Abstract
Urine concentrating ability is reduced during normal aging in people and rats. The abundance of many of the key transport proteins that contribute to urine concentrating ability is reduced in the kidney medulla of aged rats. The reductions in water, sodium, and urea transport protein abundances, and their reduced response to water restriction, contribute to the reduced ability of aged rats to concentrate their urine and conserve water. If similar mechanisms occur in human kidneys, it would provide a molecular explanation for the reduced urine concentrating ability in aging and may provide opportunities for novel therapeutic approaches to improve urine concentrating ability and/or nocturnal polyuria.
Keywords: Vasopressin, Aquaporin, Water channels, Urea transporters, sodium transport
There is a decrease in maximal urine concentrating ability during normal aging in humans (1–3). This physiological response to aging was clearly demonstrated in the Baltimore Longitudinal Study of Aging (1). Rowe and colleagues measured maximal urine concentrating ability in healthy individuals in three age groups: 20–39, 40–59, and 60–79 years. When compared with the two younger age groups, individuals aged 60–79 years had approximately a 20% reduction in maximal urine osmolality, a 50% decrease in the ability to conserve solute, and a 100% increase in minimal urine flow rate (1). Aged individuals do have a diminished thirst response, but the relationship between vasopressin (also named antidiuretic hormone) secretion and plasma osmolality is preserved and may even be enhanced (3). The change in urine concentrating ability could not be explained by a decrease in glomerular filtration rate (1). Thus, neither an abnormality in vasopressin secretion nor a reduction in renal function (as measured by glomerular filtration rate) appears to be the mechanism that explains the decrease in urine concentrating ability during aging in people or Wag/Rij rats (1,3,4).
A challenge in the field is distinguishing between normal aging and superimposed pathologies. Kidney function deteriorates at very different rates with aging in humans (1). In rats, there are strain (rather than environment) dependent changes with the F344/BN and Wag/Rij rats showing minimal, F344 showing moderate, and Sprague Dawley showing marked age-dependent loss of GFR and structural damage. These strain differences make it difficult to know which animal model best mimics human aging.
The cloning of many of the key kidney medullary water channel (aquaporins) and solute (sodium and urea) transport proteins that are involved in the urine concentrating mechanism, and the type 2 vasopressin receptor, have resulted in studies into the molecular mechanisms underlying the reduction in urine concentrating ability that occurs during aging (reviewed in [5–7]). Understanding the aging-related changes that occur in vasopressin levels, adenosine 3’,5’-cyclic monophosphate (cAMP) production, and the abundances of the key transport proteins involved in urine concentration may also be relevant to understanding the pathophysiology of nocturnal polyuria (8–10).
URINE CONCENTRATING MECHANISM
The region of the kidney that is responsible for the generation of concentrated or dilute urine is the medulla (Figure 1). To produce concentrated urine, a hypertonic medullary interstitium must be generated by the nephron segments located in the loops of Henle, and the collecting duct must be permeable to water (reviewed in [11]). To generate a hypertonic medullary interstitium, a small osmotic gradient is generated at each level of the medulla and then magnified down its length by countercurrent multiplication. In the thick ascending limb of the loop of Henle (in the outer medulla), the NKCC2/BSC1 cotransporter actively reabsorbs Na+, K+, and Cl− across the apical membrane, the K+ that is reabsorbed is secreted back into the lumen via the K+ secretory channel ROMK, resulting in net NaCl reabsorption.
Figure 1.
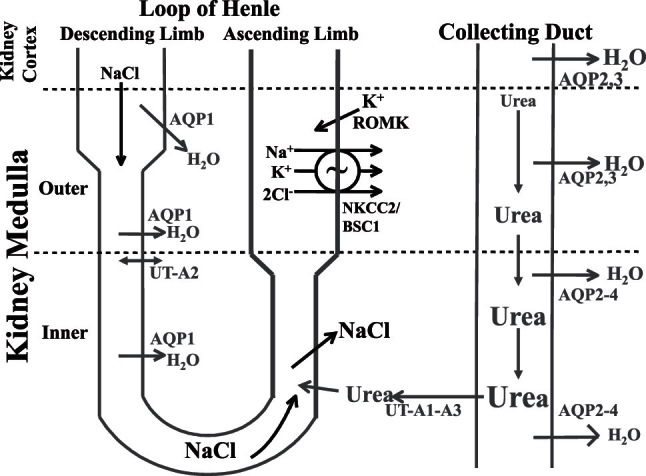
Transport proteins involved in the urinary concentrating mechanism. In the outer medulla, active NaCl reabsorption via NKCC2/BSC1 in the thick ascending limb of the loop of Henle generates a hypertonic medullary interstitium. This concentrates NaCl in the lumen of the thin descending limb of the loop of Henle by osmotically removing water via AQP1 water channels. In the inner medulla, passive NaCl reabsorption exceeds urea secretion. Water is reabsorbed from the collecting duct, in the presence of vasopressin, via AQP2 in the apical plasma membrane and AQP3 and AQP4 in the basolateral plasma membrane. Urea is concentrated in the collecting duct lumen until the fluid reaches the urea-permeable terminal inner medullary collecting duct where urea is reabsorbed into the inner medullary interstitium via the UT-A1 and UT-A3 urea transporters.
In contrast to the outer medulla, NaCl is passively reabsorbed in the inner medulla across the thin ascending limb of the loop of Henle (reviewed in [11]). The thin ascending limb luminal fluid has a higher concentration of NaCl and a lower concentration of urea than inner medullary interstitial fluid, thereby establishing chemical gradients that favor NaCl reabsorption and urea secretion, provided that the interstitial urea concentration is sufficiently high (12,13). NaCl reabsorption exceeds urea secretion in the thin ascending limb as it has a higher permeability to NaCl than urea, thereby resulting in dilution of the thin ascending limb luminal fluid as it ascends toward the outer medulla.
NaCl reabsorption in both ascending limb portions of the loop of Henle results in both a hypertonic medullary interstitium and delivery of a dilute fluid (relative to plasma) to the distal tubule because both ascending limb segments are water impermeable. In the absence of vasopressin, the collecting duct is impermeable to water resulting in excretion of this dilute fluid as dilute urine. However, in the presence of vasopressin, the collecting duct becomes highly permeable to water, and if a hypertonic medulla is present, water is reabsorbed resulting in excretion of a concentrated urine (reviewed in [11]).
VASOPRESSIN (ADH) RECEPTORS
There are two types of vasopressin receptors: type 1 and type 2 (reviewed in [14]). The V2 receptor is involved in urinary concentration and is expressed in the collecting duct and thick ascending limb. It is a G-protein–coupled 7 transmembrane spanning receptor whose activation results in the generation of the second messenger cyclic AMP. The V1 receptor is involved in increasing systemic blood pressure and has two subtypes: V1a and V1b (also called V3). It is expressed in vasculature, brain, and liver, and its activation results in an increase in intracellular calcium.
Water reabsorption along the entire collecting duct is regulated by vasopressin binding to the V2 receptor and stimulating cAMP production (reviewed in [11,15,16]). During water deprivation, plasma osmolality increases. Hypothalamic osmoreceptors, which can sense an increase of as little as 2 mOsm/kg H2O, stimulate vasopressin secretion from the posterior pituitary. Vasopressin binds to V2 receptors in the basolateral plasma membrane of collecting duct principal cells and inner medullary collecting duct cells (but not intercalated cells), and in the basolateral plasma membrane of cells in the medullary thick ascending limb, which stimulates adenylyl cyclase to produce cAMP and in turn activates protein kinase A.
One potential mechanism for the aging-related decrease in urine concentrating ability would be a reduction in V2 receptors in the aged kidney and/or the ability of vasopressin to bind to the receptor and stimulate cAMP production, even though vasopressin secretion may not be abnormal (1,3,4). There is conflicting data on the effect of aging on V2 receptor messenger RNA (mRNA) abundance: A decrease in V2 receptor mRNA abundance has been detected in one study of F344/BN rats (17), but no effect on V2 receptor mRNA abundance or vasopressin-stimulated cAMP production was detected in other studies using Wag/Rij or Sprague Dawley rats (18–20). There is a decrease in vasopressin-dependent cAMP production in 24-month-old rats, as compared with 6- or 12-month-old F344 rats, with a rightward shift in the dose–response to vasopressin and an increase in the ED50 (21). The decrease in vasopressin-dependent cAMP production could contribute to the reduced urine concentrating ability in aging. However, inner medullary cAMP content is similar in 10- and 30-month-old Wag/Rij rats, both under control conditions and following 2 days of water deprivation (22). The lack of change in inner medullary cAMP content was surprising as plasma vasopressin was significantly higher in both the 10- and 30-month-old water deprived Wag/Rij rats (22). Thus, the data on aging-related changes in V2 receptor abundance and/or the ability of vasopressin to bind to the receptor and stimulate cAMP production vary depending upon the ages and strains of rats studied. Therefore, it is difficult to assess the contribution of a decrease in vasopressin-stimulated cAMP production to the urine concentrating defect in aging.
WATER CHANNELS (AQUAPORINS)
At present, there are 13 cloned water channels or aquaporins (AQPs), six of which are expressed in the kidney (reviewed in [5,11]). AQP1 is expressed in the proximal tubule and descending limb of the loop of Henle; AQP7 is also expressed in the proximal tubule. AQP2 is primarily expressed in the apical plasma membrane and subapical vesicles of the collecting duct and is the “vasopressin-regulated” water channel. AQP3 and APQ4 are expressed in the basolateral plasma membrane of the collecting duct. AQP6 is expressed in the collecting duct in intracellular vesicles associated with H+-ATPase and is not thought to be involved in water homeostasis.
The primary mechanism by which vasopressin rapidly regulates water reabsorption in the collecting duct is by regulating the accumulation of AQP2 in the apical plasma membrane (reviewed in [11,15,16,23]). Vasopressin regulation involves both AQP2 phosphorylation at serines 256, 261, 264, and 269 (24–27) and regulated trafficking of AQP2 between subapical vesicles and the apical plasma membrane (reviewed in [5,23]). Wade and colleagues (28) originally proposed the “membrane shuttle hypothesis” in 1979, at a time when water channels had not been cloned or identified. They proposed that the (putative) water channels were stored in subapical vesicles and inserted into the apical plasma membrane in response to vasopressin. After AQP2 was cloned, the “membrane shuttle hypothesis” was confirmed experimentally by Knepper and colleagues (7) in rat inner medullary collecting ducts (reviewed in [5,23]). The water that is reabsorbed through AQP2 exits the collecting duct principal cells through AQP3 and AQP4. When vasopressin stimulation ends, water reabsorption is stopped by endocytosing AQP2 back into the cell, where it is recycled into endosomes until the next stimulation by vasopressin (reviewed in [11,15,16]).
Elderly individuals are often prescribed medications that can interfere with vasopressin release, vasopressin function, or affect the regulation of aquaporins (reviewed in [29]). This can result in an increased incidence of either hyponatremia or hypernatreia in elderly individuals. The reader is referred to a recent review for a detailed discussion of this topic (29).
Several studies show that the abundance of some of the aquaporin proteins is reduced in the aged Wag/Rij rat kidney (18,22,30), which could contribute to the reduction in concentrating ability during aging. These studies show that AQP2 protein abundance is reduced in 24- to 30-month-old rats (which are very old rats), when compared with 10-month-old rats, in both the inner and outer medulla, in both Wag/Rij and F344/BN rats (17,18,22,30). The abundance of AQP2 that is phosphorylated at serine 256 is also markedly reduced in the older Wag/Rij rats (22,30); phosphorylation of serines 261, 264, and 269 has not been studied to date. AQP3 protein abundance is also reduced in the inner medulla of 30-month-old rats, compared with 10-month-old rats, but not in the outer medulla (18,30). Transepithelial water reabsorption across the collecting duct of aged rats is likely to be reduced by the reductions in AQP2 and AQP3 protein abundances. These changes appear to be specific for AQP2 and AQP3 because neither AQP4 nor AQP1 protein abundances differ between 10- and 30-month-old Wag/Rij and Sprague Dawley rats (18,29,31). This is consistent with AQP1 and AQP4 being constitutively expressed, whereas AQP2 and AQP3 gene expression is regulated by vasopressin.
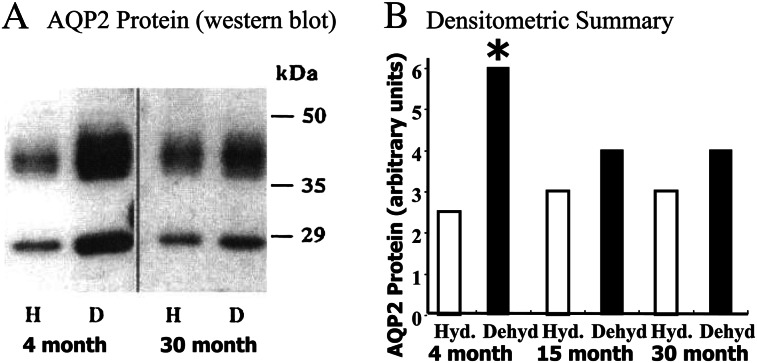
The preceding studies pertain to rats receiving food and water ad libitum, that is euvolemic rats. Because elderly individuals are more susceptible to dehydration than younger ones, an important clinical issue is the response of aged people to dehydration. To model this situation in rats, we compared the ability of 30-month-old rats to respond to 3 days of water restriction, as compared with the response of 4-month-old F344/BNF1 rats (32); 4-month-old rats are young adult animals (Figure 2). Both the 4- and 30-month-old F344/BNF1 rats lost 8% of their body weight and had similar increases in hematocrit, but only the older rats became significantly hypernatremic (serum sodium > 160 mEq/L) (32). Urine osmolality and AQP2 protein abundance increased significantly in the 4-month-old rats but not in the 30-month-old F344/BN F1 and Sprague Dawley rats (17,32,33). Somewhat surprisingly, AQP2 protein abundance did not increase in dehydrated 15-month-old F344/BNF1 rats, similar to the response in the 30-month-old rats (33). AQP2 mRNA abundance also increased in dehydrated 2-month-old rats but not in dehydrated 7-month-old Sprague Dawley rats (19). Thus, the age at which concentrating ability is lost in rats may be significantly younger than 24–30 months and emphasizes the need to study animals at several ages in order to understand the physiology of aging.
Figure 2.
Water restriction does not increase AQP2 protein abundance in 15- or 30-month-old F344/BNF1 rats. Panel A: Western blot showing that AQP2 protein (a glycosylated smear from 35 to 45 kDa and a nonglycosylated 29 kDa band) abundance is not different between hydrated (H) and dehydrated (D) 30-month-old rats but is increased significantly increased in dehydrated (D) vs hydrated (H) 4-month-old rats. Panel B: Densitometric summary. There was no significant difference between hydrated (Hyd.) vs dehydrated (Dehyd) in the 15- or 30-month-old rats. Asterisk indicates a significant difference between hydrated (Hyd.) vs dehydrated (Dehyd) 4-month-old rats. Data from references (31,32).
In contrast to the preceding findings, another study using a different strain of rats found that 2 days of water deprivation significantly increased total AQP2 and phosphoserine 256-AQP2 protein abundances in both 10- and 30-month-old Wag/Rij rats (22). In this study, AQP2 mRNA abundance was increased in the outer medulla of the 30-month-old rats, compared with the 10-month-old rats, but was unchanged in the inner medulla (22). This study concluded that AQP2 is downregulated by a posttranscriptional mechanism in aging, but water deprivation eliminates this downregulation (22). The different findings in studies that employ rats of different ages and/or strains makes it difficult to extrapolate these findings to humans. Nitric oxide synthase activity decreases AQP2 protein abundance and apical plasma membrane trafficking in the inner medullary collecting duct in response to hemorrhage in Sprague Dawley rats (34). This effect is lower in 12-month-old rats than in 2-month-old Sprague Dawley rats (34).
The preceding studies suggest that an important mechanism that contributes to the reduction in urine concentrating ability is a decrease in AQP2 protein abundance during aging. To determine whether this defect could be corrected pharmacologically, supraphysiologic concentrations of dDAVP (Desmopressin), a selective V2 receptor agonist that does not increase blood pressure, were administered to 10- and 30-month-old Wag/Rij rats (35). dDAVP administration caused similar decreases in urine flow rate and increases in urine osmolality in 10- and 30-month-old rats, although the maximal urine osmolality in the older rats was lower than in the younger Wag/Rij rats (35). dDAVP administration also increased the protein abundances of both AQP2 and AQP3 (35), suggesting that the reduced maximal urine osmolality in the aged rats is related, at least in part, to the reduced level of these AQP proteins.
SODIUM TRANSPORTERS
NaCl reabsorption through the Na+-K+-2Cl− cotransporter NKCC2/BSC1 is critical for the establishment of the hypertonic medullary interstitium that is needed to concentrate urine (reviewed in [11]). Vasopressin regulates NKCC2 both acutely and chronically. Acutely, vasopressin increases NaCl reabsorption in the medullary thick ascending limb by regulating the trafficking of NKCC2 to the apical plasma membrane and by increasing the phosphorylation of the N-terminal tail of NKCC2 at Thr-184 and Thr-189 (reviewed in [11]). Chronically, vasopressin increases NKCC2 protein abundance in the thick ascending limb (reviewed in [11]). NKCC2/BSC1 protein abundance is reduced in the outer medulla of older F344/BN rats (36). NKCC2/BSC1 protein abundance is increased by water restriction in older F344/BN and Sprague Dawley rats, but the increase is less than in younger rats (31,36). The decrease in NKCC2/BSC1 protein will reduce NaCl reabsorption across the thick ascending limb of the loop of Henle, thereby reducing the generation of a hypertonic medulla and urine concentrating ability.
The protein abundances of the β and γ subunits (70 kDa band) of the epithelial sodium channel (ENaC) are also reduced in F344/BN rats (36). Water restriction resulted in either no increase or a reduced increase, in the protein abundances of ENaC, the Na+-Cl− cotransporter (NCC/TSC), the sodium-protein exchanger 3 (NHE3), and the sodium pump Na+-K+-ATPase in F344/BN or Sprague Dawley rats (31,36). Thus, the reduced maximal urine osmolality in aged rats may also be related, at least in part, to the reduced abundances of these sodium transporter proteins. However, the interstitial concentration gradients for sodium (and urea) are similar in 4- and 23-month-old F344 rats, despite an impaired concentrating response to vasopressin, suggesting that the reduction in concentrating ability is due primarily to a decrease in water permeability in the collecting duct (37).
UREA TRANSPORTERS
Urea is the other major solute, in addition to NaCl, that contributes to medullary interstitial hyperosmolality and hence to urine concentrating ability (12,13,38). In rats, a diet with less than 8% protein results in a urine concentrating defect (39). Protein malnutrition reduces urine concentrating ability (40–44). Elderly individuals may be at risk for protein malnutrition, especially those on fixed incomes or reduced appetite due to other medical conditions. Two human (and two rat) urea transporter genes have been cloned: UT-A, which has six protein isoforms, and UT-B, which has two protein isoforms (reviewed in [45]). UT-A1 protein is expressed in the apical plasma membrane of the inner medullary collecting duct (46). UT-A3 protein is expressed in the same segment of the collecting duct as UT-A1 (47). Vasopressin increases urea permeability in the perfused terminal inner medullary collecting duct by increasing UT-A1 and UT-A3 phosphorylation and UT-A1 and UT-A3 accumulation in the apical plasma membrane (reviewed in [45]). Vasopressin phosphorylates UT-A1 at serines 486 and 499 (48). The abundance of both UT-A1 and UT-A3 proteins is significantly reduced in 30-month-old versus 10-month-old Wag/Rij rats (29,49); phosphorylation of serines 486 and 499 has not been studied to date (Figure 3).
Figure 3.
Urea transporters are reduced in 30-month-old Wag/Rij rats compared with 10-month-old rats. Panel A: UT-A1 protein abundance. Panel B: UT-A3 protein abundance. Panel C: UT-B protein abundance. Asterisk indicates a significant difference between 10- and 30-month-old rats. Data from references (29,45).
Administering a supraphysiologic concentration of dDAVP increases UT-A1 protein abundance in the 30-month-old rats but to a lesser degree than in the 10-month-old Wag/Rij rats (35). Water restricting 30-month-old rats increased inner medullary interstitial urea concentration but to a lesser degree than in the 10-month-old Wag/Rij rats (49). The reduced levels of UT-A1 and UT-A3 proteins will decrease urea reabsorption and inner medullary interstitial urea accumulation, thereby reducing the hyperosmolality of the inner medulla and urine concentrating ability. Thus, reductions in UT-A1 and UT-A3 protein abundances, along with reductions in water channel and sodium transporter protein abundances (discussed earlier), likely contribute to the reduced urine concentrating ability in the aged rats.
Changes in glucocorticoid levels may be a mechanism that contributes to the decrease in UT-A1 abundance in aged rats. Older (30-month-old) rats have elevated plasma corticosterone levels as compared with 10-month-old Wag/Rij rats (35). Glucocorticoids decrease UT-A1 transcription, mRNA abundance, and protein abundance (50,51). These findings suggest the possibility that increased glucocorticoid levels in aged rats may contribute to the reduction in UT-A1 protein, but this has not been tested experimentally.
UT-B protein is expressed in the descending vasa recta and on erythrocytes. A reduction in UT-B would reduce urine concentrating ability by decreasing intrarenal urea recycling and/or reducing the efficiency of countercurrent exchange. People who lack the Kidd blood group antigen, which is also UT-B, and knockout mice lacking UT-B are unable to concentrate their urine to normal levels (52,53). Thus, UT-B protein expression in the descending vasa recta and/or on erythrocytes is necessary to produce maximally concentrated urine (52,54–56).
UT-B protein abundance is significantly reduced in aged Wag/Rij rats (30,49), and administration of supraphysiological amounts of dDAVP increases it (35). Thus, the reduced level of UT-B protein is another factor that may contribute to reduced urine concentrating ability in aged rats and possibly humans.
SUMMARY
Urine concentrating ability is reduced during normal aging in people and rats. Many of the key proteins that contribute to urine concentrating ability, specifically the V2 receptor, AQP2, serine 256-phosphorylated AQP2, AQP3, NKCC2/BSC1, UT-A1, and UT-B, are reduced in the kidney medulla of aged rats. The reductions in the abundances of these proteins, and their reduced response to water restriction or administration of a supraphysiologic dose of dDAVP, may contribute to the reduced ability of aged rats to concentrate their urine and conserve water. If similar mechanisms occur in human kidneys, it would provide a molecular explanation for the reduced concentrating ability in aging and may provide opportunities for novel therapeutic approaches to improve urine concentrating ability and/or nocturnal polyuria.
FUNDING
This work was supported by National Institutes of Health grants R01-DK41707, R01-DK89828, and R21-DK91147.
Acknowledgments
A previous version of this review article was published as Sands JM, Urinary concentration and dilution in the aging kidney. Semin Nephrol. 29(6):579–586, copyright Elsevier Inc, 2009.
References
- 1.Rowe JW, Shock NW, DeFronzo RA. The influence of age on the renal response to water deprivation in man. Nephron. 1976;17:270–278. doi: 10.1159/000180731. [DOI] [PubMed] [Google Scholar]
- 2.Sporn IN, Lancestremere RG, Papper S. Differential diagnosis of oliguria in aged patients. N Engl J Med. 1962;267(3):130–132. doi: 10.1056/NEJM196207192670304. [DOI] [PubMed] [Google Scholar]
- 3.O’Neill PA, McLean KA. Water homeostasis and ageing. Med Lab Sci. 1992;49:291–298. [PubMed] [Google Scholar]
- 4.Geelen G, Corman B. Relationship between vasopressin and renal concentrating ability in aging rats. Am J Physiol Regul Integr Comp Physiol. 1992;262:R826–R833. doi: 10.1152/ajpregu.1992.262.5.R826. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen S, Frokiaer J, Marples D, Kwon ED, Agre P, Knepper M. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82:205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 6.Bagnasco SM. Gene structure of urea transporters. Am J Physiol Renal Physiol. 2003;284(1):F3–F10. doi: 10.1152/ajprenal.00260.2002. [DOI] [PubMed] [Google Scholar]
- 7.Knepper MA, Kim GH, Fernández-Llama P, Ecelbarger CA. Regulation of thick ascending limb transport by vasopressin. J Am Soc Nephrol. 1999;10(3):628–634. doi: 10.1681/ASN.V103628. [DOI] [PubMed] [Google Scholar]
- 8.Miller M. Nocturnal polyuria in older people: pathophysiology and clinical implications. J Am Geriatr Soc. 2000;48(10):1321–1329. doi: 10.1111/j.1532-5415.2000.tb02608.x. [DOI] [PubMed] [Google Scholar]
- 9.Johnson TM, II, Miller M, Pillion DJ, Ouslander JG. Arginine vasopressin and nocturnal polyuria in older adults with frequent nighttime voiding. J Urol. 2003;170(2, pt 1):480–484. doi: 10.1097/01.ju.0000071406.18453.5f. [DOI] [PubMed] [Google Scholar]
- 10.Johnson TMI, Sands JM, Ouslander JG. A prospective evaluation of the glomerular filtration rate in older adults with frequent nighttime urination. J Urol. 2002;167(1):146–150. [PubMed] [Google Scholar]
- 11.Sands JM, Layton HE, Fenton RA. Urine concentration and dilution. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu ASL, Brenner BM, editors. Brenner and Rector's The Kidney. 9th ed. Philadelphia, PA: Elsevier; 2011. pp. 326–352. [Google Scholar]
- 12.Kokko JP, Rector FC. Countercurrent multiplication system without active transport in inner medulla. Kidney Int. 1972;2:214–223. doi: 10.1038/ki.1972.97. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson JL. Concentration of urine in a central core model of the renal counterflow system. Kidney Int. 1972;2:85–94. doi: 10.1038/ki.1972.75. [DOI] [PubMed] [Google Scholar]
- 14.Bankir L. Antidiuretic action of vasopressin: quantitative aspects and interaction between V1a and V2 receptor-mediated effects. Cardiovasc Res. 2001;51(3):372–390. doi: 10.1016/s0008-6363(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 15.Hasler U, Leroy V, Martin PY, Feraille E. Aquaporin-2 abundance in the renal collecting duct: new insights from cultured cell models. Am J Physiol Renal Physiol. 2009;297:F10–F18. doi: 10.1152/ajprenal.00053.2009. [DOI] [PubMed] [Google Scholar]
- 16.Brown D, Fenton RA. The cell biology of vasopressin action. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu ASL, Brenner BM, editors. Brenner and rector's The Kidney. 9th ed. Philadelphia, PA: Elsevier; 2011. pp. 353–383. [Google Scholar]
- 17.Tian Y, Serino R, Verbalis JG. Downregulation of renal vasopressin V2 receptor and aquaporin-2 expression parallels age-associated defects in urine concentration. Am J Physiol Renal Physiol. 2004;287(4):F797–F805. doi: 10.1152/ajprenal.00403.2003. [DOI] [PubMed] [Google Scholar]
- 18.Preisser L, Teillet L, Aliotti S, et al. Downregulation of aquaporin-2 and-3 in aging kidney is independent of V2 vasopressin receptor. Am J Physiol Renal Physiol. 2000;279(1):F144–F152. doi: 10.1152/ajprenal.2000.279.1.F144. [DOI] [PubMed] [Google Scholar]
- 19.Terashima Y, Kondo K, Inagaki A, et al. Age-associated decrease in response of rat aquaporin-2 gene expression to dehydration. Life Sci. 1998;62(10):873–882. doi: 10.1016/s0024-3205(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 20.Klingler C, Preisser L, Barrault MB, et al. Vasopressin V2 receptor mRNA expression and cAMP accumulation in aging rat kidney. Am J Physiol Regul Integr Comp Physiol. 1997;272(6):R1775–R1782. doi: 10.1152/ajpregu.1997.272.6.R1775. [DOI] [PubMed] [Google Scholar]
- 21.Beck N, Yu BP. Effect of aging on urinary concentrating mechanism and vasopressin-dependent cAMP in rats. Am J Physiol Renal Physiol. 1982;243:F121–F125. doi: 10.1152/ajprenal.1982.243.2.F121. [DOI] [PubMed] [Google Scholar]
- 22.Combet S, Gouraud S, Gobin R, et al. Aquaporin-2 downregulation in kidney medulla of aging rats is posttranscriptional and is abolished by water deprivation. Am J Physiol Renal Physiol. 2008;294(6):F1408–F1414. doi: 10.1152/ajprenal.00437.2007. [DOI] [PubMed] [Google Scholar]
- 23.Brown D. The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol. 2003;284:F893–F901. doi: 10.1152/ajprenal.00387.2002. [DOI] [PubMed] [Google Scholar]
- 24.Hoffert JD, Fenton RA, Moeller HB, et al. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem. 2008;283(36):24617–24627. doi: 10.1074/jbc.M803074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffert JD, Pisitkun T, Wang GH, Shen RF, Knepper MA. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol. 2007;292(2):F691–F700. doi: 10.1152/ajprenal.00284.2006. [DOI] [PubMed] [Google Scholar]
- 26.Hoffert JD, Pisitkun T, Wang G, Shen R-F, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci U S A. 2006;103(18):7159–7164. doi: 10.1073/pnas.0600895103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fenton RA, Moeller HB, Hoffert JD, Yu MJ, Nielsen S, Knepper MA. Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc Natl Acad Sci U S A. 2008;105(8):3134–3139. doi: 10.1073/pnas.0712338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wade JB, Stetson DL, Lewis SA. ADH action: evidence for a membrane shuttle mechanism. Ann N Y Acad Sci. 1981;372:106–117. doi: 10.1111/j.1749-6632.1981.tb15464.x. [DOI] [PubMed] [Google Scholar]
- 29.Schlanger LE, Bailey JL, Sands JM. Electrolytes in the Aging. Adv Chronic Kid Dis. 2010;17:308–319. doi: 10.1053/j.ackd.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Combet S, Teillet L, Geelen G, et al. Food restriction prevents age-related polyuria by vasopressin-dependent recruitment of aquaporin-2. Am J Physiol Renal Physiol. 2001;281(6):F1123–F1131. doi: 10.1152/ajprenal.0139.2001. [DOI] [PubMed] [Google Scholar]
- 31.Amlal H, Wilke C. Resistance of mTAL Na+-dependent transporters and collecting duct aquaporins to dehydration in 7-month-old rats. Kidney Int. 2003;64(2):544–554. doi: 10.1046/j.1523-1755.2003.00110.x. [DOI] [PubMed] [Google Scholar]
- 32.Swenson KL, Sands JM, Jacobs JD, Sladek CD. Effect of aging on the vasopressin and aquaporin responses to dehydration in Fischer 344/Brown-Norway F1 rats. Am J Physiol. 1997;273(1):R35–R40. doi: 10.1152/ajpregu.1997.273.1.R35. [DOI] [PubMed] [Google Scholar]
- 33.Catudioc-Vallero J, Sands JM, Sidorowicz HE, Klein JD, Sladek CD. Effect of age and testosterone in the vasopressin response to dehydration in F344BNF1 male rats. Adv Exp Med Biol. 1998;449:183–185. doi: 10.1007/978-1-4615-4871-3_22. [DOI] [PubMed] [Google Scholar]
- 34.Arreche N, Fellet A, Lopez M, Lopez-Costa J, Arranz C, Balaszczuk AM. Hypovolemic state: involvement of nitric oxide in the aged related alterations of aquaporins-2 abundance in rat kidney. Vascul Pharmacol. 2008;49:19–25. doi: 10.1016/j.vph.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Combet S, Geffroy N, Berthonaud V, et al. Correction of age-related polyuria by dDAVP: molecular analysis of aquaporins and urea transporters. Am J Physiol Renal Physiol. 2003;284(1):F199–F208. doi: 10.1152/ajprenal.00167.2002. [DOI] [PubMed] [Google Scholar]
- 36.Tian Y, Riazi S, Khan O, et al. Renal ENaC subunit, Na-K-2Cl and Na-Cl cotransporter abundances in aged, water-restricted F344 x Brown Norway rats. Kidney Int. 2006;69(2):304–312. doi: 10.1038/sj.ki.5000076. [DOI] [PubMed] [Google Scholar]
- 37.Bengele HH, Mathias RS, Perkins JH, Alexander EA. Urinary concentrating defect in the aged rat. Am J Physiol Renal Physiol. 1981;240:F147–F150. doi: 10.1152/ajprenal.1981.240.2.F147. [DOI] [PubMed] [Google Scholar]
- 38.Gamble JL, McKhann CF, Butler AM, Tuthill E. An economy of water in renal function referable to urea. Am J Physiol. 1934;109:139–154. [Google Scholar]
- 39.Schmidt-Nielsen B, Barrett JM, Graves B, Crossley B. Physiological and morphological responses of the rat kidney to reduced dietary protein. Am J Physiol. 1985;248:F31–F42. doi: 10.1152/ajprenal.1985.248.1.F31. [DOI] [PubMed] [Google Scholar]
- 40.Levinsky NG, Berliner RW. The role of urea in the urine concentrating mechanism. J Clin Invest. 1959;38:741–748. doi: 10.1172/JCI103854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peil AE, Stolte H, Schmidt-Nielsen B. Uncoupling of glomerular and tubular regulations of urea excretion in rat. Am J Physiol. 1990;258:F1666–F1674. doi: 10.1152/ajprenal.1990.258.6.F1666. [DOI] [PubMed] [Google Scholar]
- 42.Epstein FH, Kleeman CR, Pursel S, Hendrikx A. The effect of feeding protein and urea on the renal concentrating process. J Clin Invest. 1957;36:635–641. doi: 10.1172/JCI103463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klahr S, Alleyne GAO. Effects of chronic protein-calorie malnutrition on the kidney. Kidney Int. 1973;3:129–141. doi: 10.1038/ki.1973.21. [DOI] [PubMed] [Google Scholar]
- 44.Hendrikx A, Epstein FH. Effect of feeding protein and urea on renal concentrating ability in the rat. Am J Physiol. 1958;195(3):539–542. doi: 10.1152/ajplegacy.1958.195.3.539. [DOI] [PubMed] [Google Scholar]
- 45.Klein JD, Blount MA, Sands JM. Urea transport in the kidney. Compr Physiol. 2011;1(2):699–729. doi: 10.1002/cphy.c100030. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen S, Terris J, Smith CP, Hediger MA, Ecelbarger CA, Knepper MA. Cellular and subcellular localization of the vasopressin-regulated urea transporter in rat kidney. Proc Natl Acad Sci U S A. 1996;93:5495–5500. doi: 10.1073/pnas.93.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blount MA, Klein JD, Martin CF, Tchapyjnikov D, Sands JM. Forskolin stimulates phosphorylation and membrane accumulation of UT-A3. Am J Physiol Renal Physiol. 2007;293(4):F1308–F1313. doi: 10.1152/ajprenal.00197.2007. [DOI] [PubMed] [Google Scholar]
- 48.Blount MA, Mistry AC, Fröhlich O, et al. Phosphorylation of UT-A1 urea transporter at serines 486 and 499 is important for vasopressin-regulated activity and membrane accumulation. Am J Physiol Renal Physiol. 2008;295(1):F295–F299. doi: 10.1152/ajprenal.00102.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trinh-Trang-Tan MM, Geelen G, Teillet L, Corman B. Urea transporter expression in aging kidney and brain during dehydration. Am J Physiol Regul Integr Comp Physiol. 2003;285(6):R1355–R1365. doi: 10.1152/ajpregu.00207.2003. [DOI] [PubMed] [Google Scholar]
- 50.Peng T, Sands JM, Bagnasco SM. Glucocorticoids inhibit transcription and expression of the rat UT-A urea transporter gene. Am J Physiol Renal Physiol. 2002;282(5):F853–F858. doi: 10.1152/ajprenal.00262.2001. [DOI] [PubMed] [Google Scholar]
- 51.Naruse M, Klein JD, Ashkar ZM, Jacobs JD, Sands JM. Glucocorticoids downregulate the rat vasopressin-regulated urea transporter in rat terminal inner medullary collecting ducts. J Am Soc Nephrol. 1997;8(4):517–523. doi: 10.1681/ASN.V84517. [DOI] [PubMed] [Google Scholar]
- 52.Sands JM, Gargus JJ, Fröhlich O, Gunn RB, Kokko JP. Urinary concentrating ability in patients with Jk(a-b-) blood type who lack carrier-mediated urea transport. J Am Soc Nephrol. 1992;2:1689–1696. doi: 10.1681/ASN.V2121689. [DOI] [PubMed] [Google Scholar]
- 53.Yang B, Bankir L, Gillespie A, Epstein CJ, Verkman AS. Urea-selective concentrating defect in transgenic mice lacking urea transporter UT-B. J Biol Chem. 2002;277:10633–10637. doi: 10.1074/jbc.M200207200. [DOI] [PubMed] [Google Scholar]
- 54.Macey RI, Yousef LW. Osmotic stability of red cells in renal circulation requires rapid urea transport. Am J Physiol. 1988;254:C669–C674. doi: 10.1152/ajpcell.1988.254.5.C669. [DOI] [PubMed] [Google Scholar]
- 55.Edwards A, Pallone TL. Facilitated transport in vasa recta: theoretical effects on solute exchange in the medullary microcirculation. Am J Physiol Renal Physiol. 1997;272(4):F505–F514. doi: 10.1152/ajprenal.1997.272.4.F505. [DOI] [PubMed] [Google Scholar]
- 56.Edwards A, Pallone TL. A multiunit model of solute and water removal by inner medullary vasa recta. Am J Physiol Heart Circ Physiol. 1998;274(4):H1202–H1210. doi: 10.1152/ajpheart.1998.274.4.H1202. [DOI] [PubMed] [Google Scholar]