Abstract
Thermotaxis is a model to elucidate how nervous systems sense and memorize environmental conditions to regulate behavioral strategies in Caenorhabditis elegans. The genetic and neural imaging analyses revealed molecular and cellular bases of this experience-dependent behavior. Surprisingly, thermosensory neurons themselves memorize the sensed temperatures. Recently developed techniques for optical manipulation of neuronal activity have facilitated the revelation that there is a sophisticated information flow between sensory neurons and interneurons. Further studies on thermotaxis will allow us to understand the fundamental logics of neural processing from sensory perceptions to behavioral outputs.
Keywords: behavior, learning and memory, neural plasticity, optogenetics, sensory signal transduction
Introduction
How the nervous system regulates animal behavior depending on environmental stimuli is a fundamental question in biology. Because of the anatomically characterized nervous system that is composed of only 302 neurons, C. elegans is suitable for dissecting behavioral regulation at a small circuit and single neuronal level. Despite its simple nervous system, C. elegans perceives a number of environmental stimuli, such as chemicals, mechanical stretch, light, and temperature, and produce appropriate behavioral outputs by utilizing different sets of behavioral strategies.1-4
Hedgecock and Russell (1975) first reported an interesting behavior, in which the animals remember the ambient temperature in association with its past cultivation conditions, and migrate to and move isothermally around that temperature on a temperature gradient.5 Since this behavior, called thermotaxis, is experience-dependent, thermotaxis provides a great opportunity to study sensory perceptions, behavioral regulations, neural plasticity such as learning and memory and so forth.6-8 Through the application of calcium imaging and optogenetics in addition to molecular genetics, our knowledge of these mechanisms has advanced in the last several years. In this review, we first discuss different thermotaxis responses observed in different assay systems. Second, we describe the recent findings of thermosensory neurons acting both as a temperature signal receptor and a memory device, new thermosensation systems and the complex flow of temperature information from thermosensory neurons to downstream interneurons. Finally, we show the importance of thermosensation on the physiology of animals and the usefulness of thermotaxis for genetic identification of new molecules that function in the nervous system.
Thermotaxis Assays: The Steepness of Thermal Gradient Makes a Difference
Hedgecock and Russell (1975) originally analyzed worm thermotaxis using two types of temperature gradients.5 One is a uniform linear temperature gradient of 0.5°C/cm, that examines the accumulation of worms. The other is a nonlinear radial temperature gradient on 9 cm Petri dish for examining tracks of individual worms (Fig. 1A). In both assays, a few hour-cultivations of animals with food at a certain temperature drove them to move toward warmer or colder regions until they reached the region that nearly corresponded to their previous cultivation temperature, and then moved isothermally (IT behavior).
Figure 1.
Thermotaxis behavior in C. elegans. (A) C. elegans cultivated at a particular temperature with food migrates to the cultivation temperature both on linear (left) and radial (right) thermal gradients. Start points of assay are shown with X. White dots on pictures of linear thermal gradient indicate terminal points of individual animal movements after 1 h thermotaxis test. Typical tracks of animals on the radial thermal gradient are shown. The figures are modified from previous reports.9,10 (B) A model of thermotaxis neural circuit. Temperature stimuli are sensed by AFD, AWC, ASI and unidentified “X” thermosensory neurons, and the thermal information transmitted to AIY and AIZ interneurons and integrated in RIA interneuron. AIY and AIZ interneurons mediate migration to higher temperature (thermophilic, T) and to lower temperature (cryophilic, C and c), respectively. The diagram is modified from previous reports.11-13
Many studies that used similar assay systems to Hedgecock and Russell (1975) reproduced the original results (Fig. 1A and Table 1).6-11,14 However, some studies using different assay systems showed that worms do not possess the ability to move to a higher temperature on a temperature gradient (Table 1).7,15-18 To understand this discrepancy, differences in assay systems such as steepness of the temperature gradient, difference in wild type strains, or temperature of starting point, were systematically analyzed.19 Jurado et al. (2010) showed that the steepness of the temperature gradient is a key parameter, which explains the different results observed in different assay systems.19 When the steepness of the temperature gradient is less than 1.0°C/cm, the distribution spectra of animals on the thermal gradient are similar to those reported in Hedgecook and Russell (1975). By contrast, when the steepness of the temperature gradient was more than 1.0°C/cm, the distribution spectra changed: while animals cultivated at low temperatures accumulated to the cultivation temperature, animals cultivated at high temperatures showed an almost athermotactic phenotype. Given that the steepness is important for thermotaxis, particularly in the case for cultivation at high temperatures, the different results reported in several studies (Table 1) are reasonably explained. It is thus necessary to consider the assay conditions carefully, particularly the steepness of a temperature gradient, when comparing thermotactic responses in different studies. Computer simulation analyses based on random walk theory also suggested that the steepness is an important factor to determine whether worms show migration toward warmer temperatures (thermophilic movement) or not.20,21 One of the simulation studies hypothesized that animals become athermotactic in a region much colder than the cultivation temperature, because calcium imaging analyses showed that thermosensory neurons AFD and AWC do not respond to temperature change at this region (discussed below).21 The simulation showed that if the temperature gradient is steep, animals are easily trapped in the colder region, resulting in athermotactic rather than thermophilic behavior.21 Although this behavioral model is solely based on random walk theory, it is possible that worms also utilize other types of behavioral strategies including “weathervane strategy.”22 Revealing such strategies would be essential to fully understand why the steep gradient abolishes thermophilic drives.
Table 1. Different assay systems for analyzing thermotaxis.
| Experimental conditions |
|
Evaluations * |
References |
||
|---|---|---|---|---|---|
| Thermal gradients | Assay times | ||||
|
Spacial |
nonlinear |
0.5°C/cm–3.5°C/cma |
1–2 h |
track |
5, 10, 11, 12, 32, 34, 35, 45, 59, 63, 64 |
| |
linear |
0.5°C/cm |
1 h |
distribution |
5, 9, 12, 26, 32, 37, 45 |
| |
(~0.5°C/cm) |
0.5°C/cm |
to 1 h (time course) |
distribution |
18 |
| |
|
0.5°C/cm |
1 h |
run duration |
24 |
| |
|
0.5°C/cm |
30 min |
run duration |
61 |
| |
|
0.5°C/cm |
30 min |
IT duration |
24 |
| |
|
0.5°C/cm |
25 min |
IT temperature |
18 |
| |
|
0.4°C/cm |
23 min |
IT temperature |
15 |
| |
linear |
1.15°C/cm |
to 1 h (time course), 8 h |
distribution |
17 |
| |
(~1°C/cm) |
1.0°C/cm |
35 min |
run duration |
13 |
| |
|
0.8°C/cm |
30 min |
IT duration |
24 |
| |
|
1.1°C/cm |
30 min |
IT temperature |
39 |
| |
|
1.0°C/cm |
25 min |
IT temperature |
18 |
| |
|
0.9°C/cm |
35 min |
IT length and temperature |
33 |
| |
linear |
1.4°C/cm |
1 h |
distribution |
16 |
| |
(1.4°C/cm) |
1.4°C/cm |
10 min |
instantaneous velocities |
16 |
| |
linear (various) |
0.2°C/cm, 0.4°C/cm, 0.6°C/cm, 0.8°C/cm, 1.0°C/cm, 1.2°C/cm |
1 h |
distribution |
19 |
| |
|
0.3°C/cm, 0.5°C/cm, 1°C/cm, 1.5°C/cm |
10 min |
distribution |
20 |
| |
|
0.40°C/cm, 0.75°C/cm, 2°C/cm |
23 min |
IT amplitude |
15 |
|
Temporal |
crawling |
± 0.5°C/min |
5 min |
run duration |
15 |
| |
swimming |
± 4°C/min |
30 sec |
turning rate |
15 |
| |
|
± 2°C/min |
15 minc |
turning rate |
36 |
| various stimulib ex. from 0.4°C/min to 6.4°C/min |
5–15 mind | turning rate | 61 | ||
Values were calculated from these ‘evaluations’ by respective equations in some articles.
a Cassata et al., Genesis, 2000.
b Sine-wave, upstep and downstep.
c repeated up (1min) and down (1min).
d involved the presentation of multiple stimulus waveforms.
Neural Circuit that Regulates Thermotaxis
To understand neural mechanisms regulating thermotaxis, it is important to understand the underlying neural circuit. The original neural circuit model was established by a series of laser ablation experiments (Fig. 1B).11 In the model, temperature information sensed by a major thermosensory neuron AFD and other unidentified neuron X is transmitted to two downstream interneurons, AIY and AIZ, which drive two opposite thermotaxis responses, migration toward warmer temperatures (thermophilic movements) and migration toward colder temperatures (cryophilic movements), respectively. Then, these two drives are integrated in the RIA interneuron. Consistent with the notion that the AFD neuron acts as a thermosensory neuron (Fig. 2A), AFD responded to thermal stimuli in calcium imaging using yellow cameleon 2.12, a genetically encoded calcium sensor (Fig. 2B).23
Figure 2.
Neural response to thermal stimuli. (A) Thermosensory systems in C. elegans. Thermosensory neurons and tissues. AFD, AWC and ASI head sensory neurons and PVD and FLP multidendritic neurons respond to temperature stimuli.11-13,23-25 A transcriptional mechanism mediated by HSF-1 in non-neuronal cells responds to cultivation temperature and modifies thermotaxis.26 (B–D) Physiological responses in AFD (B) and AWC (C and D) thermosensory neurons. Relative increases or decreases in the intracellular Ca2+ concentration have been measured with yellow cameleon (YC) and GCaMP. Schematic colored lines indicate the ratio (YFPF535/CFPF480) change of YC (ΔR/R; B and C) and the fluorescence change of GCaMP (ΔF/F; D). Temperature change are shown with solid black line. Broken black line shows Ca2+ event rates (/min) measured with GCaMP (below in D). Ca2+ event rates were low when non-variable temperature stimulus was applied, but event rates increased monotonically with increasing amplitude of the temperature stimulus. The figures are modified from previous reports.12,23,24 (E) Distinct subsets of thermosensory neurons function for robust cryophilic movement in different experimental conditions. Neurons whose ablation impairs the cryophilic movement (necessary) and neurons in which expression of TAX-4 cDNA is sufficient for rescuing the defect of tax-4 mutants (sufficient) are listed. The table is modified from a previous report.13
Genetic and physiological studies showed that the AWC olfactory neuron is also involved in temperature sensation (Figs. 1B and 2A).12,24,27 The mutant for EAT-16, a homolog of a mammalian regulator of G protein signaling (RGS) proteins, was isolated as a thermotaxis defective mutant that migrates toward colder temperatures than the previous cultivation temperature (cryophilic defect). The thermotactic defect of eat-16 mutants was not rescued by expressing the cDNA in the originally identified thermotaxis neurons, but was rescued by the expression in the well-characterized olfactory neuron, AWC.12,27 Calcium imaging using yellow cameleon 2.12 showed that the calcium concentration of AWC deterministically increased as much as the AFD thermosensory neuron in response to warming (Fig. 2C), while the responses of ASH and ASE neurons were much smaller.12 These results suggest that the AWC neuron is part of the thermotaxis neural circuit (Figs. 1B and 2A). An independent study using a calcium indicator, GCaMP and a different temperature stimulus showed that the AWC neuron did respond to temperature change but the individual responses were stochastic; the event rate of stochastic calcium spike monotonically increased with amplitude of temperature change (Fig. 2D).24 Different observations might be caused by the different calcium indicators. GCaMP3 has a shorter decay time than FRET-based calcium probes like yellow cameleon.28 Thus, an array of small calcium spikes may be integrated with yellow cameleon, while each of such spikes may be detectable with GCaMP. Also, the different results were obtained with different temperature stimuli. For example, AWC may change its response depending on the patterns of temperature stimuli. Further, in contrast to the results obtained from the imaging analyses, the electrophysiological analysis showed that ionic current was not detected upon temperature change in AWC.29 One possible explanation for this discrepancy is that thermosensing properties physiologically differ between AFD and AWC neurons, which should be clarified in future investigations.
Recently, ASI neurons previously designated as pheromone sensing neurons, were also reported to be involved in thermosensation in rather unusual assay conditions including a quite sharp steepness of the gradient (1.0°C/cm) (Fig. 2A).13 Animals cultivated at either 15°C or 20°C are set at the starting points where the temperature is 4, 5, 6, 7, 8, 9 or 10°C higher than the cultivation temperature, respectively. In this assay, wild type animals showed robust cryophilic movement, while tax-4 mutant animals defective in cGMP-dependent cation channel moved randomly. The defects of tax-4 mutants were rescued by expressing tax-4 cDNA in different combinations of AFD, AWC and ASI neurons (Fig. 2E). The rescues required quite complex combinations of these neurons and changed in accordance with the assay parameters such as cultivation temperature and starting point temperature (Fig. 2E). Further, inactivating the different set of these three neurons caused defects in the cryophilic movement depending on the assay parameters (Fig. 2E). The results from cell-inactivation experiments are not necessarily consistent with the results from rescue experiments (Fig. 2E). Beverly et al. (2011) suggested that the degeneracy within these three neurons contribute to generate robust cold navigated behavior. Calcium imaging showed that ASI could respond to temperature change and that the operating temperature range of ASI changed in the absence of AFD. These results may implicate the regulation of ASI thermosensitivity through neuroendocrine signaling from AFD.13
Thermosensory Neurons as Temperature Signal Receptor and Memory Device of Environmental Temperature
Recent physiological studies progressed our knowledge on properties of the AFD thermosensory neuron. According to the first calcium imaging of the AFD neuron using yellow cameleon 2.12,23 the calcium concentration of AFD transiently increased in response to warming above the threshold that was set by a previous cultivation temperature; the AFD of animals cultivated at 20°C responded to warming above 19°C (Fig. 2B). Similarly, the AFD of animals cultivated at 15°C or 25°C responded to warming above the threshold temperature that was near the cultivation temperature 15°C or 25°C, respectively (Fig. 2B).23 When various patterns of temperature stimuli were used,30 AFD responded to both warming and cooling, and discriminated temperature changes as small as 0.05°C. Interestingly, the sensory ending of AFD, that is disconnected from the cell body, still retained the ability to respond to the temperatures above the threshold temperature. These results suggest that the threshold temperature for calcium influx is stored at the sensory endings of the AFD neuron, thereby acting as the primary site for temperature memory. A recent electrophysiological analysis also showed that AFD responds to both warming or cooling by opening or closing ion channels, respectively.29 Because of the ability to store temperature memory and the multiple responsive properties to temperature, the AFD neuron can be used as a model to understand the mechanisms of a single-cell memory, which would be as important as modulation of synaptic strength for memory formation.
As described before, calcium imaging of AWC showed that the calcium concentration of AWC is increased in response to temperature changes (Fig. 2C and D).12,24 Kuhara et al. (2008) further showed that the temperature threshold for calcium influx in AWC exhibits the dependency on the past cultivation temperature, suggesting that AWC possesses the similar plasticity as AFD does (Fig. 2C).
Molecular Analysis of Thermosensory Neurons
Recent molecular analysis of AFD neurons certainly advanced our understanding of the molecular mechanisms underlying AFD function (Fig. 3A). Three guanylate cyclases (GCY-8, GCY-18, GCY-23) and cGMP-dependent cation channel (CNG channel), consisting of TAX-2 and TAX-4 subunits, were identified for thermosensation in the AFD neuron.31,32 A recent study demonstrated that disruption of any of the three guanylate cyclases causes distinct abnormalities in the IT behavior and AFD responses:33 mutations in gcy-8 gene caused defects in IT execution and AFD responsiveness to temperature stimuli, while mutations in gcy-18 or gcy-23 changed temperature range where IT is executed to lower or higher range, respectively. These results suggest the intricate regulation of cGMP levels is required for proper thermosensory function of AFD. TTX-4/Protein kinase C epsilon/eta and TAX-6/Calcineurin were identified as negative regulators of AFD activity and plasticity.34,35 Loss of DGK-3, one of the diacylglycerol kinases in C. elegans, impairs the adaptation speed for a new cultivation temperature, but does not alter the adaptation speed of a temperature threshold for calcium influx in the AFD neuron. This defect was rescued by AFD-specific expression of dgk-3 gene, suggesting that DGK-3 regulates the output of AFD for the behavioral plasticity rather than regulation of the thermosensitivity per se.39 CMK-1, a homolog of Ca2+/calmodulin-dependent protein kinase I/IV (CaMK I/IV), is required for activity-dependent AFD-specific gene expressions.36 Further, the mutant for the gene crh-1 encoding the cAMP response element-binding protein (CREB), which is regarded as a key transcription factor for memory formation across species,40,41 was found to show abnormal thermotactic behavior and lowered the magnitude of AFD calcium responses as compared with wild type animals.37 The behavioral defects were completely rescued by AFD-specific expression of crh-1 cDNA.37 Animals expressing the dominant negative form of CRH-1 in AFD needed longer conditioning time to change their preferred temperatures than wild type animals.37 It remains to be elucidated as to how CREB regulates the excitability of the AFD neuron. Also, it is important to address how these molecules orchestrate the full functions of the AFD neuron. Microarray analysis of cultivation temperature-dependent AFD specific gene expressions may be a potent way to obtain clues on the complex molecular interactions. The sensory ending of AFD is embedded into the sheath cell that is thought to be equivalent to glia.42 Ablation of sheath glia does not eliminate AFD function but results in thermophilic behavior, suggesting that the interaction between AFD and sheath glia is essential for thermosensation.43
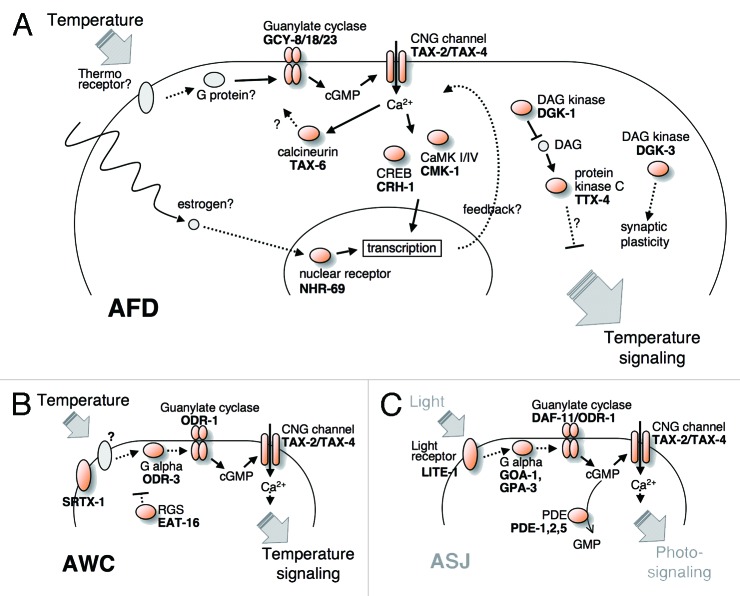
Figure 3.
A possible molecular mechanism of thermosensory signaling. (A) Thermal stimuli are sensed by unidentified receptors at the sensory ending of AFD, then unidentified G protein activates guanylate cyclases GCY-8, GCY-18, and GCY-23, leading to change of cGMP concentration and regulating the TAX-4/TAX-2 channel.31–33 TAX-6/Calcineurin, DGK-1/DAG kinase, TTX-4/Protein kinase C and CMK-1/CaMK I/IV act as regulators for the temperature signaling.34–36 CRH-1/CREB and NHR-69/nuclear receptor also regulate the temperature signaling through transcription.26,37 (B) SRTX-1/G protein-coupled receptor is reported to regulate the responding rate of AWC neuron to temperature change.24 ODR-3/G-α protein activates ODR-1/guanylate cyclases, leading to change of cGMP concentration and regulating the TAX-4 and TAX-2 CNG channel, and EAT-16/RGS acts as negative regulator for the temperature signaling.12 (C) A model for phototransduction cascade in the photoreceptor cell ASJ.38 LITE-1/G protein-coupled receptor may act as photoreceptor, two G-α proteins GOA-1 and GPA-3 may be coupled to the guanylate cyclases, DAF-11 and ODR-1, and upregulated cGMP open the TAX-4 and TAX-2 CNG channel.
A subset of molecules was revealed to function in temperature sensation in AWC (Fig. 3B).12,24 Temperature information is transmitted through a G protein signaling pathway that involves G α (ODR-3), guanylate cyclase (ODR-1), regulator of G protein signaling (EAT-16) and CNG channel (TAX-4/TAX-2). A homolog of G protein-coupled receptor SRTX-1 was also identified to function in the AWC neuron for IT behavior.24 Whether SRTX-1 directly perceives temperature and transmits signals to the G protein pathway is currently unknown. Most of these molecules in AWC thermosensation are shared by olfactory sensation. The question arises whether AWC discriminates temperature and odor stimuli. AWC relieves inhibition of the downstream interneuron AIY on perception of odorants.44 By contrast, AWC transmits temperature information through excitatory signals to AIY.45 It is likely that temperature and odor stimuli are discriminated in AWC. How does the AWC neuron discriminate between the two stimuli using a quite similar signaling pathway? The AFD neuron transmits either excitatory or inhibitory signals to AIY, dependent of the magnitude of the intracellular calcium concentration (discussed below).46 Similarly, AWC might send either excitatory or inhibitory signals to AIY. Calcium imaging using GCaMP showed that temperature and odor stimuli cause different patterns of calcium influx in AWC.24,44 It is plausible to assume that the two stimuli may activate the G protein signaling pathways in a different strength or frequency, thereby generating various types of transmissions from AWC to AIY.
AFD and AWC neurons seem to utilize similar G protein signaling pathways for temperature sensation (Fig. 3A and B), which resembles the signaling cascade in mammalian vision. C. elegans also perceive light using a G protein-dependent cGMP pathway in the ASJ neuron (Fig. 3C).3,4,38 Investigation of the analogy between these sensory systems in C. elegans and mammals will lead to understanding the evolution of signaling cascades for diverse sensory stimuli such as temperature and light.
Thermosensory Systems Outside of Amphid
Besides the temperature sensing neurons in the amphid, C. elegans perceives temperature in other systems. A heatshock transcription factor HSF-1 is known to be required for the heat-shock induced gene expression.47 Recently, HSF-1 was shown to be required for thermotaxis.26 The thermotaxis defect exhibited by hsf-1 mutants was restored by expression of HSF-1 in muscles and intestine without expression in any neurons. Additionally, HSF-1 in muscles and intestine regulates the physiological response of the AFD neuron to temperature through gene expression dynamics, which in part involves the estrogen hormonal pathway.26 Given that HSF-1 responds to a range of ambient cultivation temperatures,26 these results indicate that worms also utilize the systemic temperature perception mechanism through HSF-1, leading to the modulation of the activity of the AFD thermosensory neuron (Fig. 2A). The similar finding that non-neuronal cells modulate nervous system to change behavior is also demonstrated in aerotaxis, in which the animals move to a preferred ambient oxygen concentration.48 The uv1 endocrine cells in the gonad were shown to sense environmental oxygen concentrations through a hypoxia-induced transcription factor (HIF-1) and may send signals to the nervous system to modulate oxygen preference. Given the importance of temperature and oxygen concentrations, it is plausible that animals have evolved mechanisms in which to perceive such environmental information in multiple tissues.
Another recent study also showed another type of temperature sensitive neurons. The multidendritic neurons PVD and FLP that envelope the whole animal body respond to noxious cold- and heat-shock stimuli, respectively (Fig. 2A),25 indicating that these neurons may be required for noxious temperature avoidance.25,49 At least, a TRP channel (TRPA-1) appeared to play a role for the noxious cold reception.25 The temperature range for noxious stimuli is quite different from the ambient temperature range for thermotaxis, suggesting that C. elegans has independent thermosensory mechanisms that discriminate noxious and ambient temperature.
Information Processing in the Thermotaxis Neural Circuit
The temperature information received by various thermosensory systems may produce different behavioral and physiological strategies through information processing in interneurons. Both AFD and AWC neurons are connected to the postsynaptic AIY interneuron that is responsible for thermophilic movement in the neural circuit (Fig. 1B).11,12 Recent studies have unveiled sophisticated information processing in these three neurons. The study using animals mutant for the gene eat-4, encoding a vesicular glutamate transporter (VGLUT), showed that EAT-4-dependent glutamatergic transmission from AFD downregulated the activity of the AIY neuron through a glutamate-gated chloride channel GLC-3. This inhibition of AIY resulted in cryophilic movements (Fig. 4A). In contrast, EAT-4-dependent glutamatergic transmission from AWC upregulated AIY, thereby inducing thermophilic drive (Fig. 4A). Thus, the two glutamate transmissions from AFD and AWC encoding opposite information flows balance the activity of the single interneuron AIY, which may consequently process behavioral output (Fig. 4A).45
Figure 4.

A model for neural regulation in the thermotaxis neural circuit. (A) Functional connections of AFD, AWC and AIY. EAT-4-dependent glutamatergic transmission from AFD inhibits AIY and promotes cryophilic behavior, while EAT-4-dependent glutamatergic transmission from AWC excites AIY and promotes thremophilic behavior.45 A study of olfactory sensation showed that EAT-4-dependent glutamatergic transmission from AWC inhibits AIY in presence of odor stimuli.44 Unknown receptors are colored with gray. (B) A model for neural regulation of stimulatory and inhibitory neural signal controlling opposite thermotactic behavior. “T” and “C” indicate thermophilic and cryophilic driving signal, respectively. Cartoons of signal in AFD and AIY responded to thermal stimulus are shown in rectangles. A strong AFD response activates both stimulatory and inhibitory neurotransmissions to AIY, inducing a relatively weak activation of AIY. A weak AFD response causes relatively low activation of inhibitory neurotransmission, inducing a strong activation of AIY, which generate a thermophilic drive. A lack of sensory response eliminates AIY activation, whereby AIY-independent pathway generates cryophilic movement. The width of arrow indicates the strength of signal, and dotted arrows indicate weakness of signal. The figures are modified from previous report.46
Since calcium imaging showed that the AIY neuron did not respond to temperature stimuli in AFD-ablated animals, there should be excitatory signals from AFD to AIY besides the EAT-4-mediated inhibitory signal (Fig. 4A).30,45 AFD-ablated animals exhibited cryophilic or athermotactic phenotypes, whereas animals that impair EAT-4-dependent transmission exhibited a thermophilic phenotype. These behavioral differences support the EAT-4-independent transmission between AFD and AIY.11,45
The use of a recently developed technique for optical manipulation of neuronal activity50 has enabled the discovery of the bidirectional neurotransmission of AFD neuron.46,51 An analysis with simultaneous use of a light-driven cation channel channelrhodopsin-2 (ChR2) and the electrophysiological technique demonstrated the tonic and graded excitatory synaptic transmissions between AFD and AIY neurons.51 The signal from AFD is scale downed in AIY, and pulse stimulation from AFD neither facilitates nor depresses the AIY responses. This excitatory transmission requires UNC-31, that regulates neuropeptides release, suggesting that the excitatory synapse between AFD and AIY is peptidergic (Fig. 4A). The other study used a light-driven chloride channel, halorhodopsin (NpHR), to dissect the AFD to AIY transmission.46 In this study, the activation of NpHR in AFD caused the partial inactivation of the AFD neuron; the magnitude of the response to calcium influx measured by yellow cameleon 3.60 was partially lowered, which was confirmed by the lowered voltage change measured by a membrane voltage indicator, mermaid. This reduced response of AFD caused the hyperactivation of AIY, while the complete or nearly complete inactivation of AFD caused the loss of or lowered AIY response (Fig. 4B). Further, the partial inactivation of AFD did not enhance the AIY activity in mutant animals lacking eat-4 in AFD. These results suggest that partial inactivation of the AFD neuron reduces the EAT-4-mediated glutamatergic inhibitory signal, thereby leading to hyperactivation of the AIY neuron (Fig. 4B), while the complete inactivation of AFD reduces both excitatory and inhibitory signals, resulting in the loss of AIY response (Fig. 4B). It is thus plausible that the different activity states of AFD generate the diverse output that is balanced by two opposite neurotransmissions.
In contrast to the conclusion that EAT-4-dependent glutamatergic transmission from AWC upregulates AIY,45 physiological analysis using AWC hyper-activated mutants showed that activation of AWC inhibits the AIY neuron.12 Thus, it is possible that AWC utilizes EAT-4-independent neurotransmission for the inhibition of AIY (Fig. 4A). Additionally, AWC also perceives odorants and transmits the odor information to AIY, indicating that the nature of the information flow from AWC to AIY appears to be much more complicated. Interestingly, the AWC neuron inhibits the AIY neuron through the EAT-4-dependent activation of GLC-3, and exposures to odorants relieve this inhibition (Fig. 4A).44 Given that temperature information flow from AWC to AIY does not require GLC-3 but EAT-4-dependent glutamatergic transmission, temperature and odor stimuli may be discriminated not only at the level of the sensory neuron but also at the level of the neurotransmissions to the postsynaptic neuron.
In summary, despite the simple physical connections between AFD, AWC and AIY neurons (Fig. 1B),52 recent studies showed highly intricate information flow inside the circuit. More analyses should be required to fully understand the information processing in this thermotaxis circuit. In addition, the complex nature of information flows is also observed in the case of chemotaxis that partly shares neurons with thermotaxis.22,53 How different sensory inputs are discriminated in the neural circuit will be an important question in future studies.
AFD Thermosensation Affects the Physiological Process
Environmental temperature affects many aspects of physiology of organisms including life span. The recent study using C. elegans showed that the ambient temperature sensed by AFD influenced the lifespan.54,55 Inactivation of AFD either by a laser microbeam or genetic mutations led to a significant reduction in lifespan when animals were cultivated at 25°C, but did not affect lifespan when cultivated at 15°C. These results suggest that the thermosensation through AFD prevents worms from otherwise having a much shorter lifespan at high temperatures. The control of life span by AFD is independent of DAF-16/FOXO, a key transcriptional factor for aging, and instead is dependent on the activity of a steroid hormone signaling. The high temperature sensed by AFD promotes the transcription of daf-9 that encodes a cytochrome P450 in body tissues such as the XXX neurosecretory cells, the hypodermis, and the spermatheca. Steroid ligands produced by DAF-9 bind to and inhibit the nuclear hormone receptor DAF-12. Inactivation of DAF-12 contributes to the anti-aging effects at high temperature.54,55
Input of temperature is also important for developmental choice. Inactivation of AIY interneurons, downstream of AFD thermosensory neurons, largely affects dauer formation.56 The sensation and the processing of environmental temperature by AFD and AIY, respectively, might be more crucial for physiology as well as development of worms than we have previously expected.
Recent report showed that heat shock responses at 30°C and 34°C in somatic cells depend on the AFD-AIY thermosensory system.57 When worms were treated under heat shock of 30°C or 34°C, mRNA levels of hsp-70 were upregulated in wild type animals. gcy-8 gene encodes one of three guanylate cyclases that redundantly function for thermosensation in AFD, and ttx-3 gene encodes the LIM homeodomain protein essential for AIY development.31,56 The upregulations of hsp-70 mRNA by heat shock were largely reduced in gcy-8 and ttx-3 mutants, suggesting that heat shock responses in somatic cells are mediated by the AFD-AIY system.57 These observations are, however, totally inconsistent with the other reports that no changes in mRNA levels were observed both in the same and similar experimental conditions.26,54
The Usefulness of Thermotaxis for Characterizing New Neural Molecules
One of the advantages for behavioral studies in C. elegans is its powerful genetics.58 Genetic analysis of thermotaxis behavior is a particularly effective way to reveal new functions of molecules because of the high detectability of subtle behavioral abnormalities. Forward genetic screens, reverse genetic analysis and characterization of mutants defective in thermotaxis have already identified important functions of many molecules in neuronal development, synaptic plasticity and sensory transduction (Table 2).8
Table 2. Genes required for thermotactic behavior.
| Gene | Gene product | Mutant phenotype | Site of action (Neuron) | Reference |
|---|---|---|---|---|
|
tax-4 |
cGMP dependent channel (a subunit) |
athermotactic |
AFD and AWC |
12, 32, 65 |
|
tax-2 |
cGMP dependent channel (β subunit) |
athermotactic |
AFD and AWC |
|
|
ttx-1 |
OTD/OTX homeodomain protein |
cryophilic |
AFD |
64 |
|
ceh-14 |
LIM homeodomain protein |
athermotactic |
AFD |
66 |
|
cmk-1 |
CaM kinase I/IV |
abnormal |
AFD |
36 |
|
dac-1 |
SKI/SNO/DAC family |
cryophilic |
AFD |
67 |
|
tax-6 |
Calcineurin A subunit |
thermophilic |
AFD |
34 |
|
ttx-4 |
nPKC-epsilon/eta |
thermophilic |
AFD |
35 |
|
dgk-3 |
diacylglycerol kinase |
abnormal temperature memory |
AFD |
39 |
|
ncs-1 |
Neuronal calcium sensor |
abnormal isothermal tracking |
AIY |
68 |
|
ttx-3 |
LIM homeodomain protein |
cryophilic |
AIY |
56 |
|
ceh-10 |
homeodomain protein |
abnormal |
AIY |
69 |
|
lin-11 |
LIM homeodomain protein |
thermophilic |
AIZ |
70 |
|
unc-86 |
POU (transcriptional factor) |
thermophilic |
AIZ |
11 |
|
ttx-7 |
Inositol monophosphatase |
athermotactic |
RIA |
71 |
|
dgk-1 |
diacylglycerol kinase |
cryophilic |
unknown |
35 |
|
odr-3 |
G protein a subunit |
slightly thermophilic |
AWC |
12 |
|
eat-16 |
regulator of G protein signaling |
slightly cryophilic |
AWC |
12 |
|
srtx-1 |
G protein coupled receptor |
abnormal isothermal tracking |
AWC |
24 |
|
ttx-8 |
macoilin |
athermotactic |
AFD, AIY, AIZ and other neurons |
59 |
|
crh-1 |
CREB protein |
abnormal (cryophilic and athermotactic) |
AFD |
37 |
|
hsf-1 |
heat-shock transcription factor |
abnormal temperature memory |
whole body |
26 |
|
nhr-69 |
estrogen receptor |
abnormal temperature memory |
AFD |
26 |
|
eat-4 |
VGLUT homolog |
athermotactic |
AFD, AWC, RIA |
45 |
|
glc-3 |
glutamate-gated chloride channel |
slightly thermophilic |
AIY |
45 |
|
ins-1 |
insulin homolog |
defective in association between temperature and food |
neurons |
72 |
|
age-1 |
PI-3-kinase homolog |
partially defective in association between temperature and food |
thermotactic interneurons |
72 |
|
daf-16 |
forkhead-type transcriptional factor |
partially defective in association between temperature and food |
unknown (thermotactic interneurons) |
72 |
|
hen-1 |
secretory protein |
defective in association between temperature and food |
unknown |
73 |
| gcy-28 | receptor-like guanylate cyclase | defective in association between temperature and food | unknown | 14 |
The recent study genetically identified a novel molecule that functions in the nervous system. The maco-1/ttx-8 gene, encoding a homolog of human macoilin, was originally isolated as thermotaxis defective mutant and independently as suppressor for social behavior defective mutants.59,60 MACO-1 is a highly conserved novel protein with several transmembrane domains in the N-terminus and coiled-coil domains in the C-terminus. MACO-1 was broadly expressed in the nervous system and localized to ER in neurons. The calcium response of neurons for thermotaxis or social behavior, such as AFD, AIY and PQR, was largely decreased in maco-1 mutants. Further, the presynaptic structure in motor neurons of maco-1 mutants was partly disorganized. These results suggest that MACO-1 is generally required for neuronal excitability and synapse organization.59,60 FLJ10747, a predicted human homolog of MACO-1, rescued the thermotaxis defects of maco-1 mutants,59 indicating that the function of macoilin is evolutionary conserved between nematodes and humans. Thus, the functional identifications of MACO-1 in C. elegans provided important clues for the analysis of human macoilin. Further genetic screens and analyses using several assay systems of thermotaxis will facilitate understanding of such novel but evolutionary conserved neural molecules.
Concluding Remarks and Perspective
Analyses of thermotaxis in C. elegans have provided fruitful information about the mechanisms of thermosensation and neural computation from sensory perceptions to behavioral outputs. Importantly, the temperature information is memorized in a single thermosensory neuron, AFD. How does AFD store, maintain and recall memory? Comprehensive and thorough analyses of AFD will reveal the cellular logics of memory. Given that CRH-1 and CMK-1, homologs of CREB and CaMKI/IV, respectively, are involved in temperature coding in AFD, the reasonable assumption is that the logics of memory found in AFD will be conserved in human.
As demonstrated in this review, the temperature information received by discrete sensory systems is transmitted to downstream neurons in a complex manner even in C. elegans. To fully understand the mechanisms for the complex and sophisticated information flow, the recently developed techniques that enable the imaging of neural activity or to manipulate activity of a single neuron of freely moving animals61,62 will be useful. Combining these technologies and more detailed behavioral analysis will produce a systematic data set that links the activity of each neuron and behavioral output, thus enabling mathematical analyses. The future analyses of thermotaxis will continue to dissect the principles of neural operations conserved from nematode to human.
Acknowledgments
We thank Yuki Tsukada and Yukuo Nishida for comments in this manuscript and discussions. T.K., N.O. and N.N. were supported by the Japan Society for the Promotion of Science. This work was supported by CREST, Japan Science and Technology Agency and Grant-in-Aid for Scientific Research on Innovative Areas “Neural Diversity and Neocortical Organization” from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan to I.M.
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/19504
References
- 1.de Bono M, Maricq AV. Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci. 2005;28:451–501. doi: 10.1146/annurev.neuro.27.070203.144259. [DOI] [PubMed] [Google Scholar]
- 2.Bargmann CI, Kaplan JM. Signal transduction in the Caenorhabditis elegans nervous system. Annu Rev Neurosci. 1998;21:279–308. doi: 10.1146/annurev.neuro.21.1.279. [DOI] [PubMed] [Google Scholar]
- 3.Ward A, Liu J, Feng Z, Xu XZS. Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nat Neurosci. 2008;11:916–22. doi: 10.1038/nn.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards SL, Charlie NK, Milfort MC, Brown BS, Gravlin CN, Knecht JE, et al. A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol. 2008;6:e198. doi: 10.1371/journal.pbio.0060198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hedgecock EM, Russell RL. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA. 1975;72:4061–5. doi: 10.1073/pnas.72.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mori I. Genetics of chemotaxis and thermotaxis in the nematode Caenorhabditis elegans. Annu Rev Genet. 1999;33:399–422. doi: 10.1146/annurev.genet.33.1.399. [DOI] [PubMed] [Google Scholar]
- 7.Garrity PA, Goodman MB, Samuel AD, Sengupta P. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev. 2010;24:2365–82. doi: 10.1101/gad.1953710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori I, Sasakura H, Kuhara A. Worm thermotaxis: a model system for analyzing thermosensation and neural plasticity. Curr Opin Neurobiol. 2007;17:712–9. doi: 10.1016/j.conb.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Ito H, Inada H, Mori I. Quantitative analysis of thermotaxis in the nematode Caenorhabditis elegans. J Neurosci Methods. 2006;154:45–52. doi: 10.1016/j.jneumeth.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Mohri A, Kodama E, Kimura KD, Koike M, Mizuno T, Mori I. Genetic control of temperature preference in the nematode Caenorhabditis elegans. Genetics. 2005;169:1437–50. doi: 10.1534/genetics.104.036111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori I, Ohshima Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature. 1995;376:344–8. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- 12.Kuhara A, Okumura M, Kimata T, Tanizawa Y, Takano R, Kimura KD, et al. Temperature sensing by an olfactory neuron in a circuit controlling behavior of C. elegans. Science. 2008;320:803–7. doi: 10.1126/science.1148922. [DOI] [PubMed] [Google Scholar]
- 13.Beverly M, Anbil S, Sengupta P. Degeneracy and neuromodulation among thermosensory neurons contribute to robust thermosensory behaviors in Caenorhabditis elegans. J Neurosci. 2011;31:11718–27. doi: 10.1523/JNEUROSCI.1098-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsunozaki M, Chalasani SH, Bargmann CI. A behavioral switch: cGMP and PKC signaling in olfactory neurons reverses odor preference in C. elegans. Neuron. 2008;59:959–71. doi: 10.1016/j.neuron.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryu WS, Samuel ADT. Thermotaxis in Caenorhabditis elegans analyzed by measuring responses to defined Thermal stimuli. J Neurosci. 2002;22:5727–33. doi: 10.1523/JNEUROSCI.22-13-05727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada Y, Ohshima Y. Distribution and movement of Caenorhabditis elegans on a thermal gradient. J Exp Biol. 2003;206:2581–93. doi: 10.1242/jeb.00477. [DOI] [PubMed] [Google Scholar]
- 17.Anderson JL, Albergotti L, Proulx S, Peden C, Huey RB, Phillips PC. Thermal preference of Caenorhabditis elegans: a null model and empirical tests. J Exp Biol. 2007;210:3107–16. doi: 10.1242/jeb.007351. [DOI] [PubMed] [Google Scholar]
- 18.Chi CA, Clark DA, Lee S, Biron D, Luo L, Gabel CV, et al. Temperature and food mediate long-term thermotactic behavioral plasticity by association-independent mechanisms in C. elegans. J Exp Biol. 2007;210:4043–52. doi: 10.1242/jeb.006551. [DOI] [PubMed] [Google Scholar]
- 19.Jurado P, Kodama E, Tanizawa Y, Mori I. Distinct thermal migration behaviors in response to different thermal gradients in Caenorhabditis elegans. Genes Brain Behav. 2010;9:120–7. doi: 10.1111/j.1601-183X.2009.00549.x. [DOI] [PubMed] [Google Scholar]
- 20.Ramot D, MacInnis BL, Lee H-C, Goodman MB. Thermotaxis is a robust mechanism for thermoregulation in Caenorhabditis elegans nematodes. J Neurosci. 2008;28:12546–57. doi: 10.1523/JNEUROSCI.2857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakazato K, Mochizuki A. Steepness of thermal gradient is essential to obtain a unified view of thermotaxis in C. elegans. J Theor Biol. 2009;260:56–65. doi: 10.1016/j.jtbi.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 22.Iino Y, Yoshida K. Parallel use of two behavioral mechanisms for chemotaxis in Caenorhabditis elegans. J Neurosci. 2009;29:5370–80. doi: 10.1523/JNEUROSCI.3633-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura KD, Miyawaki A, Matsumoto K, Mori I. The C. elegans thermosensory neuron AFD responds to warming. Curr Biol. 2004;14:1291–5. doi: 10.1016/j.cub.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 24.Biron D, Wasserman S, Thomas JH, Samuel ADT, Sengupta P. An olfactory neuron responds stochastically to temperature and modulates Caenorhabditis elegans thermotactic behavior. Proc Natl Acad Sci USA. 2008;105:11002–7. doi: 10.1073/pnas.0805004105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chatzigeorgiou M, Yoo S, Watson JD, Lee W-H, Spencer WC, Kindt KS, et al. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat Neurosci. 2010;13:861–8. doi: 10.1038/nn.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugi T, Nishida Y, Mori I. Regulation of behavioral plasticity by systemic temperature signaling in Caenorhabditis elegans. Nat Neurosci. 2011;14:984–92. doi: 10.1038/nn.2854. [DOI] [PubMed] [Google Scholar]
- 27.Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–27. doi: 10.1016/0092-8674(93)80053-H. [DOI] [PubMed] [Google Scholar]
- 28.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–81. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramot D, MacInnis BL, Goodman MB. Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat Neurosci. 2008;11:908–15. doi: 10.1038/nn.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark DA, Biron D, Sengupta P, Samuel ADT. The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J Neurosci. 2006;26:7444–51. doi: 10.1523/JNEUROSCI.1137-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inada H, Ito H, Satterlee J, Sengupta P, Matsumoto K, Mori I. Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics. 2006;172:2239–52. doi: 10.1534/genetics.105.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komatsu H, Mori I, Rhee JS, Akaike N, Ohshima Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron. 1996;17:707–18. doi: 10.1016/S0896-6273(00)80202-0. [DOI] [PubMed] [Google Scholar]
- 33.Wasserman SM, Beverly M, Bell HW, Sengupta P. Regulation of response properties and operating range of the AFD thermosensory neurons by cGMP signaling. Curr Biol. 2011;21:353–62. doi: 10.1016/j.cub.2011.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhara A, Inada H, Katsura I, Mori I. Negative regulation and gain control of sensory neurons by the C. elegans calcineurin TAX-6. Neuron. 2002;33:751–63. doi: 10.1016/S0896-6273(02)00607-4. [DOI] [PubMed] [Google Scholar]
- 35.Okochi Y, Kimura KD, Ohta A, Mori I. Diverse regulation of sensory signaling by C. elegans nPKC-epsilon/eta TTX-4. EMBO J. 2005;24:2127–37. doi: 10.1038/sj.emboj.7600697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satterlee JS, Ryu WS, Sengupta P. The CMK-1 CaMKI and the TAX-4 Cyclic nucleotide-gated channel regulate thermosensory neuron gene expression and function in C. elegans. Curr Biol. 2004;14:62–8. doi: 10.1016/j.cub.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 37.Nishida Y, Sugi T, Nonomura M, Mori I. Identification of the AFD neuron as the site of action of the CREB protein in Caenorhabditis elegans thermotaxis. EMBO Rep. 2011;12:855–62. doi: 10.1038/embor.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Ward A, Gao J, Dong Y, Nishio N, Inada H, et al. C. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog. Nat Neurosci. 2010;13:715–22. doi: 10.1038/nn.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biron D, Shibuya M, Gabel C, Wasserman SM, Clark DA, Brown A, et al. A diacylglycerol kinase modulates long-term thermotactic behavioral plasticity in C. elegans. Nat Neurosci. 2006;9:1499–505. doi: 10.1038/nn1796. [DOI] [PubMed] [Google Scholar]
- 40.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–8. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 41.Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–60. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–87. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 43.Bacaj T, Tevlin M, Lu Y, Shaham S. Glia are essential for sensory organ function in C. elegans. Science. 2008;322:744–7. doi: 10.1126/science.1163074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, et al. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature. 2007;450:63–70. doi: 10.1038/nature06292. [DOI] [PubMed] [Google Scholar]
- 45.Ohnishi N, Kuhara A, Nakamura F, Okochi Y, Mori I. Bidirectional regulation of thermotaxis by glutamate transmissions in Caenorhabditis elegans. EMBO J. 2011;30:1376–88. doi: 10.1038/emboj.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhara A, Ohnishi N, Shimowada T, Mori I. Neural coding in a single sensory neuron controlling opposite seeking behaviours in Caenorhabditis elegans. Nat Commun. 2011;2:355. doi: 10.1038/ncomms1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–96. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 48.Chang AJ, Bargmann CI. Hypoxia and the HIF-1 transcriptional pathway reorganize a neuronal circuit for oxygen-dependent behavior in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2008;105:7321–6. doi: 10.1073/pnas.0802164105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glauser DA, Chen WC, Agin R, Macinnis BL, Hellman AB, Garrity PA, et al. Heat avoidance is regulated by transient receptor potential (TRP) channels and a neuropeptide signaling pathway in Caenorhabditis elegans. Genetics. 2011;188:91–103. doi: 10.1534/genetics.111.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang F, Wang L-P, Brauner M, Liewald JF, Kay K, Watzke N, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–9. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 51.Narayan A, Laurent G, Sternberg PW. Transfer characteristics of a thermosensory synapse in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2011;108:9667–72. doi: 10.1073/pnas.1106617108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White JG, Southgate E, Thomson JN, Brenner S. The structure of the ventral nerve cord of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275:327–48. doi: 10.1098/rstb.1976.0086. [DOI] [PubMed] [Google Scholar]
- 53.Chalasani SH, Kato S, Albrecht DR, Nakagawa T, Abbott LF, Bargmann CI. Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat Neurosci. 2010;13:615–21. doi: 10.1038/nn.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee S-J, Kenyon C. Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr Biol. 2009;19:715–22. doi: 10.1016/j.cub.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mori I, Sasakura H. Aging: shall we take the high road? Curr Biol. 2009;19:R363–4. doi: 10.1016/j.cub.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 56.Hobert O, Mori I, Yamashita Y, Honda H, Ohshima Y, Liu Y, et al. Regulation of interneuron function in the C. elegans thermoregulatory pathway by the ttx-3 LIM homeobox gene. Neuron. 1997;19:345–57. doi: 10.1016/S0896-6273(00)80944-7. [DOI] [PubMed] [Google Scholar]
- 57.Prahlad V, Cornelius T, Morimoto RI. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science. 2008;320:811–4. doi: 10.1126/science.1156093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyara A, Ohta A, Okochi Y, Tsukada Y, Kuhara A, Mori I. Novel and conserved protein macoilin is required for diverse neuronal functions in Caenorhabditis elegans. PLoS Genet. 2011;7:e1001384. doi: 10.1371/journal.pgen.1001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arellano-Carbajal F, Briseño-Roa L, Couto A, Cheung BHH, Labouesse M, de Bono M. Macoilin, a conserved nervous system-specific ER membrane protein that regulates neuronal excitability. PLoS Genet. 2011;7:e1001341. doi: 10.1371/journal.pgen.1001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clark DA, Gabel CV, Gabel H, Samuel ADT. Temporal activity patterns in thermosensory neurons of freely moving Caenorhabditis elegans encode spatial thermal gradients. J Neurosci. 2007;27:6083–90. doi: 10.1523/JNEUROSCI.1032-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leifer AM, Fang-Yen C, Gershow M, Alkema MJ, Samuel ADT. Optogenetic manipulation of neural activity in freely moving Caenorhabditis elegans. Nat Methods. 2011;8:147–52. doi: 10.1038/nmeth.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuhara A, Mori I. Molecular physiology of the neural circuit for calcineurin-dependent associative learning in Caenorhabditis elegans. J Neurosci. 2006;26:9355–64. doi: 10.1523/JNEUROSCI.0517-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Satterlee JS, Sasakura H, Kuhara A, Berkeley M, Mori I, Sengupta P. Specification of thermosensory neuron fate in C. elegans requires ttx-1, a homolog of otd/Otx. Neuron. 2001;31:943–56. doi: 10.1016/S0896-6273(01)00431-7. [DOI] [PubMed] [Google Scholar]
- 65.Coburn CM, Bargmann CI. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron. 1996;17:695–706. doi: 10.1016/S0896-6273(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 66.Cassata G, Kagoshima H, Andachi Y, Kohara Y, Dürrenberger MB, Hall DH, et al. The LIM homeobox gene ceh-14 confers thermosensory function to the AFD neurons in Caenorhabditis elegans. Neuron. 2000;25:587–97. doi: 10.1016/S0896-6273(00)81062-4. [DOI] [PubMed] [Google Scholar]
- 67.Colosimo ME, Brown A, Mukhopadhyay S, Gabel C, Lanjuin AE, Samuel ADT, et al. Identification of thermosensory and olfactory neuron-specific genes via expression profiling of single neuron types. Curr Biol. 2004;14:2245–51. doi: 10.1016/j.cub.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 68.Gomez M, De Castro E, Guarin E, Sasakura H, Kuhara A, Mori I, et al. Ca2+ signaling via the neuronal calcium sensor-1 regulates associative learning and memory in C. elegans. Neuron. 2001;30:241–8. doi: 10.1016/S0896-6273(01)00276-8. [DOI] [PubMed] [Google Scholar]
- 69.Altun-Gultekin Z, Andachi Y, Tsalik EL, Pilgrim D, Kohara Y, Hobert O. A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development. 2001;128:1951–69. doi: 10.1242/dev.128.11.1951. [DOI] [PubMed] [Google Scholar]
- 70.Hobert O, D’Alberti T, Liu Y, Ruvkun G. Control of neural development and function in a thermoregulatory network by the LIM homeobox gene lin-11. J Neurosci. 1998;18:2084–96. doi: 10.1523/JNEUROSCI.18-06-02084.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanizawa Y, Kuhara A, Inada H, Kodama E, Mizuno T, Mori I. Inositol monophosphatase regulates localization of synaptic components and behavior in the mature nervous system of C. elegans. Genes Dev. 2006;20:3296–310. doi: 10.1101/gad.1497806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kodama E, Kuhara A, Mohri-Shiomi A, Kimura KD, Okumura M, Tomioka M, et al. Insulin-like signaling and the neural circuit for integrative behavior in C. elegans. Genes Dev. 2006;20:2955–60. doi: 10.1101/gad.1479906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ishihara T, Iino Y, Mohri A, Mori I, Gengyo-Ando K, Mitani S, et al. HEN-1, a secretory protein with an LDL receptor motif, regulates sensory integration and learning in Caenorhabditis elegans. Cell. 2002;109:639–49. doi: 10.1016/S0092-8674(02)00748-1. [DOI] [PubMed] [Google Scholar]