1,4]. To increase patient compliance and decrease associated costs, these guidelines also suggest that patients bring their personal machines at the time of surgery.
We propose a clarification of the OSA perioperative management algorithm as described by Bolden et al [4]. Specifically, postoperative CPAP and/or BiPAP use may be contraindicated in cases where positive airway pressure may result in breakdown of the operative repair or pathological introduction of air into potential spaces. Such cases might include surgery of the paranasal sinuses, skull base, pituitary fossa, middle ear, retropharynx and reconstruction of large hard and/or soft tissue defects of the head and neck with or without free-tissue transfer.
To diminish the risk of postoperative hypoxemia and associated complications until CPAP may be safely reapplied, some temporizing measures include close postoperative electronic monitoring with continuous pulse oximetry, minimizing the use of opioids or other sedating medications, exploring alternative forms of analgesia (eg, nerve blocks) if possible, encouraging early ambulation, aggressive pulmonary toilet, elevating the head of the bed, using supplemental oxygen, and using mandibular prostheses or nasal airways if necessary and available and their use is not contraindicated [8]. By using such measures, CPAP-dependent patients who avoided postoperative CPAP use had statistically equivalent rates of pulmonary complications to those of a non-OSA cohort after undergoing gastric bypass [9]. Of course, individual patient characteristics and the specific surgical procedure performed may render some or all of these measures ineffective or not applicable. For this reason, continuous postoperative visual monitoring in a step-down unit with an appropriately low nurse-patient ratio is particularly important, and has been recommended for all patients with OSA2. In such settings, apneic episodes are noted and rapidly aborted with stimulation.
Previous reports of similar complications from oronasal CPAP, as experienced by this patient, are rare. There is one report of air regurgitation into the eye after dacryocystorhinostomy [10] and another report of pneumocephalus in a patient with a spontaneous CSF leak [11]. Iatrogenic pneumocephalus and/or subcutaneous emphysema after head trauma has been described in a number of case reports secondary to CPAP or even bag mask ventilation [12–15]. Sequelae in these cases vary in severity from complete resolution to persistent neurological deficits. In our case, it is unknown if the use of BiPAP contributed to the development and/or persistence of the CSF leak, hydrocephalus, or the subsequent memory deficits. Depending on the method of closure, the rate of postoperative CSF leak after transsphenoidal neurosurgery has been reported to be as low as 2.5% [16]. One recent study reported a postoperative CSF leak rate of 5% after closure of a sphenoid defect specifically with a fat graft [17]. Animal models have shown that integration of fascial dural repair may take place as soon as one week postoperatively, with a durable fibrous ingrowth as soon as two weeks [18]. This healing process, however, depends greatly on the size of the skull base defect, the nature of the material used for repair, and history of radiation treatment [19].
Currently, based on limited evidence from animal models, we empirically recommend waiting 6 weeks after dural repair for reinitiation of CPAP or BiPAP at our institution. This timeline should be tailored to the patient, carefully weighing the risk of pneumocephalus against the risk of apnea and hypoxia. Patient-specific factors to take into account include the severity of OSA, size of the skull base defect, presence of nasal packing, and type of reconstruction. Further investigation is warranted to evaluate the optimal timeline for resumption of CPAP or BiPAP so that pneumocephalus is avoided without compromising the pulmonary and cardiovascular status of postoperative OSA patients.
Footnotes
This case report should be attributed to the Department of Otolaryngology- Head and Neck Surgery of the University of Iowa, Iowa City, IA.
No departmental or institutional funding was provided for this case report.
University of Iowa Institutional Review Board approval #201008739 was obtained to review this patient’s chart and for publication.
Benumof J. Creation of observational unit may decrease sleep apnea risk. Anesthesia Patient Safety Foundation Newsletter. Park Ridge (IL): American Society of Anesthesiologists; 2002;17:39.
References
- 1.Gross JB, Bachenberg KL, Benumof JL, et al. American Society of Anesthesiologists. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–1093. doi: 10.1097/00000542-200605000-00026. [DOI] [PubMed] [Google Scholar]
- 2.Fidan H, Fidan F, Unlu M, Ela Y, Ibis A, Tetik L. Prevalence of sleep apnoea in patients undergoing operation. Sleep Breath. 2006;10:161–165. doi: 10.1007/s11325-006-0067-9. [DOI] [PubMed] [Google Scholar]
- 3.Lopez PP, Stefan B, Schulman CI, Byers PM. Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation: more evidence for routine screening for obstructive sleep apnea before weight loss surgery. Am Surg. 2008;74:834–838. [PubMed] [Google Scholar]
- 4.Bolden N, Smith CE, Auckley D. Avoiding adverse outcomes in patients with obstructive sleep apnea (OSA): development and implementation of a perioperative OSA protocol. J Clin Anesth. 2009;21:286–293. doi: 10.1016/j.jclinane.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. 2001;76:897–905. doi: 10.4065/76.9.897. [DOI] [PubMed] [Google Scholar]
- 6.Chung SA, Yuan H, Chung F. A systemic review of obstructive sleep apnea and its implications for anesthesiologists. Anesth Analg. 2008;107:1543–1563. doi: 10.1213/ane.0b013e318187c83a. [DOI] [PubMed] [Google Scholar]
- 7.Liao P, Yegneswaran B, Vairavanathan S, Zilberman P, Chung F. Postoperative complications in patients with obstructive sleep apnea: a retrospective matched cohort study. Can J Anaesth. 2009;56:819–828. doi: 10.1007/s12630-009-9190-y. [DOI] [PubMed] [Google Scholar]
- 8.Schnoor J, Ilgner J, Hein M, Westhofen M, Rossaint R. Perioperative management of patients with obstructive sleep apnoea. Anaesthesist. 2009;58:189–198. doi: 10.1007/s00101-009-1519-y. [DOI] [PubMed] [Google Scholar]
- 9.Jensen C, Tejirian T, Lewis C, Yadegar J, Dutson E, Mehran A. Postoperative CPAP and BiPAP use can be safely omitted after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2008;4:512–514. doi: 10.1016/j.soard.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Cannon PS, Madge SN, Selva D. Air regurgitation in patients on continuous positive airway pressure (CPAP) therapy following dacrocystorhinostomy with or without Lester-Jones tube insertion. Br J Ophthalmol. 2010;94:891–893. doi: 10.1136/bjo.2009.166082. [DOI] [PubMed] [Google Scholar]
- 11.Jarjour NN, Wilson P. Pneumocephalus associated with nasal continuous positive airway pressure in a patient with sleep apnea syndrome. Chest. 1989;96:1425–1426. doi: 10.1378/chest.96.6.1425. [DOI] [PubMed] [Google Scholar]
- 12.Klopfenstein CE, Forster A, Suter PM. Pneumocephalus. A complication of continuous positive airway pressure after trauma. Chest. 1980;78:656–657. doi: 10.1378/chest.78.4.656. [DOI] [PubMed] [Google Scholar]
- 13.Young AE, Nevin M. Tension pneumocephalus following mask CPAP. Intensive Care Med. 1994;20:83. doi: 10.1007/BF02425065. [DOI] [PubMed] [Google Scholar]
- 14.Kramer NR, Fine MD, McRae RG, Millman RP. Unusual complication of nasal CPAP: subcutaneous emphysema following facial trauma. Sleep. 1997;20:895–897. [PubMed] [Google Scholar]
- 15.Nicholson B, Dhindsa H. Traumatic tension pneumocephalus after blunt head trauma and positive pressure ventilation. Prehosp Emerg Care. 2010;14:499–504. doi: 10.3109/10903120903564522. [DOI] [PubMed] [Google Scholar]
- 16.Esposito F, Dusick JR, Fatemi N, Kelly DF. Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery. Neurosurgery. 2007;60(4) Suppl 2:295–303. doi: 10.1227/01.NEU.0000255354.64077.66. [DOI] [PubMed] [Google Scholar]
- 17.Rabadán AT, Hernández D, Ruggeri CS. Pituitary tumors: our experience in the prevention of postoperative cerebrospinal fluid leaks after transsphenoidal surgery. J Neurooncol. 2009;93:127–131. doi: 10.1007/s11060-009-9858-8. [DOI] [PubMed] [Google Scholar]
- 18.Tachibana E, Saito K, Fukuta K, Yoshida J. Evaluation of the healing process after dural reconstruction achieved using a free fascial graft. J Neurosurg. 2002;96:280–286. doi: 10.3171/jns.2002.96.2.0280. [DOI] [PubMed] [Google Scholar]
- 19.El-Banhawy OA, Halaka AN, Altuwaijri MA, Ayad H, El-Sharnoby MM. Long-term outcome of endonasal endoscopic skull base reconstruction with nasal turbinate graft. Skull Base. 2008;18:297–308. doi: 10.1055/s-0028-1086055. [DOI] [PMC free article] [PubMed] [Google Scholar]