Abstract
The nematode C. elegans has during the past decade proven to be a valuable model organism to identify and examine molecular mechanisms regulating lipid storage and metabolism. While the primary approach has been to identify genes and pathways conferring alterations in lipid accumulation, only a few recent studies have recognized the central role of fatty acid degradation in cellular lipid homeostasis. In the present study, we show how complete oxidation of fatty acids can be determined in live C. elegans by examining oxidation of tritium-labeled fatty acids to tritiated H2O that can be measured by scintillation counting. Treating animals with sodium azide, an inhibitor of the electron transport chain, reduced 3H2O production to approximately 15%, while boiling of animals prior to assay completely blocked the production of labeled water. We demonstrate that worms fed different bacterial strains exhibit different fatty acid oxidation rates. We show that starvation results in increased fatty acid oxidation, which is independent of the transcription factor NHR-49. On the contrary, fatty acid oxidation is reduced to approximately 70% in animals lacking the worm homolog of the insulin receptor, DAF-2. Hence, the present methodology can be used to delineate the role of specific genes and pathways in the regulation of β-oxidation in C. elegans.
Keywords: C. elegans, Fatty acid oxidation, bacterial diet, daf-2, nhr-49
Introduction
Any living organism must be able to regulate its production, utilization, and storage of energy. If more energy is consumed than expended, the excess energy will be stored in the body, mainly in triacylglycerols, potentially leading to obesity and related disorders. During times of nutrient scarcity, these fat depots can be catabolized to release energy. During fasting, lipases hydrolyze triacylglycerol to yield glycerol and free fatty acids. Fatty acids must be activated to acyl-CoA esters by acyl-CoA synthetases to participate in both anabolic and catabolic processes. Acyl-CoA esters destined for ATP production are transported to the mitochondria or peroxisomes for β-oxidation. In eukaryotes, it is commonly accepted that very long-chain fatty acids (VLCFAs) (> C20) are almost exclusively metabolized in peroxisomes, because only peroxisomes possess the acyl-CoA oxidases necessary to initiate the degradation of very long-chain fatty acyl-CoA esters. After approximately ten rounds of β-oxidation, the shortened acyl-CoAs are transported to the mitochondria, where these, along with other short- (< C8), medium- (C8-C12), and long-chain (C12-C20) fatty acids, are degraded to C2 acetyl-CoA moieties by mitochondrial β-oxidation (reviewed in refs. 1 and 2). Subsequently, acetyl-CoA enters the citric acid cycle to generate NADH and FADH2, which eventually transfer electrons to the electron transport chain to generate a proton motive force that drives the generation of ATP.
Dys-regulation of fatty acid degradation leads to a range of disorders generally associated with either energetic deficiency or the toxicity of accumulating metabolic substrates or intermediates. Mitochondrial β-oxidation is comprised of at least 25 enzymes and transport proteins, and deficiencies in 18 of them have been demonstrated to cause diseases in humans.3,4 Lack of functional peroxisomes results in Zellweger syndrome, a rare congenital disorder in which accumulation of VLCFAs and branched-chain fatty acids as well as lack of ether lipids results in impaired brain development, hypomyelination, and impaired function of various organs.5 Moreover, the worldwide increase in the prevalence of overweight and obesity has intensified the search to identify genes that control the development, differentiation, and function of fat-storing tissues. A number of invertebrate genetic model systems including C. elegans have accelerated the discovery of new genes important for maintaining lipid homeostasis including evolutionary conserved signaling pathways like insulin-, TGF-β-, and serotonin-signaling, as well as several transcription factors; SREBP, C/EBP, KLF and PPARα.6 However, while these studies have primarily addressed how lipid accumulation is affected in response to genetic perturbations, the degradation of fatty acids has only been addressed indirectly via transcriptional analyses.
In the present work, we have established a method to determine complete fatty acid oxidation in living C. elegans and show how this method can be applied to address how various perturbations affect fatty acid oxidation in C. elegans.
Results
In an effort to identify factors that are important for fatty acid oxidation in living C. elegans we have established an assay that monitors the oxidation of tritiated fatty acids to tritiated H2O in C. elegans. A simplified illustration of the biochemical process is shown in Figure 1. The formation of 3H2O from oxidation of oleic acid was found to be linear up to approximately 1,400 μg of C. elegans protein in each sample, at which point the assay began to saturate (results not shown). Using this assay we have found that the specific activity for palmitic acid and oleic acid is 0.90 ± 0.17 (n = 12) and 0.70 ± 0.32 (n = 34) pmol fatty acid oxidized/min/mg protein, respectively, as shown in Figure 2A. To further validate the assay, we killed animals by boiling (15 min at 95°C) which completely ablated the generation of labeled water from palmitic acid (Fig. 2B). Moreover, addition of 10 mM sodium azide, a potent inhibitor of Complex IV of the electron transport chain, to live C. elegans inhibited the generation of labeled H2O from oleic acid to approximately 15% of untreated worms (Fig. 2C).
Figure 1.
Schematic illustration of the fatty acid oxidation assay in live C. elegans. The radioactively labeled fatty acids are complexed to BSA in order to be soluble in water-based buffer. Worms ingest FA-BSA complexes and the labeled fatty acids are absorbed by intestinal cells. Once imported, the labeled fatty acids are activated to acyl-CoA esters, which are transported across the mitochondrial double membrane by the carnitine shuttle system. In the mitochondrial matrix, the labeled acyl-CoA esters are metabolized through β-oxidation, the citric acid cycle and the electron transport chain to ultimately produce tritiated water.
Figure 2.
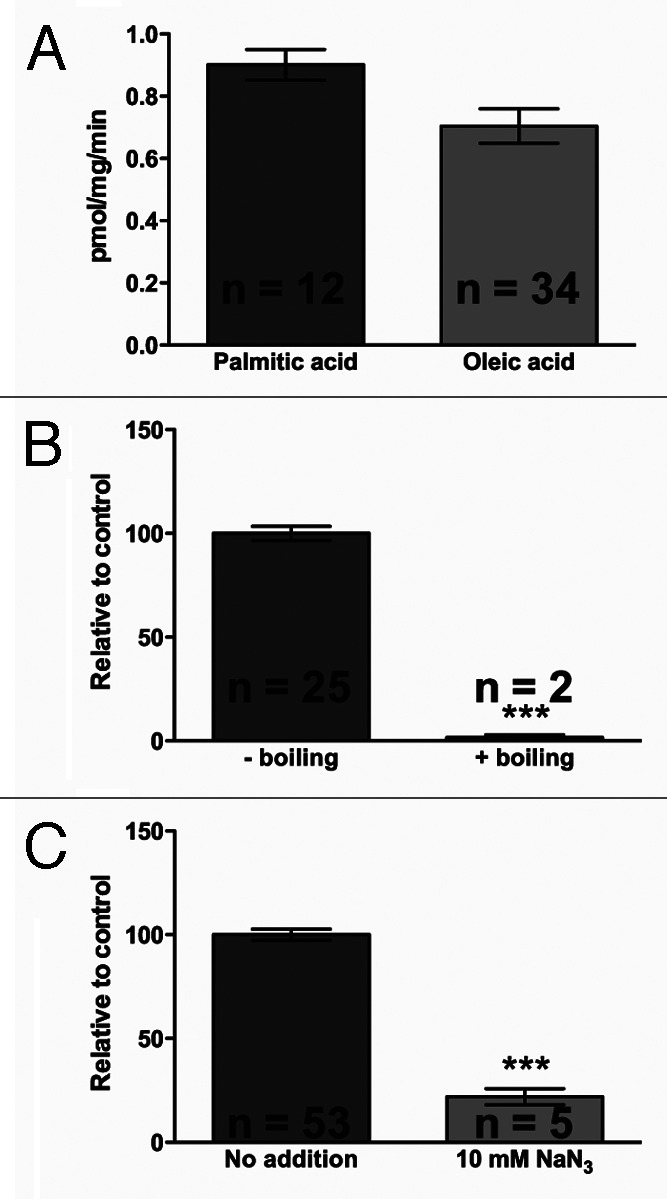
Control experiments. (A) Specific activity of palmitic or oleic acid oxidiation in C. elegans wild type animals. (B) β-oxidation of palmitic acid in live and heat-killed worms. L4 worms were incubated with tritiated oleic acid complexed to BSA for one hour, and the amount of tritiated water generated by oxidation was determined, normalized to protein levels, and expressed relative to control levels. For the boiling experiments, worms were boiled in 5 mL S-basal for 15 min at 95°C. (C) β-oxidation of oleic acid in controls vs. azide-treated worms. Azide to 10mM was added to the samples just after addition of assay mix. Average ± SEM is shown; n indicates the number of determinations. Asterisks indicate statistical significance (***: p < 0.0001).
Assay Applications
To demonstrate the relevance of our assay, we investigated changes in β-oxidation rates in starved animals, mutants previously shown to have altered lipid metabolism and in response to different bacterial diets.
When deprived of food, many organisms engage a set of evolutionary conserved behavioral, physiological and structural responses to reduce overall metabolism, which involves the activation of lipolysis and fatty acid degradation.7 Hence, we hypothesized that food deprivation would result in increased degradation of fatty acids. Therefore, we starved worms for three or six hours to subsequently determine the level of labeled H2O generated from oxidation of oleic acid. Consistent with our hypothesis, we found that wild-type animals subjected to three hours of starvation, increased oxidation of exogenous oleic acid by 2.5-fold compared with fed animals (Fig. 3). Oxidation was not increased further after six hours of food deprivation, indicating that the maximum fatty acid oxidation capacity is reached after only three hours.
Figure 3.

β-oxidation of oleic acid increases during starvation. L4 worms were incubated with tritiated oleic acid complexed to BSA for one hour, and the amount of tritiated water generated by oxidation was determined, normalized to protein levels, and expressed relative to control levels. The starvation experiments were performed, when non-starved animals were L4 (see Experimental Section for details). Average ± SEM is shown; n indicates the number of determinations. Asterisks represent statistical significance (**: p < 0.01; ***: p ≤ 0.0001). Black asterisks indicate a significant difference from non-starved mutants.
The expression of genes involved in fatty acid degradation in C. elegans is orchestrated by a number of transcription factors including the nuclear hormone receptor NHR-49, which is orthologous to the mammalian HNF-4α nuclear hormone receptor and functionally similar to peroxisome proliferator-activated receptor PPARα. Since NHR-49 has previously been shown to be required for full induction of β-oxidation genes in response to starvation,8 we hypothesized that animals lacking functional NHR-49 would have decreased capacity to degrade exogenous fatty acids. However, we found that disruption of NHR-49 function did not affect oleic acid oxidation under fed conditions (Fig. 3). We also found that nhr-49 animals are able to upregulate their ability to degrade exogenous oleic acid in response to three hours starvation to a similar level as wild-type animals.
Ablation of insulin receptor function (e.g., daf-2 mutants) has been shown to result in increased stress resistance, longevity, and accumulation of triacylglycerols compared with wild-type N2 animals.9 Not surprisingly, we found that daf-2 animals oxidize approximately 30% less oleic acid under fed conditions compared with N2 (Fig. 3). However, relative to their fatty acid oxidation under fed conditions, oxidation of oleic acid increased to a similar extent in response to starvation.
It has recently been shown that different bacterial diets have profound effects on C. elegans lipid storage and composition10,11 that may be associated to the presence of vaccenic acid (C18:1n7) in the bacterial diet.11 While these studies addressed only the amount of triacylglycerols in C. elegans cultured on different bacterial strains, they did not determine their ability to degrade fatty acids. OP50 is the bacterial strain most commonly used as C. elegans food source in the laboratory. It is derived from E. coli B and is a uracil auxotroph and therefore growth-limited. HB101 is another E. coli strain used in the laboratory, which is a hybrid of E. coli strains K-12 and B and forms a thicker lawn than OP50. HB101 is characterized by having a high content of carbohydrates, increased monounsaturated fatty acids and decreased cyclopropane fatty acids.10
When animals were fed HB101, oxidation of oleic acid increased significantly compared with animals fed OP50 (Fig. 4). Although other factors are likely to contribute to storage of triacylglycerols, increased fatty acid oxidation may contribute to less triacylglycerol accumulation seen in animals feeding on HB101 compared with OP50-fed animals.
Figure 4.

β-oxidation of oleic acid is increased in worms grown on HB101 compared with standard OP50 diet. N2 animals were grown on OP50 or HB101 for one generation. L4 animals were incubated with tritiated oleic acid complexed to BSA for one hour, and the amount of tritiated water generated by oxidation was determined, normalized to protein levels, and expressed relative to control levels. Average ± SEM is shown; n indicates the number of determinations. Asterisks represent statistical significance (**: p < 0.01).
Discussion
In the present work, we describe how oxidation of long-chain fatty acids can be determined in live C. elegans. We have used this assay to show that fatty acid oxidation increases, as expected, when animals are deprived of food for three and six hours. Surprisingly, we found that loss of NHR-49 function affected neither fatty acid oxidation in fed worms, nor oxidation in response to starvation. Although we and others12,13 have shown that NHR-49 is required for expression of genes involved in fatty acid degradation, the present observation suggests that other factors than NHR-49 are required for induction of β-oxidation enzymes during starvation. This is consistent with the fact that van Gilst et al. found the expression of only 7 of the 18 fasting-regulated genes is impaired upon nhr-49 deletion.8 We have recently shown by quantitative proteomics that knock down of nhr-49 results in reduced levels of enzymes involved in β-oxidation, which are predicted to reside primarily in the peroxisomes, suggesting that NHR-49 is required for degradation of fatty acids in the peroxisomes.13 Since degradation of very long-chain fatty acids predominantly takes place in the peroxisomes,14 NHR-49 may mainly be required for peroxisomal β-oxidation of very long-chain fatty acids rather than mitochondrial degradation of long-chain fatty acids as oleic acid. Furthermore, as our assay relies on the degradation of exogenous fatty acids, we cannot rule out that loss of NHR-49 function primarily affects degradation of fatty acids from internal lipid stores.
Interestingly, we found that impaired insulin signaling lowers fatty acid oxidation in fed animals, but does not affect induction of β-oxidation in response to starvation. These data are in line with a recent study by Brys et al. demonstrating that disruption of insulin signaling [daf-2 (e1370)] increases the efficiency of aerobic ATP production and preserves the bio-energetic competence of mitochondria in aging worms.15 A relevant question in relation to the daf-2 metabolic phenotype is whether daf-2 worms live longer because they β-oxidize less and therefore produce less ROS, or if they β-oxidize less to preserve adequate energy stores for their long lifespan. The results obtained by Brys et al. indicate that the latter is more plausible, since aging daf-2 worms actually have an increased ROS production compared with aging N2 animals but with little mitochondrial damage.15
Collectively, in the present work, we describe a method to directly determine oxidation of exogenously supplied fatty acids in C. elegans and verified that this assay can be used to examine the effect of various inhibitors and stimulators on fatty acid oxidation. Fatty acid degradation in C. elegans has previously been examined indirectly by measuring the expression level of β-oxidation enzymes by quantitative PCR,8 however, mRNA levels may not relate directly to β-oxidation rates as enzyme activities also are regulated at the post-transcriptional level. Moreover, substrate availability may also change the overall flux through a specific pathway independent of the mRNA levels of specific enzymes.
We show that fatty acid oxidation is upregulated in response to food deprivation independent of the transcription factor NHR-49. The present method provides an overall measurement of the complete oxidation of fatty acid to H2O and CO2. The assay does not allow us to distinguish between changes in fatty acid uptake, import to mitochondria, β-oxidation, citric acid cycle, or the electron transport chain, that may affect the amount of tritiated H2O generated by complete oxidation of fatty acids. The activities of these pathways/cellular processes should therefore be determined by other means, e.g., Mullaney et al. have recently shown that short-term incubation with fluorescently labeled fatty acids can be used to assay fatty acid uptake in C. elegans.16 Further work is also needed, before we can quantitatively determine oxidation of fatty acids derived from endogenous storage- and structural lipids. Such a methodology would clearly complement the described assay and would undoubtedly contribute to a more detailed understanding of lipid metabolism and disorders caused by alterations in cellular fatty acid homeostasis.
Materials and Methods
Culturing and handling of C. elegans and E. coli strains
Worms were cultured using standard protocols17 on plates seeded with different bacterial strains including OP50. Wild-type N2 Bristol and daf-2(e1370) were obtained from the Caenorhabditis elegans Genetics Center. The nhr-49(nr2041) mutant was kindly provided by Dr. Kaveh Ashrafi, UCSF Mission Bay Campus, San Francisco, USA.
Determination of complete oxidation of fatty acid to H2O
Synchronized L4 stage worms were washed off five to ten 9 cm plates into 15 mL Falcon tubes and washed three times in 0.9% NaCl. Worms were incubated end-over-end for 20 min to empty their intestines and washed once in sterile S-basal (5.85 g/L NaCl, 1 g/L K2 HPO4, 6 g/L KH2PO4, 5 ug/mL cholesterol). Worms were allowed to settle at the bottom of the tubes, and 520 µl were transferred to a 2.0 ml centrifuge tube. From each sample, 20 µl were transferred to 1.5 mL centrifuge tubes, which received 80 µl of water and were subsequently stored at -80°C for protein determination. Blanks, containing only S-basal, were treated as samples. The assay mix was prepared as follows: [9,10-3H(N)] labeled- (Perkin Elmer, specific activity 45–52 Ci/mmol) and unlabeled fatty acid were mixed 1:150 and dried under N2. The fatty acid was then saponified by the addition of 1.1 molar excess NaOH and subsequently complexed to fatty acid-free BSA in a 4:1 ratio dissolved in phosphate-buffered saline to reach a final concentration of 20 mM. The labeled fatty acid was added to C. elegans to a final concentration of 20 μM, and samples were incubated end-over-end for one hour at ambient temperature. After incubation, 540 µL freshly prepared 10% w/v TCA were added, and the samples were vortexed briefly. Samples were centrifuged at 9,300 x g for 5 min at room temperature, and the supernatants were transferred to fresh tubes. PBS (250 µl) and 5M NaOH (100 µl) were added, and the samples were vortexed briefly and loaded onto freshly prepared 1ml Dowex 1 × 8 200–400 MESH CI columns. Samples were eluted using water (1 ml) directly into scintillation tubes. The amount of tritiated H2O generated was determined by scintillation counting (DPM for 10 min.) and expressed as pmol oxidized fatty acid per mg protein per min. Data were analyzed in GraphPad Prism 5 (GraphPad Software) using Student’s t-tests.
Two negative controls were included; heat-killed worms and worms treated with NaN3. To heat-kill animals, worms were suspended in 5 ml S-basal, transferred to a glass tube and left for 15–20 min. at 95°C, while control worms emptied their intestines. To inhibit respiration with NaN3, animals were incubated with 10 mM NaN3, which was added to the samples just after the addition of labeled fatty acids. When assaying worms, which are slightly developmentally delayed compared with N2 [nhr-49(nr2041) and daf-2(e1370)], synchronized nhr-49 L1 larvae were plated three hours before N2, while daf-2 were plated 12 h before N2 to ensure that all worms were L4 at the time of the experiment. The starvation experiments were timed so that non-starved worms were L4 at the time of the experiment. To starve worms, late L3-early L4 animals were washed and placed on unseeded plates for three or six hours before they were assayed.
Protein determination
Fatty acid oxidation rates were normalized to the total protein content of the samples. Protein samples were diluted in sonication buffer (10 mM TRIS-HCl pH 7.5, 40 mM NaCl, 0.1% SDS) (200 µl) and sonicated 3 × 30 sec at maximum intensity in a Bioruptor UCD-200 (Diagenode). Additional sonication buffer (200 µl), was added, and the samples were incubated end-over-end at 4°C for one hour. Samples were spun 18,000 x g for 5 min at 4°C, and protein concentrations were measured using the Qubit Quant-iT® Protein Assay kit according to the manufacturer (Invitrogen).
Acknowledgments
We thank Anne Sofie Braun Olsen (Department of Biochemistry and Molecular Biology, University of Southern Denmark) for excellent technical assistance. This work was supported by funds from The Danish Research Councils and The Novo Nordisk Foundation.
Glossary
Abbreviations:
- C/EBP
CCAAT/enhancer binding protein
- DPM
disintegrations per minute
- FA
fatty acid
- KLF
Krüppel-like factor
- PPARα
peroxisome proliferator-activated receptor α
- SREBP
sterol regulatory element binding protein
- TGF-β
transforming growth factor β
- VLCFA
very long-chain fatty acid
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/19564
References
- 1.Reddy JK, Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu Rev Nutr. 2001;21:193–230. doi: 10.1146/annurev.nutr.21.1.193. [DOI] [PubMed] [Google Scholar]
- 2.Wanders RJ, Ruiter JP, IJLst L, Waterham HR, Houten SM. The enzymology of mitochondrial fatty acid beta-oxidation and its application to follow-up analysis of positive neonatal screening results. J Inherit Metab Dis. 2010;33:479–94. doi: 10.1007/s10545-010-9104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillingham MB, Purnell JQ, Jordan J, Stadler D, Haqq AM, Harding CO. Effects of higher dietary protein intake on energy balance and metabolic control in children with long-chain 3-hydroxy acyl-CoA dehydrogenase (LCHAD) or trifunctional protein (TFP) deficiency. Mol Genet Metab. 2007;90:64–9. doi: 10.1016/j.ymgme.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moczulski D, Majak I, Mamczur D. An overview of beta-oxidation disorders. Postepy Hig Med Dosw (Online) 2009;63:266–77. [PubMed] [Google Scholar]
- 5.Steinberg SJ, Dodt G, Raymond GV, Braverman NE, Moser AB, Moser HW. Peroxisome biogenesis disorders. Biochim Biophys Acta 2006; 1763:1733-48. [DOI] [PubMed]
- 6.Elle IC, Olsen LC, Pultz D, Rødkaer SV, Faergeman NJ. Something worth dyeing for: molecular tools for the dissection of lipid metabolism in Caenorhabditis elegans. FEBS Lett. 2010;584:2183–93. doi: 10.1016/j.febslet.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 7.Wang T, Hung CC, Randall DJ. The comparative physiology of food deprivation: from feast to famine. Annu Rev Physiol. 2006;68:223–51. doi: 10.1146/annurev.physiol.68.040104.105739. [DOI] [PubMed] [Google Scholar]
- 8.Van Gilst MR, Hadjivassiliou H, Yamamoto KR. A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc Natl Acad Sci U S A. 2005;102:13496–501. doi: 10.1073/pnas.0506234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs S, Bundy JG, Davies SK, Viney JM, Swire JS, Leroi AM. A metabolic signature of long life in Caenorhabditis elegans. BMC Biol. 2010;8:14. doi: 10.1186/1741-7007-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks KK, Liang B, Watts JL. The influence of bacterial diet on fat storage in C. elegans. PLoS One. 2009;4:e7545. doi: 10.1371/journal.pone.0007545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang SO, Box AC, Xu N, Le Men J, Yu J, Guo F, et al. Genetic and dietary regulation of lipid droplet expansion in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2010;107:4640–5. doi: 10.1073/pnas.0912308107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Gilst MR, Hadjivassiliou H, Jolly A, Yamamoto KR. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol. 2005;3:e53. doi: 10.1371/journal.pbio.0030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredens J, Engholm-Keller K, Giessing A, Pultz D, Larsen MR, Højrup P, et al. Quantitative proteomics by amino acid labeling in C. elegans. Nat Methods. 2011;8:845–7. doi: 10.1038/nmeth.1675. [DOI] [PubMed] [Google Scholar]
- 14.Lazarow PB. The role of peroxisomes in mammalian cellular metabolism. J Inherit Metab Dis. 1987;10(Suppl 1):11–22. doi: 10.1007/BF01812843. [DOI] [PubMed] [Google Scholar]
- 15.Brys K, Castelein N, Matthijssens F, Vanfleteren JR, Braeckman BP. Disruption of insulin signalling preserves bioenergetic competence of mitochondria in ageing Caenorhabditis elegans. BMC Biol. 2010;8:91. doi: 10.1186/1741-7007-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullaney BC, Blind RD, Lemieux GA, Perez CL, Elle IC, Faergeman NJ, et al. Regulation of C. elegans fat uptake and storage by acyl-CoA synthase-3 is dependent on NR5A family nuclear hormone receptor nhr-25. Cell Metab. 2010;12:398–410. doi: 10.1016/j.cmet.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]



