Abstract
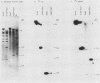
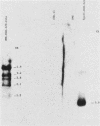
A strain of herpesvirus saimiri containing a bovine growth hormone (bGH) gene under the control of the simian virus 40 (SV40) late-region promoter was constructed. This strain, bGH-Z20, was replication competent and stably harbored the bGH gene upon serial passage. Nonpermissive marmoset T cells persistently infected with bGH-Z20 produced a 0.9-kilobase RNA which contained all of the bGH exon sequences and appeared to initiate within the SV40 promoter region. However, in permissively infected owl monkey kidney cells, RNAs containing growth hormone sequences appeared to initiate from herpesvirus saimiri promoters positioned upstream from the SV40-growth hormone gene. Persistently infected T cells in culture secreted 500 ng of bGH protein per 10(6) cells per 24 h during the several months of testing. The secreted protein was 21 kilodaltons, the size of authentic bGH. New World primates experimentally infected with bGH-Z20 produced circulating bGH and developed immunoglobulin G antibodies directed against bGH. Because herpesviruses characteristically remain latent in the infected host, these observations suggest a means for replacing gene products missing or defective in hereditary genetic disorders.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F. Prospects for human gene therapy. Science. 1984 Oct 26;226(4673):401–409. doi: 10.1126/science.6093246. [DOI] [PubMed] [Google Scholar]
- Bornkamm G. W., Delius H., Fleckenstein B., Werner F. J., Mulder C. Structure of Herpesvirus saimiri genomes: arrangement of heavy and light sequences in the M genome. J Virol. 1976 Jul;19(1):154–161. doi: 10.1128/jvi.19.1.154-161.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
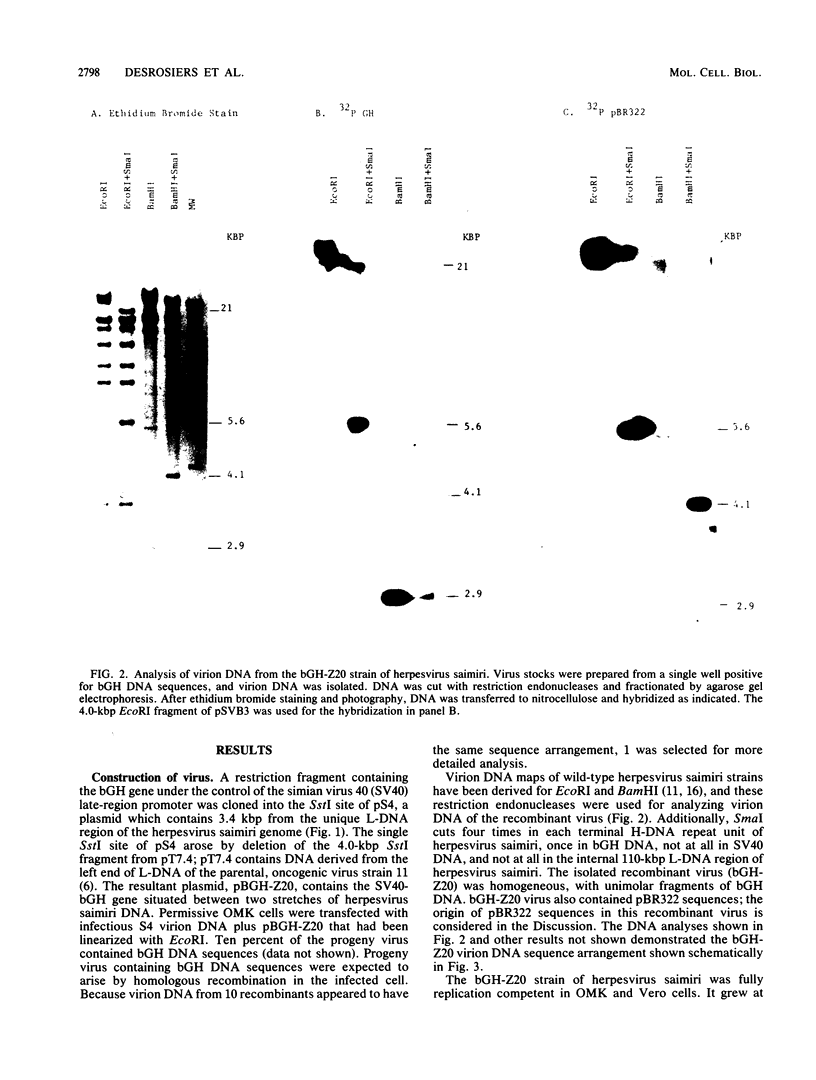
- Desrosiers R. C., Bakker A., Kamine J., Falk L. A., Hunt R. D., King N. W. A region of the Herpesvirus saimiri genome required for oncogenicity. Science. 1985 Apr 12;228(4696):184–187. doi: 10.1126/science.2983431. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Burghoff R. L., Bakker A., Kamine J. Construction of replication-competent Herpesvirus saimiri deletion mutants. J Virol. 1984 Feb;49(2):343–348. doi: 10.1128/jvi.49.2.343-348.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C., Falk L. A., Jr Herpesvirus tamarinus and its relation to herpes simplex virus. J Gen Virol. 1981 Sep;56(Pt 1):119–130. doi: 10.1099/0022-1317-56-1-119. [DOI] [PubMed] [Google Scholar]
- Doehmer J., Barinaga M., Vale W., Rosenfeld M. G., Verma I. M., Evans R. M. Introduction of rat growth hormone gene into mouse fibroblasts via a retroviral DNA vector: expression and regulation. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2268–2272. doi: 10.1073/pnas.79.7.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Herz C., Roizman B. The alpha promoter regulator-ovalbumin chimeric gene resident in human cells is regulated like the authentic alpha 4 gene after infection with herpes simplex virus 1 mutants in alpha 4 gene. Cell. 1983 May;33(1):145–151. doi: 10.1016/0092-8674(83)90343-4. [DOI] [PubMed] [Google Scholar]
- Kamine J., Bakker A., Desrosiers R. C. Mapping of RNA transcribed from a region of the Herpesvirus saimiri genome required for oncogenicity. J Virol. 1984 Nov;52(2):532–540. doi: 10.1128/jvi.52.2.532-540.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Knust E., Schirm S., Dietrich W., Bodemer W., Kolb E., Fleckenstein B. Cloning of Herpesvirus saimiri DNA fragments representing the entire L-region of the genome. Gene. 1983 Nov;25(2-3):281–289. doi: 10.1016/0378-1119(83)90232-9. [DOI] [PubMed] [Google Scholar]
- Koomey J. M., Mulder C., Burghoff R. L., Fleckenstein B., Desrosiers R. C. Deletion of DNA sequence in a nononcogenic variant of Herpesvirus saimiri. J Virol. 1984 May;50(2):662–665. doi: 10.1128/jvi.50.2.662-665.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong A. D., Frenkel N. Herpes simplex virus amplicon: effect of size on replication of constructed defective genomes containing eucaryotic DNA sequences. J Virol. 1984 Sep;51(3):595–603. doi: 10.1128/jvi.51.3.595-603.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong A. D., Frenkel N. The herpes simplex virus amplicon. IV. Efficient expression of a chimeric chicken ovalbumin gene amplified within defective virus genomes. Virology. 1985 Apr 30;142(2):421–425. doi: 10.1016/0042-6822(85)90351-4. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Devillers-Thiery A., Blobel G. Nascent prehormones are intermediates in the biosynthesis of authentic bovine pituitary growth hormone and prolactin. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2432–2436. doi: 10.1073/pnas.74.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupker J. H., Roskam W. G., Miloux B., Liauzun P., Yaniv M., Jouannau J. Abundant excretion of human growth hormone by recombinant-plasmid-transformed monkey kidney cells. Gene. 1983 Oct;24(2-3):281–287. doi: 10.1016/0378-1119(83)90088-4. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Smith G. L., Gerin J. L., Purcell R. H. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature. 1984 Sep 6;311(5981):67–69. doi: 10.1038/311067a0. [DOI] [PubMed] [Google Scholar]
- O'Hare P., Honess R. W. Identification of a subset of herpesvirus saimiri polypeptides synthesized in the absence of virus DNA replication. J Virol. 1983 Apr;46(1):279–283. doi: 10.1128/jvi.46.1.279-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicali D., Paoletti E. Construction of poxviruses as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4927–4931. doi: 10.1073/pnas.79.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlakis G. N., Hamer D. H. Regulation of a metallothionein-growth hormone hybrid gene in bovine papilloma virus. Proc Natl Acad Sci U S A. 1983 Jan;80(2):397–401. doi: 10.1073/pnas.80.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlakis G. N., Hizuka N., Gorden P., Seeburg P., Hamer D. H. Expression of two human growth hormone genes in monkey cells infected by simian virus 40 recombinants. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7398–7402. doi: 10.1073/pnas.78.12.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Delery M., Rook A. H., Frederick W. R., Epstein J. S., Manischewitz J. F., Jackson L., Ramsey K. M., Mittal K., Plotkin S. A. Comparative virulence and immunogenicity of the Towne strain and a nonattenuated strain of cytomegalovirus. Ann Intern Med. 1984 Oct;101(4):478–483. doi: 10.7326/0003-4819-101-4-478. [DOI] [PubMed] [Google Scholar]
- Randall R. E., Honess R. W., O'Hare P. Proteins specified by herpesvirus saimiri: identification and properties of virus-specific polypeptides in productively infected cells. J Gen Virol. 1983 Jan;64(Pt 1):19–35. doi: 10.1099/0022-1317-64-1-19. [DOI] [PubMed] [Google Scholar]
- Shih M. F., Arsenakis M., Tiollais P., Roizman B. Expression of hepatitis B virus S gene by herpes simplex virus type 1 vectors carrying alpha- and beta-regulated gene chimeras. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5867–5870. doi: 10.1073/pnas.81.18.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Moss B. Infectious poxvirus vectors have capacity for at least 25 000 base pairs of foreign DNA. Gene. 1983 Nov;25(1):21–28. doi: 10.1016/0378-1119(83)90163-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tackney C., Cachianes G., Silverstein S. Transduction of the Chinese hamster ovary aprt gene by herpes simplex virus. J Virol. 1984 Nov;52(2):606–614. doi: 10.1128/jvi.52.2.606-614.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner F. J., Bornkamm G. W., Fleckenstein B. Episomal viral DNA in a Herpesvirus saimiri-transformed lymphoid cell line. J Virol. 1977 Jun;22(3):794–803. doi: 10.1128/jvi.22.3.794-803.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woychik R. P., Camper S. A., Lyons R. H., Horowitz S., Goodwin E. C., Rottman F. M. Cloning and nucleotide sequencing of the bovine growth hormone gene. Nucleic Acids Res. 1982 Nov 25;10(22):7197–7210. doi: 10.1093/nar/10.22.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woychik R. P., Lyons R. H., Post L., Rottman F. M. Requirement for the 3' flanking region of the bovine growth hormone gene for accurate polyadenylylation. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3944–3948. doi: 10.1073/pnas.81.13.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying S. Y., Guillemin R. Dried Staphylococcus aureus as a rapid immunological separating agent in radioimmunoassays. J Clin Endocrinol Metab. 1979 Feb;48(2):360–362. doi: 10.1210/jcem-48-2-360. [DOI] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]