Abstract
Calorie restriction (CR) induces enhanced insulin-stimulated glucose uptake in fast-twitch (type II) muscle from old rats, but the effect of CR on slow-twitch (type I) muscle from old rats is unknown. The purpose of this study was to assess insulin-stimulated glucose uptake and phosphorylation of key insulin signaling proteins in isolated epitrochlearis (fast-twitch) and soleus (slow-twitch) muscles from 24-month-old ad libitum fed and CR (consuming 65% of ad libitum, intake) rats. Muscles were incubated with and without 1.2 nM insulin. CR versus ad libitum rats had greater insulin-stimulated glucose uptake and Akt phosphorylation (pAkt) on T308 and S473 for both muscles incubated with insulin. GLUT4 protein abundance and phosphorylation of the insulin receptor (Y1162/1163) and AS160 (T642) were unaltered by CR in both muscles. These results implicate enhanced pAkt as a potential mechanism for the CR-induced increase in insulin-stimulated glucose uptake by the fast-twitch epitrochlearis and slow-twitch soleus of old rats.
Keywords: Glucose transport, Aging, Insulin resistance, Muscle fiber type, Insulin signaling
Calorie restriction (CR; ie, consuming ∼60%–75% of ad libitum, AL, intake) without malnutrition leads to improved function and health in many species (1,2). One of the hallmarks of CR is an increase in whole-body insulin sensitivity (3–6). Given that skeletal muscle accounts for the greatest amount of whole body glucose disposal (7), it is not surprising that CR leads to increased insulin-mediated glucose uptake by skeletal muscle (3,8,9).
Because skeletal muscle is a heterogeneous tissue that is composed of type I (slow-twitch) and type II (fast-twitch) fibers that differ on the basis of contractile and metabolic properties, it is valuable to assess muscles with differing fiber type compositions to gain a full understanding of CR effects. Previous research on adult rats or mice has reported that CR leads to greater insulin-stimulated glucose uptake (ISGU) in skeletal muscles composed primarily of type I (soleus) or type II (epitrochlearis or extensor digitorum longus) fibers (10–13). In isolated muscle, CR increases ISGU in the primarily type II epitrochlearis muscle of 23-month-old rats (14,15), but there have been no reports on insulin-stimulated uptake in a predominantly type I muscle of old rats.
A number of earlier studies have evaluated the underlying mechanisms for improved insulin sensitivity in skeletal muscle from adult rats. Most of the previous studies have reported little or no CR effect on the abundance of GLUT4 (13,14,16,17), the insulin-regulated glucose transporter protein. We have performed a series of studies that characterized the effects of CR on key insulin signaling proteins in skeletal muscle. Insulin-induced phosphorylation of Akt is essential for ISGU (18). In this context, it is significant that the most consistent and substantial increase in insulin signaling with CR in skeletal muscle has been an increase in insulin-mediated phosphorylation of Akt (11–13, 19–21). Insulin-stimulated Akt phosphorylation was increased by CR in both the soleus (composed of ∼90% type I fibers) and the epitrochlearis (∼90% type II fibers) in 9-month-old rats (13).
In view of the striking CR effect on Akt phosphorylation in adult animals, it is clearly relevant to identify the Akt substrate(s) that regulate ISGU. This link between Akt and glucose uptake was discovered by Sano and colleagues (22,23) who identified a protein that they called Akt substrate of 160 kDa (AS160; also known as TBC1D4). They found that AS160 was phosphorylated by Akt in response to insulin and that the phosphorylation of AS160 modulated ISGU. They also convincingly demonstrated that Thr642 of AS160 was the most important insulin-regulated Akt phosphosite for insulin-mediated glucose uptake (22). Sharma and colleagues (13) recently reported a muscle-specific effect of CR in 9-month-old rats: insulin-stimulated Thr642 phosphorylation of AS160 was increased by CR in the epitrochlearis but not in the soleus.
In contrast to the relatively extensive amount of research on the mechanisms for the CR-induced elevation in ISGU in muscles from adult animals, little is known about CR effects on insulin signaling in muscle from old rats. A few studies have evaluated the influence of CR on insulin receptor function in skeletal muscle of old rats (16,24,25), but these studies did not determine if there were fiber type differences. The effects of CR on Akt or AS160 phosphorylation in insulin-stimulated muscle from old rats, regardless of fiber type, have not been reported. Furthermore, the influence of CR on GLUT4 abundance in the epitrochlearis and soleus of old rats is unknown.
The goal of the current study was to begin to fill some of these gaps in knowledge with regard to CR effects on skeletal muscle insulin signaling, GLUT4, and glucose transport in old rats. A novel aspect of the experiment was to assess CR effects on ISGU by both predominantly type I (soleus) and predominantly type II (epitrochlearis) skeletal muscles of old rats. In addition, in the same muscles, we probed the influence of CR on the phosphorylation of key insulin signaling proteins, including the insulin receptor, Akt, and AS160. Finally, we determined if CR resulted in altered expression of GLUT4 protein in the soleus and epitrochlearis of old rats.
Experimental Procedures
Materials
Unless otherwise noted, all chemicals were purchased from Sigma Chemical (St Louis, MO) or Fisher Scientific (Hanover Park, IL). Human recombinant insulin was obtained from Eli Lilly (Indianapolis, IN). Reagents and apparatus for sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotting were from Bio-Rad Laboratories (Hercules, CA). Anti-phospho-insulin receptorTyr1162/1163 (pIRTyr1162/1163; # 44-804G) and anti-insulin receptor (IR; #AHR0271) were from Invitrogen (Camarillo, CA). Anti-Akt (#9272), anti-phospho AktSer473 (pAktSer473; #9271), anti-phospho AktThr308 (pAktThr308; #9275), anti-GLUT4 (#2213), and anti-rabbit IgG horseradish peroxidase (#7074) were from Cell Signaling Technology (Danvers, MA). Anti-phospho-AS160Thr642 (#07-802) and anti-AS160 (#07-741) were from Millipore (Billerica, MA). 2-Deoxy- D -[3H] glucose ([3H]2-DG) and [14C]mannitol were from Perkin Elmer (Boston, MA).
Animal Care
Procedures for animal care were approved by the University of Michigan Committee on Use and Care of Animals. CR and AL male Fischer 344 × Brown Norway, F1 generation rats were obtained at 23 months of age from the National Institute of Aging Calorie Restriction Colony and were individually housed for a month prior to experimentation. CR was initiated at 14 weeks of age with 90% of AL, increased to 75% of AL at 15 weeks, and to 60% of AL at 16 weeks, a level maintained until 23 months of age. Upon arrival at the Michigan animal facility, rats were housed individually in shoebox cages and maintained on a 12:12-hours light–dark cycle (lights out at 17:00 PM) in specific pathogen-free conditions. The AL group had AL access to the NIH31 chow for the duration of the study. The CR group received NIH31/NIA-fortified chow (Test Diet), which contains extra vitamin supplementation to provide CR animals with a level of vitamins similar to that of animals allowed AL access to the NIH31 diet. The CR group received 60%–65% of the intake of the AL group daily during the final month of the study. All rats were fed between 15:30 PM and 16:30 PM each day, and food intake of both groups was measured daily. All rats were weighed weekly at the same time of day. Muscle experiments were performed on AL (N = 12) and CR (N = 13) fed rats at 24 months of age.
Muscle Dissection and Incubation
Food was removed from the cages of all rats on the morning of the experimental day between 07:00 and 08:00 hours. Rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg) between 10:30 and 13:30 hours. Upon loss of pedal reflexes, soleus and epitrochlearis muscles were removed and rapidly rinsed in warm (35°C) Krebs-Henseleit buffer (KHB). Muscles were longitudinally split into strips of similar size for each muscle (two strips for each epitrochlearis and four strips for each soleus). Muscles strips were subsequently placed in vials containing the appropriate media shaking and continuous gassing (95% O2/5% CO2) in a heated (35°C) water bath. In the first incubation step, all muscles were incubated in vials containing 2 mL KHB supplemented with 0.1% bovine serum albumin (BSA), 2 mM sodium pyruvate, 6 mM mannitol as a rinse step for 30 minutes. In the second incubation step, all muscles were incubated in vials containing 2 mL KHB supplemented with 0.1% BSA, 2 mM sodium pyruvate, 6 mM mannitol, and either 0 nM (basal) or 1.2 nM insulin for 30 minutes. All muscles were then transferred to a third vial containing 2 mL of KHB/BSA solution, the same insulin concentration as the previous step, 1 mM 2-DG (including a final specific activity of 2.25 mCi/mmol [3H]-2-DG), and 9 mM mannitol (including a final specific activity of 0.022 mCi/mmol [14C]-mannitol) for 20 minutes. Following the third incubation step, muscles were rapidly blotted on filter paper moistened with ice-cold KHB, trimmed, freeze-clamped using aluminum tongs cooled in liquid nitrogen, and stored at −80oC for later processing and analysis.
Muscle Lysate Preparation
Frozen muscles were weighed, transferred to microfuge tubes, and homogenized in ice-cold lysis buffer (1 mL/muscle) using a Qiagen TissueLyser II (Valencia, CA). The lysis buffer contained Tissue Protein Extraction Reagent (Thermo Scientific, Rockford, IL; #78510) supplemented with 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM sodium vanadate (Na3VO4), 1 mM β-glycerophosphate, 1 μg/mL leupeptin, and 1 mM phenylmethylsulfonyl fluoride. Homogenates were transferred to microfuge tubes, rotated for 1 hours at 4°C, and then centrifuged (15,000g) for 15 minutes (4oC) to remove insoluble material. Protein concentration was measured using the bicinchoninic acid method (Pierce Biotechnology, Rockford, IL; #23225).
Immunoblotting
Equal amounts of protein from each sample were mixed with 6× Laemmli buffer, boiled with sodium dodecyl sulfate loading buffer for 5 minutes, separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and then transferred to nitrocellulose. Membranes were rinsed with Tris-buffered saline plus Tween-20 (TBST; 0.14 mol/L NaCl, 0.02 mol/L Tris base, pH 7.6, and 0.1% Tween-20), blocked with 5% BSA in TBST for 1 hours at room temperature, and transferred to primary antibody 1:1000 in TBST plus 5% BSA overnight at 4°C. Blots were washed 3 × 5 minutes with TBST and incubated in buffer containing the appropriate secondary antibody (1:20,000 dilution) for 1 hours at room temperature. Membranes were then washed 3 × 5 minutes with TBST and subjected to enhanced chemiluminescence with West Dura Extended Duration Substrate (Pierce; #34075) for visualization of protein bands. Immunoreactive proteins were quantified by densitometry (AlphaEase FC; Alpha Innotech, San Leandro, CA).
2-Deoxy-D-Glucose Uptake
Aliquots (200 μL) of the supernatants were combined in a vial with 10 mL of scintillation cocktail (Research Products International, Mount Prospect, IL), and a scintillation counter (Perkin Elmer, Waltman, MA) was used to determine 3H and 14C disintegrations per minutes. These values were used to determine [3H]-2-DG uptake as previously described (26,27).
Statistical Analysis
A student’s t test was used to compare AL and CR groups. Data are presented as mean ± SEM. A p value < .05 was considered statistically significant.
RESULTS
Food Intake, Body Mass, and Mass of Muscle Strips
As intended, daily food intake for AL rats (18.4 ± 1.46 g) was greater (p < .01) than for CR rats (12.0 ± 1.02 g; 65% of AL), and as expected, body mass was greater (p < .05) for AL (533 ± 7 g) versus CR (302.5 ± 1.8 g) rats. Also as expected, the masses of the muscle strips used for ex vivo incubation were greater for the soleus (p < .01) of AL (51.7 ± 2.5 mg) versus CR (42.0 ± 2.4 mg) rats and for the epitrochlearis (p < .001) of AL (84.2 ± 3.2 mg) versus CR (58.5 ±2.2 mg) rats.
2-Deoxy-D-Glucose Uptake
2-DG uptake in the absence of insulin for both the epitrochlearis (p = .34) and soleus (p = .16) was not significantly different between AL and CR rats (Figure 1). 2-DG uptake with insulin was significantly greater (p < .05) for CR versus AL rats in both the epitrochlearis (56% increase) and the soleus (40% increase).
Figure 1.
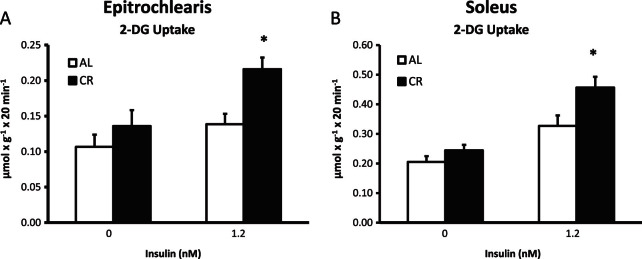
2-Deoxy-D-glucose (2-DG) uptake in epitrochlearis (A) and soleus (B) muscles with 0 or 1.2 nM insulin. *p < .05, CR versus AL in the same insulin treatment group. Data are means ± SEM. n = 9–11 muscles per diet group and insulin concentration. Open bars = ad libitum (AL) fed. Closed bars = calorie restriction (CR) fed.
Protein Abundance
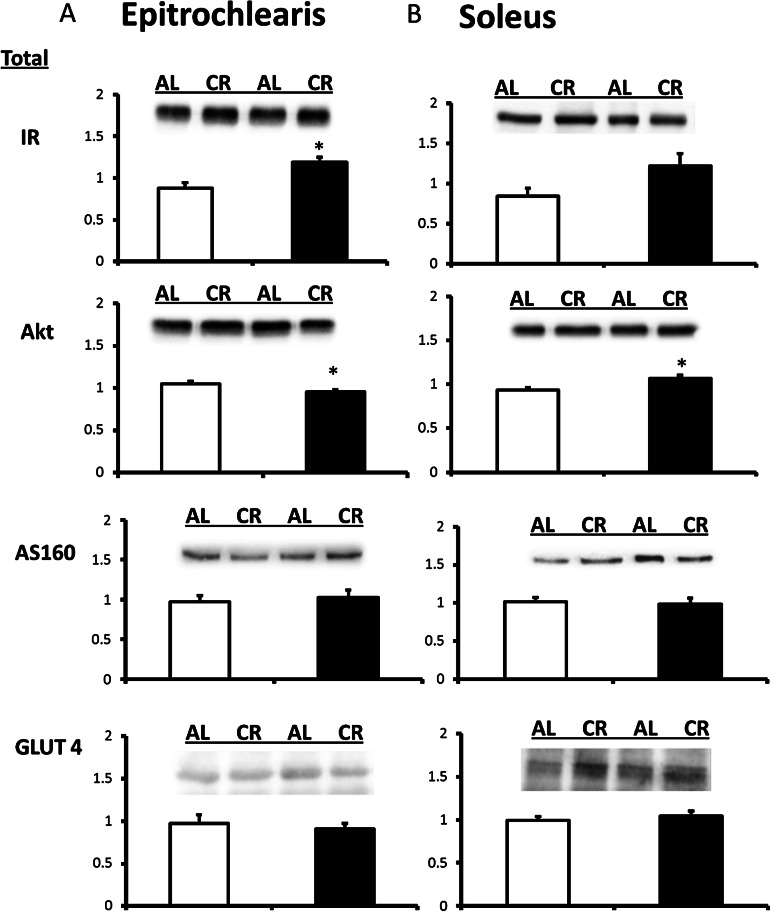
Insulin receptor abundance for the CR versus AL group was increased significantly in the epitrochlearis (35% increase; p < .005) and tended to increase in the soleus (p = .056; Figure 2). Akt abundance was decreased slightly for the CR compared with AL group in the epitrochlearis (9% decrease; p < .05; Figure 2). Akt abundance was increased with CR in the soleus (15% increase; p < .01). Neither total AS160 nor GLUT4 abundance in either muscle differed for AL versus CR rats (Figure 2).
Figure 2.
IR (insulin receptor) protein abundance in epitrochlearis and soleus muscles. Akt protein abundance in the epitrochlearis and soleus muscles AS160 protein abundance in epitrochlearis and soleus muscles. GLUT4 protein abundance in the epitrochlearis and soleus muscles. *p < .05, calorie restriction (CR) versus ad libitum (AL).
Insulin Receptor Phosphorylation
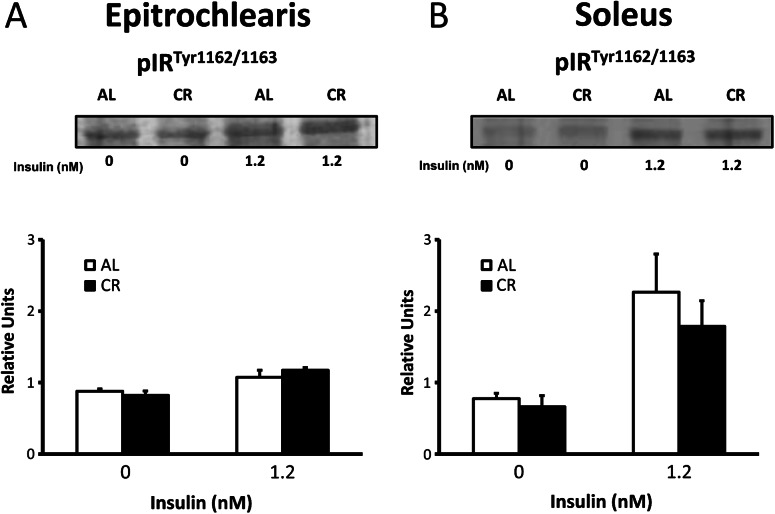
In the epitrochlearis, there were no diet effects on insulin receptor phosphorylation either with or without insulin (Figure 3). There were also no significant diet effects on insulin receptor phosphorylation in the soleus with or without insulin.
Figure 3.
Insulin receptor tyrosine phosphorylation (IRTyr1162/1163) in the epitrochlearis (A) and soleus (B) muscles with 0 or 1.2 nM insulin. *p < .05, calorie restriction (CR) versus ad libitum (AL) in the same insulin treatment group. Data are means ± SEM. n = 9–11 muscles per diet group and insulin concentration.
Akt Phosphorylation
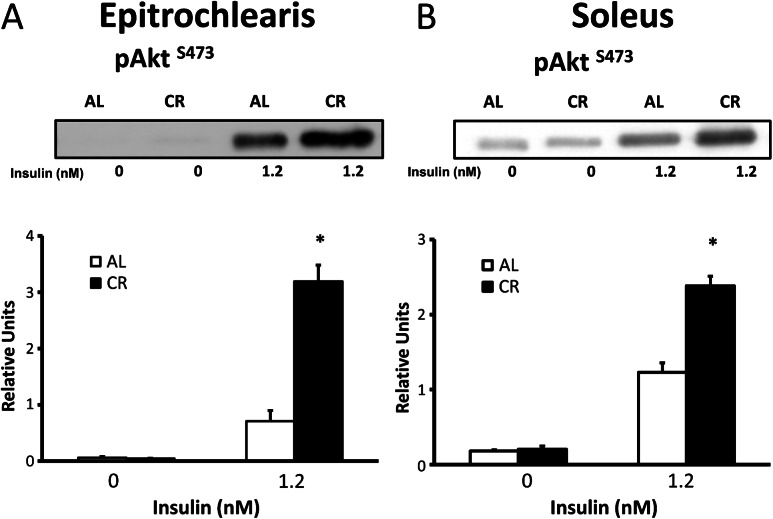
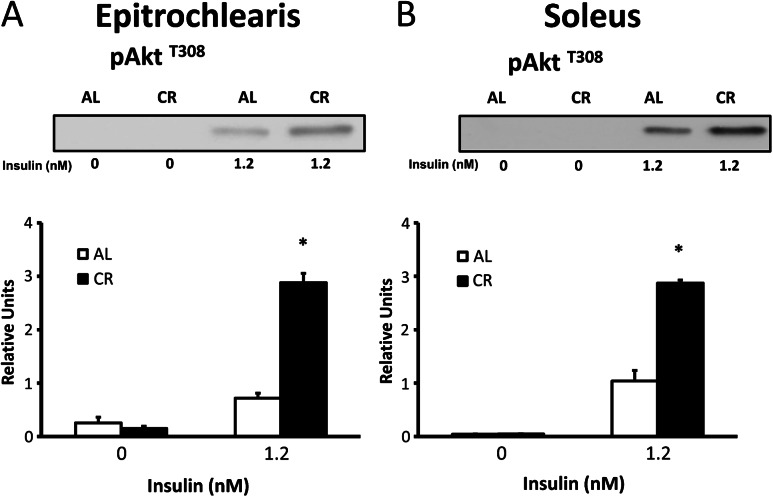
In the absence of insulin, there was no diet effect observed on Akt phosphorylation on either T308 or S473 in the epitrochlearis or soleus (Figures 4 and 5). In both the epitrochlearis and the soleus under insulin-stimulated conditions, Akt phosphorylation was increased on both T308 (300% in epitrochlearis, p < .001; and 177% in soleus, p < .001) and S473 (350% increase in epitrochlearis, p < .001; and 94% in soleus, p < .001) for CR versus AL rats.
Figure 4.
AktThr308 phosphorylation in epitrochlearis (A) and soleus (B) muscles with 0 or 1.2 nM insulin. *p < .05, calorie restriction (CR) versus ad libitum (AL) in the same insulin treatment group. Data are means ± SEM. n = 9–11 muscles per diet group and insulin concentration.
Figure 5.
AktSer473 phosphorylation in epitrochlearis (A) and soleus (B) muscles with 0 or 1.2 nM insulin. Data are means ± SEM. n = 9–11 muscles per diet group and insulin concentration.
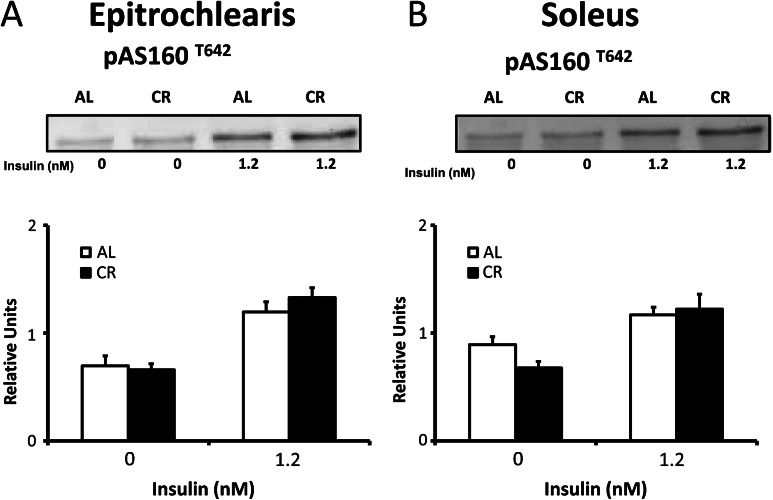
AS160 Phosphorylation
In both the epitrochlearis and soleus muscle, phosphorylation of AS160 at the T642 site did not differ between AL and CR animals in either the absence of insulin or with insulin stimulation (Figure 6).
Figure 6.
AS160Thr642 phosphorylation in epitrochlearis (A) and soleus (B) muscles with 0 or 1.2 nM insulin. Data are means ± SEM. n = 9–11 muscles per dietary group and insulin concentration.
DISCUSSION
We previously demonstrated in 23-month-old rats that CR leads to increased ISGU by the predominantly type II epitrochlearis muscle (14,15), but the influence of CR on glucose uptake in primarily type I skeletal muscle from old rats had not been previously reported. It was important to fill this gap in knowledge because the ISGU rates of muscles composed of primarily type I fibers are approximately twofold greater than muscles composed of predominantly type II fibers (13,28,29). Furthermore, relatively greater age-related insulin resistance has been reported for predominantly type I versus predominantly type II muscles from older rats (29,30). The most important new findings of the current study for isolated skeletal muscles with a physiological insulin concentration, old (24-month-old) CR versus AL rats were: (a) glucose uptake was enhanced for both type I soleus and type II epitrochlearis; (b) GLUT4 abundance was unchanged in both the soleus and epitrochlearis; (c) insulin receptor phosphorylation was not altered by diet in either soleus or epitrochlearis; (d) Akt phosphorylation was enhanced in both the soleus and epitrochlearis; and (e) AS160 phosphorylation at T642 was unchanged by diet in both the soleus and epitrochlearis.
Total GLUT4 abundance is highly correlated to the capacity for ISGU in rat skeletal muscle (28). However, total GLUT4 content was unaltered by CR in both the epitrochlearis and the soleus. These results were consistent with the previously published data for CR effect on GLUT4 in skeletal muscle of old rats. Wang and colleagues (16) reported that CR did not alter total GLUT4 content in the diaphragm of 29-month-old rats. Because the diaphragm contracts continuously and chronic contraction can increase GLUT4 expression (31), the results of the current study for the soleus and epitrochlearis provided valuable new information. The absence of a CR effect on GLUT4 abundance in the soleus of 24-month-old rats is consistent with the results that we previously reported for the soleus of 9-month-old rats (13). GLUT4 abundance in the epitrochlearis was also unaltered by CR in 24-month-old rats, consistent with our previous results for the epitrochlearis of 8-month-old rats (14). However, in a recent study, we found a modest (22%) CR-induced increase in GLUT4 abundance in the epitrochlearis of 9-month-old rats (13). CR did not result in altered GLUT4 abundance in the vastus lateralis muscle from adult rhesus monkeys (32). GLUT4 abundance in hind limb muscles from mice were unaltered (33,34) or increased (35) in response to CR. Taken together, a number of previous studies have established that even though skeletal muscle GLUT4 abundance is often unaltered by CR, improved insulin sensitivity is consistently reported with CR in adults. Furthermore, the current results clearly demonstrate that enhanced ISGU can occur in both type I and type II muscles of old rats in the absence of increased GLUT4 expression. The CR-induced enhancement on insulin-mediated glucose uptake in isolated epitrochlearis muscles from 5.5-month-old rats is attributable to a proportional increase in GLUT4 translocation to the cell surface (19). It seems reasonable to suspect that greater GLUT4 translocation may also mediate the CR-related elevation in ISGU of muscles from old rats. Because GLUT4 translocation is regulated by the insulin-signaling pathway, it was important that we also evaluated CR effects on key insulin signaling steps in muscles from old rats.
There was no CR effect on tyrosine1162/1163 phosphorylation of the insulin receptor for either muscle with a physiologic insulin dose. These tyrosine residues on the insulin receptor’s β subunit are major regulatory sites that account for most of the receptor’s insulin-mediated autophosphorylation and tyrosine kinase activity (36). Furthermore, replacement of both of these tyrosine residues with phenylalanine residues results in a marked of reduction insulin-stimulated 2-DG uptake (36). The lack of a CR-related increase in insulin receptor tyrosine phosphorylation was found despite a moderate (35%) increase for insulin receptor abundance of the epitrochlearis of CR compared with AL rats. These results for insulin receptor abundance are reminiscent of the previously reported modest increase (∼19%) in insulin receptor binding capacity (B max) for CR versus AL rats in the diaphragm of 29-month-old rats (16). Zhu and colleagues (24,25) reported that after 25-month-old rats were injected with a high dose of insulin into the portal vein, gastrocnemius muscle (predominantly type II fibers) from CR compared with AL animals had greater insulin receptor tyrosine phosphorylation. In 9-month-old rats, we previously reported no significant CR compared with AL differences for tyrosine phosphorylation of the insulin receptor from soleus or epitrochlearis muscles incubated with a physiologic insulin dose, but there was a CR-related increase in both muscles with a supraphysiologic insulin concentration (13). These results suggest that CR effects on tyrosine phosphorylation of the insulin receptor in skeletal muscle may be insulin dose-dependent. Furthermore, the CR-related amplification of postreceptor signaling and glucose uptake in muscle stimulated by a physiologic insulin dose in the current study do not appear to be attributable to greater tyrosine phosphorylation of the insulin receptor.
We have consistently found a robust CR-induced increase in skeletal muscle Akt phosphorylation in skeletal muscle (both soleus and epitrochlearis) of young adult rats or mice (11–13,37). The current study was the first to assess the influence of CR on Akt phosphorylation in skeletal muscle from old rats. Consistent with earlier results, CR versus AL rats had a striking increase in insulin-stimulated phosphorylation of the key regulatory sites of Akt (T308 and S473) in both muscles. The small CR effects on Akt abundance (15% increase in soleus and 9% decrease in epitrochlearis) were insufficient to account for the marked (177% increase in soleus and 300% increase in epitrochlearis for T308; 94% increase in soleus and 350% increase in epitrochlearis for S473) increases for Akt phosphorylation with CR. The dramatic increases in Akt phosphorylation with CR at a physiologic insulin dose occurred without an apparent change in upstream signaling at the insulin receptor, and these data are consistent with our previous results for the effect of CR on the soleus and epitrochlearis for 9-month-old rats (13).
AS160 is an Akt substrate and the most distal insulin signaling protein that is clearly implicated in insulin-mediated activation of glucose uptake in skeletal muscle (38,39). The ability to phosphorylate AS160 on T642 is essential for the full effect of insulin on GLUT4 translocation and glucose uptake (22,23,38,40). However, we found no evidence for diet-related changes in insulin-stimulated T642 phosphorylation in either the epitrochlearis or soleus muscle of old rats. Consistent with the current results, we previously reported that T642 phosphorylation of the insulin-stimulated soleus was unaltered by CR for 9-month-old rats (13). The lack of a CR effect on T642 phosphorylation of AS160 in the epitrochlearis with physiologic insulin in 24-month-old rat differs from the CR-related increase in T642 for the epitrochlearis of 9-month-old rats (13). The explanation for the different results for T642 in the epitrochlearis of adult compared with old rats remains to be determined.
In conclusion, the most significant new result was that the CR effect on ISGU was well preserved for both type I soleus and type II epitrochlearis of old rats. Furthermore, in both muscles, it seems likely that this increase is secondary, at least in part, to greater insulin-stimulated Akt phosphorylation. However, in neither muscle was insulin-stimulated AS160 T642 phosphorylation enhanced by CR. These results suggest that the mechanisms for improved ISGU in the epitrochlearis with CR may not be completely identical for adult compared with old rats. One plausible scenario is that CR may enhance AS160 phosphorylation on other phosphosites and/or elevate phosphorylation of other Akt substrates that are implicated in the regulation of glucose uptake (eg, TBC1D1 and CDP-138) (38,39,41).
FUNDING
This work was supported by the National Institute on Aging grants AG-010026, AG-013283 and T32-AG000114.
References
- 1.Weindruch R. Caloric restriction and aging. Sci Am. 1996;274:46–52. doi: 10.1038/scientificamerican0196-46. [DOI] [PubMed] [Google Scholar]
- 2.Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 3.Gupta G, She L, Ma XH, et al. Aging does not contribute to the decline in insulin action on storage of muscle glycogen in rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R111–R117. doi: 10.1152/ajpregu.2000.278.1.R111. [DOI] [PubMed] [Google Scholar]
- 4.Kemnitz JW, Roecker EB, Weindruch R, Elson DF, Baum ST, Bergman RN. Dietary restriction increases insulin sensitivity and lowers blood glucose in rhesus monkeys. Am J Physiol. 1994;266:E540–E547. doi: 10.1152/ajpendo.1994.266.4.E540. [DOI] [PubMed] [Google Scholar]
- 5.Arciero PJ, Vukovich MD, Holloszy JO, Racette SB, Kohrt WM. Comparison of short-term diet and exercise on insulin action in individuals with abnormal glucose tolerance. J Appl Physiol. 1999;86:1930–1935. doi: 10.1152/jappl.1999.86.6.1930. [DOI] [PubMed] [Google Scholar]
- 6.Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care. 1994;17:30–36. doi: 10.2337/diacare.17.1.30. [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 8.Ivy JL, Young JC, Craig BW, Kohrt WM, Holloszy JO. Ageing, exercise and food restriction: effects on skeletal muscle glucose uptake. Mech Ageing Dev. 1991;61:123–133. doi: 10.1016/0047-6374(91)90011-n. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee S, Saenger P, Hu M, Chen W, Barzilai N. Fat accretion and the regulation of insulin-mediated glycogen synthesis after puberty in rats. Am J Physiol. 1997;273:R1534–R1539. doi: 10.1152/ajpregu.1997.273.4.R1534. [DOI] [PubMed] [Google Scholar]
- 10.Gazdag AC, Dumke CL, Kahn CR, Cartee GD. Calorie restriction increases insulin-stimulated glucose transport in skeletal muscle from IRS-1 knockout mice. Diabetes. 1999;48:1930–1936. doi: 10.2337/diabetes.48.10.1930. [DOI] [PubMed] [Google Scholar]
- 11.McCurdy CE, Davidson RT, Cartee GD. Brief calorie restriction increases Akt2 phosphorylation in insulin-stimulated rat skeletal muscle. Am J Physiol Endocrinol Metab. 2003;285:E693–E700. doi: 10.1152/ajpendo.00224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCurdy CE, Cartee GD. Akt2 is essential for the full effect of calorie restriction on insulin-stimulated glucose uptake in skeletal muscle. Diabetes. 2005;54:1349–1356. doi: 10.2337/diabetes.54.5.1349. [DOI] [PubMed] [Google Scholar]
- 13.Sharma N, Arias EB, Bhat AD, et al. Mechanisms for increased insulin-stimulated Akt phosphorylation and glucose uptake in fast- and slow-twitch skeletal muscles of calorie-restricted rats. Am J Physiol Endocrinol Metab. 2011;300:E966–E978. doi: 10.1152/ajpendo.00659.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cartee GD, Kietzke EW, Briggs-Tung C. Adaptation of muscle glucose transport with caloric restriction in adult, middle-aged, and old rats. Am J Physiol. 1994;266:R1443–R1447. doi: 10.1152/ajpregu.1994.266.5.R1443. [DOI] [PubMed] [Google Scholar]
- 15.Dean DJ, Cartee GD. Brief dietary restriction increases skeletal muscle glucose transport in old Fischer 344 rats. J Gerontol Biol Sci. 1996;51:B208–B213. doi: 10.1093/gerona/51a.3.b208. [DOI] [PubMed] [Google Scholar]
- 16.Wang ZQ, Bell-Farrow AD, Sonntag W, Cefalu WT. Effect of age and caloric restriction on insulin receptor binding and glucose transporter levels in aging rats. Exp Gerontol. 1997;32:671–684. doi: 10.1016/s0531-5565(97)00054-5. [DOI] [PubMed] [Google Scholar]
- 17.Wilkes JJ, Nagy LE. Chronic ethanol feeding impairs glucose tolerance but does not produce skeletal muscle insulin resistance in rat epitrochlearis muscle. Alcohol Clin Exp Res. 1996;20:1016–1022. doi: 10.1111/j.1530-0277.1996.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 18.Cho H, Mu J, Kim JK, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 19.Dean DJ, Brozinick JT, Jr, Cushman SW, Cartee GD. Calorie restriction increases cell surface GLUT-4 in insulin-stimulated skeletal muscle. Am J Physiol. 1998;275:E957–E964. doi: 10.1152/ajpendo.1998.275.6.E957. [DOI] [PubMed] [Google Scholar]
- 20.Davidson RT, Arias EB, Cartee GD. Calorie restriction increases muscle insulin action but not IRS-1-, IRS-2-, or phosphotyrosine-PI 3-kinase. Am J Physiol Endocrinol Metab. 2002;282:E270–E276. doi: 10.1152/ajpendo.00232.2001. [DOI] [PubMed] [Google Scholar]
- 21.Dean DJ, Cartee GD. Calorie restriction increases insulin-stimulated tyrosine phosphorylation of insulin receptor and insulin receptor substrate-1 in rat skeletal muscle. Acta Physiol Scand. 2000;169:133–139. doi: 10.1046/j.1365-201x.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 22.Sano H, Kane S, Sano E, et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 23.Kane S, Sano H, Liu SC, et al. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem. 2002;277:22115–22118. doi: 10.1074/jbc.C200198200. [DOI] [PubMed] [Google Scholar]
- 24.Zhu M, Miura J, Lu LX, et al. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp Gerontol. 2004;39:1049–1059. doi: 10.1016/j.exger.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Zhu M, de Cabo R, Anson RM, Ingram DK, Lane MA. Caloric restriction modulates insulin receptor signaling in liver and skeletal muscle of rat. Nutrition. 2005;21:378–388. doi: 10.1016/j.nut.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 26.Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol. 1994;76:979–985. doi: 10.1152/jappl.1994.76.2.979. [DOI] [PubMed] [Google Scholar]
- 27.Cartee GD, Bohn EE. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol. 1995;268:E902–E909. doi: 10.1152/ajpendo.1995.268.5.E902. [DOI] [PubMed] [Google Scholar]
- 28.Henriksen EJ, Bourey RE, Rodnick KJ, Koranyi L, Permutt MA, Holloszy JO. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am J Physiol. 1990;259:E593–E598. doi: 10.1152/ajpendo.1990.259.4.E593. [DOI] [PubMed] [Google Scholar]
- 29.Sharma N, Arias EB, Sajan MP, et al. Insulin resistance for glucose uptake and Akt2 phosphorylation in the soleus, but not epitrochlearis, muscles of old vs. adult rats. J Appl Physiol. 2010;108:1631–1640. doi: 10.1152/japplphysiol.01412.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Escriva F, Agote M, Rubio E, et al. In vivo insulin-dependent glucose uptake of specific tissues is decreased during aging of mature Wistar rats. Endocrinology. 1997;138:49–54. doi: 10.1210/endo.138.1.4862. [DOI] [PubMed] [Google Scholar]
- 31.Ivy JL. Muscle insulin resistance amended with exercise training: role of GLUT4 expression. Med Sci Sports Exerc. 2004;36:1207–1211. [PubMed] [Google Scholar]
- 32.Gazdag AC, Sullivan S, Kemnitz JW, Cartee GD. Effect of long-term caloric restriction on GLUT4, phosphatidylinositol-3 kinase p85 subunit, and insulin receptor substrate-1 protein levels in rhesus monkey skeletal muscle. J Gerontol Biol Sci. 2000;55:B44–B46. doi: 10.1093/gerona/55.1.b44. ; discussion B47–B48. [DOI] [PubMed] [Google Scholar]
- 33.Argentino DP, Dominici FP, Al-Regaiey K, Bonkowski MS, Bartke A, Turyn D. Effects of long-term caloric restriction on early steps of the insulin-signaling system in mouse skeletal muscle. J Gerontol A Biol Sci Med Sci. 2005;60:28–34. doi: 10.1093/gerona/60.1.28. [DOI] [PubMed] [Google Scholar]
- 34.Argentino DP, Munoz MC, Rocha JS, Bartke A, Turyn D, Dominici FP. Short-term caloric restriction does not modify the in vivo insulin signaling pathway leading to Akt activation in skeletal muscle of Ames dwarf (Prop1(df)/Prop1(df)) mice. Horm Metab Res. 2005;37:672–679. doi: 10.1055/s-2005-870577. [DOI] [PubMed] [Google Scholar]
- 35.Argentino DP, Dominici FP, Munoz MC, Al-Regaiey K, Bartke A, Turyn D. Effects of long-term caloric restriction on glucose homeostasis and on the first steps of the insulin signaling system in skeletal muscle of normal and Ames dwarf (Prop1df/Prop1df) mice. Exp Gerontol. 2005;40:27–35. doi: 10.1016/j.exger.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Ellis L, Clauser E, Morgan DO, Edery M, Roth RA, Rutter WJ. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986;45:721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- 37.McCurdy CE, Davidson RT, Cartee GD. Calorie restriction increases the ratio of phosphatidylinositol 3-kinase catalytic to regulatory subunits in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:E996–E1001. doi: 10.1152/ajpendo.00566.2004. [DOI] [PubMed] [Google Scholar]
- 38.Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab. 2007;32:557–566. doi: 10.1139/H07-026. [DOI] [PubMed] [Google Scholar]
- 39.Cartee GD, Funai K. Exercise and insulin: convergence or divergence at AS160 and TBC1D1? Exerc Sport Sci Rev. 2009;37:188–195. doi: 10.1097/JES.0b013e3181b7b7c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem. 2006;281:31478–31485. doi: 10.1074/jbc.M605461200. [DOI] [PubMed] [Google Scholar]
- 41.Xie X, Gong Z, Mansuy-Aubert V, et al. C2 domain-containing phosphoprotein CDP138 regulates GLUT4 insertion into the plasma membrane. Cell Metab. 2011;14:378–389. doi: 10.1016/j.cmet.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]