Abstract
Rationale
A key role has been identified for the circadian locomotor output cycles kaput (Clock) gene in the regulation of drug reward. Mice bearing a dominant negative mutation in the Clock gene (ClockΔ19 mice) exhibit increased cocaine-induced conditioned place preference, reduced anxiety- and depression-like behavior, increased sensitivity to intracranial self-stimulation, and increased do-paminergic cell activity in the ventral tegmental area.
Objectives
We sought to determine if this hyperhedonic phenotype extends to cocaine self-administration and measures of motivation.
Methods
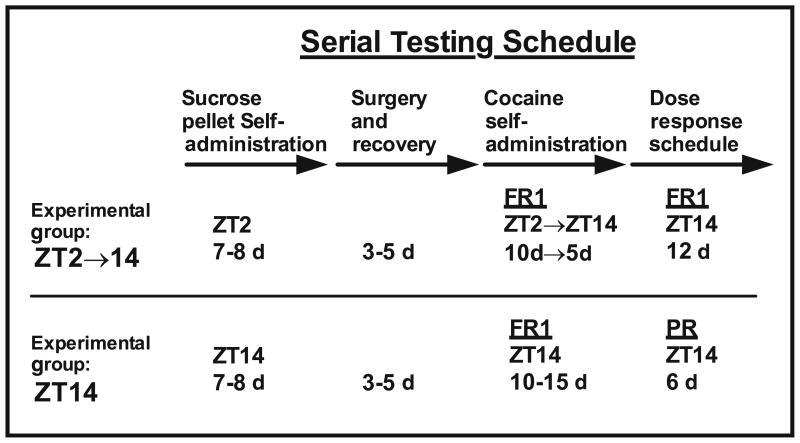
Two separate serial testing procedures were carried out (n=7–10/genotype/schedule). Testing began with acquisition of sucrose pellet self-administration, implantation of intravenous catheter, acquisition of cocaine self-administration, and dose–response testing (fixed ratio or progressive ratio). To evaluate diurnal variations in acquisition behavior, these sessions occurred at Zeitgeber 2 (ZT2) or ZT14.
Results
WT and ClockΔ19 mice exhibited similar learning and readily acquired food self-administration at both ZT2 and ZT14. However, only ClockΔ19 mice acquired cocaine self-administration at ZT2. A greater percentage of ClockΔ19 mice reached acquisition criteria at ZT2 and ZT14. ClockΔ19 mice self-administered more cocaine than WT mice. Using fixed ratio and progressive ratio schedules of reinforcement dose–response paradigms, we found that cocaine is a more efficacious reinforcer in ClockΔ19 mice than in WT mice.
Conclusion
Our results demonstrate that the Clock gene plays an important role in cocaine reinforcement and that decreased CLOCK function increases vulnerability for cocaine use.
Keywords: Clock gene, Cocaine self-administration, Reinforcer, Addiction, Circadian, Genetic animal models
Introduction
Physiological functions and behaviors exhibit endogenous rhythms, which enable organisms to adapt to their environment and anticipate a variety of stimuli. Circadian rhythms are regulated by a molecular circadian locomotor output cycles kaput (clock), which consists of several Clock genes that are present throughout the body. These proteins interact with each other through a series of transcriptional and translational feedback loops that are regulated over a 24-h period in the absence of environmental input (Takahashi et al. 2008). In mammals, the CLOCK and brain and muscle Arnt-like protein-1 (BMAL-1) proteins are transcriptional activators that heterodimerize and promote transcription of the Period (Per1, Per2, Per3) and Cryptochrome genes (Cry1, Cry2), as well as many other genes by binding to E-box elements (CANNTG) in their promoters (Reppert and Weaver 2001).
Disruption of sleep and circadian rhythms is a symptom common to many psychiatric disorders, including drug dependence. Drug dependence may be more prevalent in individuals with a compromised circadian clock, or with mood disorders that may have a circadian basis (Falcón and McClung 2009; Grandin et al. 2006; Kandel et al. 2001; McClung 2007). Several pre-clinical studies have identified an important role for circadian genes in drug-related behaviors (Falcón and McClung 2009; Perreau-Lenz and Spanagel 2008). Additionally, there are well-described diurnal variations in behavioral responses to drugs of abuse and the propensity to self-administer these drugs (Abarca et al. 2002; Lynch et al. 2008; Roberts et al. 2002). Rodent studies have shown prominent diurnal patterns of cocaine self-administration, with greater intake during the active (dark) phase (Lynch et al. 2008; Roberts et al. 2002). Further, when animals are given high doses of cocaine or access to more self-administration trials, cocaine intake is increased with a significant reduction in diurnal patterns of intake (Roberts et al. 2002). The loss of rhythm in intake may be important in the development of dependence, where there is a loss of control and escalation of drug intake that interferes with normal activities (Ahmed and Koob 1998).
Previously, we identified a key role for the Clock gene in mediating the response to drugs of abuse. Mice bearing a dominant negative mutation in the Clock gene (ClockΔ19 mice) display increased locomotor responses to novelty and express conditioned place preference for cocaine to a greater extent at lower cocaine doses (McClung et al. 2005). In addition to the increased preference for cocaine, these mice exhibit an overall “manic-like” phenotype characterized by reduced anxiety-like and depression-like behavior, increased intracranial self-stimulation at a lower threshold, and increased dopaminergic cell activity in the ventral tegmental area (VTA) (McClung et al. 2005; Roybal et al. 2007). Many of these behavioral phenotypes are rescued by expressing functional CLOCK in the VTA of CLOCKΔ19 mutants or are recapitulated by reducing Clock expression in the VTA of wild-type (WT) mice via RNA interference (Coque et al. 2011; Mukherjee et al. 2010; Roybal et al. 2007). Since these behavioral phenotypes are correlated with increased vulnerability to cocaine’s rewarding effects, we wanted to test ClockΔ19 mice and their WT littermates in the cocaine self-administration paradigm under fixed ratio (FR) and progressive ratio (PR) schedules of reinforcement (Marinelli and White 2000). This paradigm is clinically relevant and is a measure of behavioral sensitivity to the reinforcing and motivational properties of cocaine (Caine et al. 1999; Grahame et al. 1995; Richardson and Roberts 1995; Sanchis-Segura and Spanagel 2006).
Materials and methods
Mice
Clock mutant mice were created by N-ethyl-N-nitrosourea mutagenesis, resulting in a dominant negative CLOCK protein (King et al. 1997; Vitaterna et al. 1994). The mutant CLOCK has an internal deletion of 51 amino acids (due to deletion of exon 19) in the C-terminal transcriptional activation domain of the CLOCK protein. As a dominant negative or antimorph, CLOCKΔ19 heterodimerizes with BMAL-1 and binds to E-box elements but is unable to activate transcription (unpublished observation; Katada and Sassone-Corsi 2010; King et al. 1997). This mutation is maintained on a BALB/c background (>10 generations of backcrossing). The genotyping protocol is provided by Jackson Labs (http://jaxmice.jax.org/strain/002923.html). Male Clock mutant (ClockΔ19/ClockΔ19) and wild-type littermate controls were used to all experiments to facilitate comparison with published data (McClung et al. 2005; Roybal et al. 2007). Mice were 8–12 weeks old at the beginning of the experiment and were individually housed on a 12:12 h light/dark cycle (lights on 7 a.m., lights off 7 p.m.) with food and water ad libitum unless otherwise specified. Mice were weighed daily before each session.
Two separate serial testing procedures were carried out (n=7–10/genotype/schedule) with experiments performed at either Zeitgeber time 2 (ZT; lights on at ZT0), followed by ZT14 (termed ZT2⇒14group) or ZT14 only (Table 1). Testing began with acquisition of sucrose pellet self-administration, surgical implantation of an indwelling intravenous catheter, acquisition of cocaine self-administration, and either self-administration dose–response testing on a FR or on a PR schedule of cocaine reinforcement (Ruiz-Durántez et al. 2006; Sanchis-Segura and Spanagel 2006). Sucrose pellet self-administration was carried out to facilitate subsequent operant responding for cocaine. All experimental procedures were approved by the University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee.
Table 1.
Experimental design
|
Acquisition of sucrose pellet self-administration
Mice were weighed (free feeding weight) and then food deprived for 16 h. Afterward, mice were weighed and placed in an operant conditioning chamber equipped with a pellet dispenser, a food trough, and two levers, one on each side of the trough (Med Associates, VT, USA) for daily 1-h sessions (or until mice consumed 30 pellets). Mice achieved acquisition criteria for a session if they earned ≥25 pellets. During the session, the house light was on and there was continuous illumination of a cue light above the active (left) lever. A single response (FR1) at the active (left) lever resulted in delivery of a single sucrose pellet (20 mg, Bio-Serv, Frenchtown, NJ, USA) into the food trough. Mice were maintained at 85–90 % of their free feeding weight until they achieved ≥25 pellets for three sessions in a row.
Acquisition of cocaine self-administration
Following acquisition of sucrose pellet self-administration, mice were returned to free feeding levels and surgically implanted with a chronic indwelling intravenous catheter in the right jugular vein, as described earlier (Colby et al. 2003; Ruiz-Durántez et al. 2006). Catheters were flushed daily with 0.05 mL enrofloxacin and 0.05 mL heparinized saline containing gentamicin (20 IU/mL heparin; 0.33 mg/mL gentamicin), and catheter patency was verified once a week by administration of 0.05 mL methohexital sodium (0.5 mg/mL) followed by brief anesthesia. Three days after surgery, mice were tested for acquisition of cocaine self-administration in daily 1 h sessions on an FR1 schedule. An active (left) lever press resulted in concurrent delivery 50 μL of a cocaine solution over 2.5 s and presentation of a compound stimulus (cue light above lever and 2.9 kHz tone for 2.5 s, house light was off), after which there was an 8-s timeout when the house light remained off and additional responding had no programmed consequences. Inactive (right) lever pressing was recorded without any consequences. Acquisition of cocaine self-administration was conducted over 10 to 15 sessions (5 days/week) at a dose of 0.5 mg/kg cocaine per infusion using an FR1 schedule of reinforcement. Active lever responding (infusions+ timeout responding), inactive lever responding, and number of infusions were recorded for each session. Mice were considered to have acquired cocaine self-administration if they self-administered ≥15 infusions/session for three consecutive sessions (with ≥2:1 active/inactive lever press ratio). Acquisition latency was defined as the number of sessions until three consecutive sessions of ≥15 infusions/ session had been achieved. The acquisition criteria and dose selection are well described by Ruiz-Durantez et al. (2006).
Cocaine self-administration fixed ratio dose–response: ZT2⇒14group
After 10 sessions of acquisition training at ZT2, followed by five sessions at ZT14, mice (n=7/genotype) were subsequently tested on an FR1 schedule with descending unit doses (1.0, 0.5, 0.25, 0.0125, 0.063, and 0 mg/kg/infusion) at ZT14. Mice self-administered each unit dose for two consecutive sessions and each session lasted 1 h. Active lever responding (infusions+timeout responding), inactive lever responding, and the total number of infusions were recorded for each session.
Cocaine self-administration progressive ratio dose–response: ZT14 group
After 10–15 sessions of acquisition training at ZT14, only mice that met acquisition criteria (n=4–5/genotype) were subsequently tested at ZT14 on a progressive ratio schedule with three unit doses (1.0, 0.5, and 0.25 mg/kg/infusion; counterbalance dose order presentation). In this schedule, each successive cocaine infusion required increased lever press responding according to the following progression: 1, 2, 4, 6, 9, 12, 16, 20, 25, 30, 36, 42, 49, 56, 64, 72, 81, 90, 100, 110, 121, 132, 144, 156, 169, 182, 196, 210, 225, and 240 (limit). Mice self-administered each unit dose for two consecutive sessions and each session lasted 5 h. Active lever responding (infusions+timeout responding), inactive lever responding, and the total number of infusions were recorded for each session. The breakpoint was defined as the step value associated with the last completed ratio and was proportional to the number of reinforcers earned.
Drugs
Cocaine HCl (provided by the National Institute on Drug Abuse) was dissolved in 0.9 % saline in concentrations appropriate to doses. Drug dose was varied by varying the concentration of the drug solution.
Data analysis
Data are presented as group means±SEM. Two-way analysis of variance (ANOVA) was used to compare the following data (factors identified in parentheses): acquisition (genotype×ZT), latency to acquire (genotype×ZT; where only data from mice which acquired were included in analysis), infusions earned during maintenance (genotype×ZT), FR1 schedule dose–response (genotype×dose), and progressive ratio schedule sessions (genotype×dose). Three-way ANOVA was used to analyze the following data: acquisition of sucrose pellet self-administration (genotype× ZT×session), lever discrimination during cocaine self-administration (genotype×ZT×lever type for Fig. 2c; geno-type×dose×lever type for Figs. 3b and 4b). Repeated measures were employed where appropriate. Significant effects (defined as p≤0.05) were further analyzed by Bonferroni post hoc testing. Specifically, we hypothesized that ClockΔ19 mice would earn more cocaine infusions and exhibit increased lever pressing during acquisition and maintenance, as well as for each dose tested during the FR1 and PR schedule. Graphs were produced and statistics were performed using GraphPad Prism version 4.00 (Graph-Pad Software, San Diego, CA, USA) and SPSS Statistics 20 (IBM, Armonk, NY, USA).
Fig. 2.
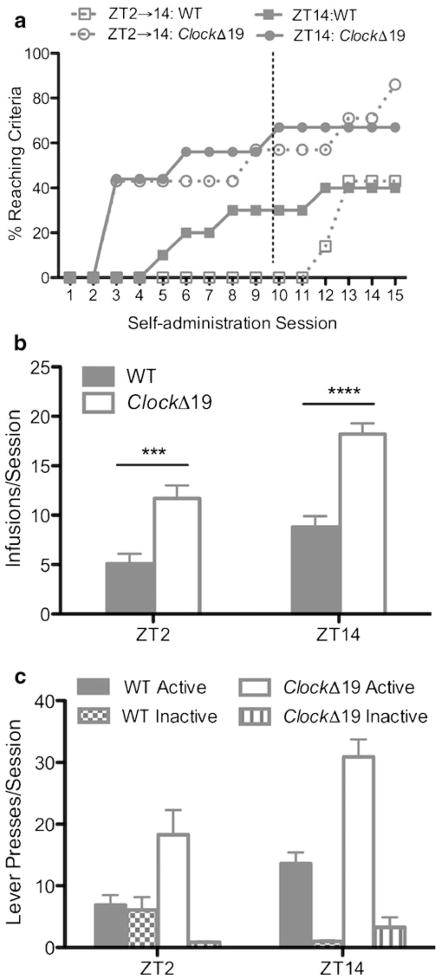
ClockΔ19 mice exhibit a greater propensity to initiate cocaine use, reduced latency to acquire cocaine self-administration, and increased cocaine intake. a Percent of mice reaching acquisition criteria. Open (ZT2⇒14 group) and closed (ZT14 group) circles denote ClockΔ19 mice, and open (ZT2⇒14 group) and closed (ZT14 group) squares denote WT mice. Dashed vertical line between sessions 10 and 11 denotes the transition ZT2⇒14 (described in the “Materials and methods” section). b Cocaine infusions earned for ZT2 and ZT14 sessions. Open bars denote ClockΔ19 mice and gray bars denote WT mice. c Lever discrimination (mean number of active and inactive lever presses). Open bars denote ClockΔ19 mice active lever presses, bars with vertical lines denote ClockΔ19 mice inactive lever presses, gray bars denote WT mice active lever presses, and checkered bars denote WT mice inactive lever presses. ***p<0.001; ****p<0.0001. N=7–10/genotype/ZT
Fig. 3.
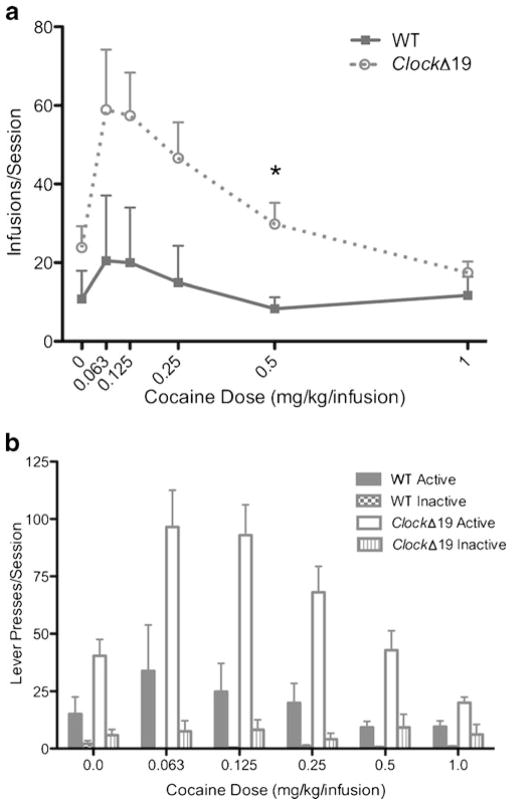
Cocaine is a more efficacious reinforcer in ClockΔ19 mice than in WT mice. a Cocaine infusions earned in an FR1 descending dose–response schedule. b Dose–response lever discrimination (mean number of active and inactive lever presses). Open bars denote ClockΔ19 mice active lever presses, bars with vertical lines denote ClockΔ19 mice inactive lever presses, gray bars denote WT mice active lever presses, and checkered bars denote WT mice inactive lever presses. Open circles denote ClockΔ19 mice and gray squares denote WT mice. N=7/genotype
Fig. 4.
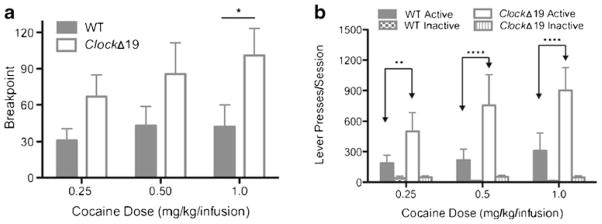
ClockΔ19 mice displayed greater motivation to obtain cocaine infusions in a progressive ratio schedule of reinforcement. a Number of lever presses at which the breakpoint occurred. b Dose–response lever discrimination (mean number of active and inactive lever presses). Open bars denote ClockΔ19 mice and gray bars denote WT mice. *p<0.05; **p<0.01; ****p<0.0001. N=4–5/genotype
Results
Acquisition of sucrose pellet self-administration
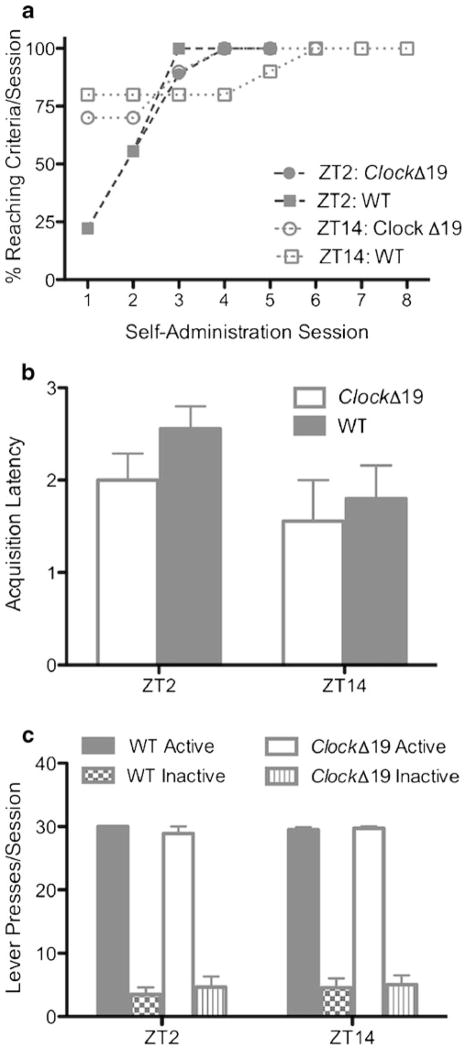
The percent of mice that reached acquisition criteria (>25 pellets) on the first training day was greater for mice undergoing sessions during ZT14 than ZT2 (Fig. 1a). A three-way ANOVA (genotype×ZT×session) was performed to analyze the sucrose pellet acquisition data. There was a significant genotype×ZT interaction (F(1,147)=6.74, p<0.01), as well as a significant ZT×session interaction (F(4,147)=6.78, p<0.0001). These interactions were not followed by significant main effects for other variables. Although there was a significant interaction genotype×ZT interaction noted, these are largely due to the first training session. Further, the latency to acquire sucrose pellet self-administration did not significantly differ for the time of day the sessions occurred (either ZT2 or ZT14) or for the genotypes tested (Fig. 1b). Mean responding for each lever for the three consecutive sessions that enabled each subject to meet acquisition criteria is presented in Fig. 1c. Mice that achieved acquisition criteria for three consecutive days were no longer subjected to subsequent sessions. These data suggest that normal CLOCK function is not required for acquisition of responding for a natural (non-drug) reinforcer and that initial differences in training can be seen during the early phase of the light and dark cycle.
Fig. 1.
Normal CLOCK function is not required for acquisition of responding for sucrose pellet reinforcers during the early phase of the light or dark cycle. a The percent of mice that reached acquisition criteria for each session (presented as a cumulative percentage). Open (ZT14 group) and closed (ZT2 group) circles denote ClockΔ19 mice, and open (ZT14 group) and closed (ZT2 group) squares denote WT mice. b Latency to acquire sucrose pellet self-administration at ZT2 and ZT14. Open bars denote ClockΔ19 mice and gray bars denote WT mice. c Lever discrimination (mean number of active and inactive lever presses) for the three consecutive sessions that enabled each subject to meet acquisition criteria. Open bars denote ClockΔ19 mice active lever presses, bars with vertical lines denote ClockΔ19 mice inactive lever presses, gray bars denote WT mice active lever presses, and checkered bars denote WT mice inactive lever presses. Open (inactive lever) and closed (active lever) circles denote ClockΔ19 mice, and open (inactive lever) and closed (active lever) squares denote WT mice. N=9–10/genotype/ZT
Acquisition of cocaine self-administration
Two serial testing procedures were carried out (termed ZT2⇒14and ZT14). In the ZT2⇒14 testing procedure, none of the WT mice tested at ZT2 had acquired cocaine self-administration after 10 sessions. Subsequent sessions occurred at ZT14, when both genotypes were more likely to self-administer cocaine, thus facilitating genotype comparisons in approaching dose–response schedules (Roberts et al. 2002). For both serial testing procedures (ZT2⇒14 and ZT14), the percent of ClockΔ19 mice that reached acquisition criteria for cocaine self-administration was greater than that of WT mice after 15 sessions (main effect of genotype—F(1,56)=41.25, p<0.001; Fig. 2a). For mice that acquired cocaine self-administration, acquisition latencies were analyzed using a two-way ANOVA (genotype×ZT). ClockΔ19 mice had significantly shorter acquisition latencies (ZT2⇒14, 4.5±1.5 sessions; ZT14, 4.7±1.2 sessions) than WT mice (ZT2⇒14, 12.7±0.3 sessions; ZT14, 7.8±1.5 sessions; main effect of genotype—F(1,13)=16.83, p<0.01). We compared the first 10 self-administration sessions to determine whether the time of day a session occurred (ZT2 or ZT14) had an effect on cocaine self-administration. Both genotypes self-administered more cocaine at ZT14 than at ZT2 (main effect of testing time—F (1, 36) =20.4, p < 0.0001). However, ClockΔ19 mice earned a greater number of cocaine infusions per session than WT mice (main effect of genotype—F(1,36)=50.1, p<0.0001; Fig. 2b). The average number of responses (active and inactive lever presses) was analyzed by three-way ANOVA (genotype×ZT×lever type) (Fig. 2c). There was a significant genotype×lever type interaction (F(1,60)=7.96, p<0.01), an expected significant main effect of lever type (F(1,60)=27.03, p<0.0001), and a significant main effect of genotype (F(1,60)=5.26, p<0.05). Together, these data show that a single mutation in the Clock gene produced a greater propensity to initiate cocaine use, reduced latency to acquire cocaine self-administration during the early phase of the light or dark cycle, and increased cocaine intake.
Cocaine self-administration FR1 schedule dose–response analysis
After 15 days of cocaine self-administration, mice from the ZT2⇒14group were tested on descending unit doses using an FR1 schedule of reinforcement (Fig. 3a). The average number of infusions per session was analyzed using two-way ANOVA (genotype×dose). The number of infusions depended on the dose available, where both ClockΔ19 and WT mice earned more cocaine infusions at the lower end of the dose scale (main effect of dose—F(5, 60)=6.51, p<0.0001). The number of infusions earned was dependent on genotype, where ClockΔ19 mice earned more infusions overall than did WT mice (main effect of genotype—F(1, 12)=4.79, p<0.05). Post hoc analysis revealed a significant increase in infusions earned for ClockΔ19 mice at the 0.5 mg/kg/infusion dose. An upward shift in the dose–response curve is seen for ClockΔ19 mice, suggesting that cocaine is a more efficacious reinforcer in ClockΔ19 mice than in WT mice. Lever discrimination data was analyzed by three-way ANOVA (genotype × dose × lever type) (Fig. 3b). There was a significant dose×lever type (F(5, 311)=6,41, p<0.0001) and genotype×lever type (F(1, 311)=30.11, p<0.0001) interaction, as well as a main effect of genotype (F(1,311)=54.11, p<0.001), dose (F(5,311)= 6.33, p<0.001), and lever type (F(1,311)=121.9, p<0.001). There was a strong trend toward significance for the genotype×dose interaction (F(5,311)=2.17, p=0.058).
Cocaine self-administration progressive ratio schedule dose–response analysis
After 10–15 days of cocaine self-administration, mice that acquired cocaine self-administration from the ZT14 group were tested on three unit doses (with counterbalanced dose presentation) using a progressive ratio schedule of reinforcement. The number of lever presses at which the breakpoint occurred was significantly greater for ClockΔ19 mice than for WT mice (main effect of genotype—F(1,48)= 7.87; p < 0.01; Fig. 4a). Post hoc analysis revealed ClockΔ19 mice had a significantly higher breakpoint then WT mice at the 1.0 mg/kg/infusion dose. Furthermore, ClockΔ19 mice earned more infusions per session than WT mice (main effect of genotype—F(1, 48) =9.16, p<0.01). Lastly, ClockΔ19 mice performed significantly more active lever presses per session than WT mice for each unit dose test (main effect of genotype—F(1,48)=79, p<0.0001; main effect of dose—F(2,48)=7.34, p<0.01; Fig. 4b). ClockΔ19 mice displayed greater motivation to obtain cocaine infusions. Together, these results show that a mutation in the Clock gene produced increased sensitivity to the reinforcing efficacy of cocaine.
Discussion
ClockΔ19 mice display a greater propensity to initiate use (regardless of time of day sessions occurred), reduced latency to acquire cocaine self-administration, and elevated cocaine intake during acquisition as compared with WT mice. ClockΔ19 mice exhibit an upward shift in an FR1 descending dose–response schedule, evidence of increased cocaine efficacy. Further, ClockΔ19 mice exhibit higher break points than WT mice in a progressive ratio dose–response schedule, suggesting that they have increased motivation for cocaine. Together, this indicates that ClockΔ19 mice are more sensitive to the reinforcing efficacy of cocaine (Caine et al. 1999; Grahame et al. 1995; Richardson and Roberts 1995; Sanchis-Segura and Spanagel 2008). Moreover, these differences are not likely attributed to performance or operant learning capacity, since both genotypes readily acquired lever press behavior for sucrose pellets with similar latencies.
It is well-known that the extent to which mice will self-administer cocaine depends heavily on its genetic background and BALB/c mice, the genetic background of the mice used in these experiments, normally exhibit a very low propensity to acquire cocaine self-administration, thus making the effects of the Clock mutation on this background more striking (Deroche et al. 1997; Ruiz-Durántez et al. 2006; Thomsen and Caine 2006). WT mice exhibit cocaine self-administration behaviors that may in large part be attributed to the BALB/c genetic background (including low rates of acquisition, low infusions during maintenance, and low breakpoints that do not change with increasing dose). The increased responding for cocaine seen with ClockΔ19 mice further supports their phenotypic characterization as hyperhedonic, where ClockΔ19 mice exhibit reduced anxiety-like and depression-like behaviors, increased intracranial self-administration at a lower threshold, increased sucrose preference, and increased alcohol intake (McClung et al. 2005; Ozburn and McClung 2010; Roybal et al. 2007). Additionally, there are known diurnal variations in behavioral responses to drugs of abuse and the propensity to self-administer these drugs (Abarca et al. 2002; Lynch et al. 2008; Roberts et al. 2002). Rodent studies have shown prominent diurnal patterns of cocaine self-administration, with greater intake during the active (dark) phase (Lynch et al. 2008; Roberts et al. 2002). To facilitate comparison with published data, we examined acquisition and intake during the first 10 cocaine self-administration sessions at ZT2 or ZT14. In concordance with published data, we found that WT mice did not acquire cocaine self-administration during their inactive (light) phase, and approximately 30 % of mice acquired cocaine self-administration when sessions occurred during their active (dark) phase. In contrast, we found that 57 % of ClockΔ19 mice acquired cocaine self-administration during their inactive period, and 70 % acquire during the active period. The blunted diurnal rhythm in cocaine self-administration and increased cocaine intake that ClockΔ19 mice exhibit may be important in the development of dependence, where there is a loss of control and escalation of drug intake that interferes with normal activities (Ahmed and Koob 1998). Together, this further demonstrates that disruption of CLOCK protein function results in increased cocaine abuse liability in mice with a non-drug preferring genetic background.
There is dissociation between standard paradigms used in addiction research to assess the rewarding properties of drugs of abuse (i.e., conditioned place preference, locomotor sensitization, and self-administration). For example, Per1 mutant mice do not exhibit cocaine-induced locomotor sensitization or conditioned place preference, but they do self-administer cocaine (Abarca et al. 2002; Halbout et al. 2011). Through various genetic and pharmacological studies, it is now well accepted that these behaviors rely on different neurobiological substrates. Further, these and many behaviors are influenced by locomotor activity. ClockΔ19 mice exhibit increased home cage activity and increased locomotor activity in response to a novel environment. In contrast, ClockΔ19 mice exhibit locomotor sensitization to cocaine to a similar extent as their WT littermates (McClung et al. 2005; Roybal et al. 2007). Previously, we presented the results of noncontingent delivery of cocaine in conditioned place preference and intracranial self-stimulation studies which indicated CLOCK acts a negative regulator of the rewarding properties of cocaine. In the present study, we present results showing the contingent delivery of cocaine in self-administration studies indicate CLOCK acts as a negative regulator of the incentive motivational properties of cocaine. Thus, CLOCK has a role in the neurobiological processes involved in both the behavioral response to cocaine and the incentive motivation for cocaine. ClockΔ19 mice earn more cocaine infusions in an FR1 dose–response schedule. Since the dose–response curve is shifted upward for ClockΔ19 mice, even at the 0.0 mg/kg dose, one interpretation could be that these differences are due to locomotor activity. However, both WT and ClockΔ19 mice displayed similar and appropriate lever discrimination. Importantly, active lever pressing for the 0.0 mg/kg dose is not significantly different. The modest increase in responding may be due to the descending dose presentation, where ClockΔ19 mice seem to display somewhat perseverant behavior during what is essentially extinction. ClockΔ19 mice exhibited further evidence of increased motivation for cocaine in the PR schedule, where they performed roughly three times as many active lever presses as WT mice. Together, this suggests that the increased acquisition, intake, FR1, and PR responding seen in ClockΔ19 mice are not solely due increased locomotor activity. In the context of previous findings, it is evident that the ClockΔ19 mice have a hyperhedonic, or “manic-like” phenotype that extends to drugs of abuse.
Increased excitability of VTA DA neurons is linked with increased levels of reward (Adamantidis et al. 2011; Marinelli and White 2000). DA cell firing rates are increased in ClockΔ19 mice, a phenomenon which also occurs in mice that have reduced Clock expression in the VTA due to RNA interference (McClung et al. 2005; Mukherjee et al. 2010). Genes involved in DA synthesis, metabolism, and release, which are known to influence dopaminergic activity in the VTA, are up regulated in ClockΔ19 mutants and in mice that have reduced Clock expression in the VTA due to RNA interference (McClung et al. 2005; Mukherjee et al. 2010). ClockΔ19 mice display augmented DA synthesis and activity primarily during the light cycle (McClung et al. 2005; unpublished observation). Therefore, it is conceivable these alterations underlie the increased daytime sensitivity to the reinforcing efficacy of cocaine seen in ClockΔ19 mice.
There is strong evidence from the literature suggesting that these CLOCK-mediated effects on cocaine reward are likely related to augmented dopaminergic activity. However, there is also evidence of altered glutamatergic systems in ClockΔ19 mice that may contribute to their drug preferring phenotype (Beaulé et al. 2009; Dzirasa et al. 2010). Both of these systems have long been implicated in drug-related behaviors and drug-induced plasticity. Recent findings have revealed that glutamate is co-released from dopaminergic VTA terminals (Hnasko et al. 2010). Further, Beaulé et al. (2009) found that ClockΔ19 mice have significantly reduced glial glutamate uptake and GLAST levels, presumably resulting in increased glutamatergic tone. We also found significantly reduced Glast expression in the VTA (at ZT4 and ZT16) of ClockΔ19 mice (unpublished observation). Together, augmented dopaminergic and glutamatergic systems may represent the molecular mechanisms underlying the increased propensity for addiction-like behavior seen in ClockΔ19 mice. Ongoing and future studies in our lab are focused on determining molecular mechanisms that underlie the hyperhedonic behavioral phenotype of the ClockΔ19 mouse. These findings will help us to understand the genetic vulnerability for addiction and to develop novel treatments in the future.
Acknowledgments
This study was supported by NIH grants DA-07290 and AA-020452 (to ARO), DA-023988 (to CAM), and DA-010460 (to DWS). The authors thank Edgardo Falcon, Andrea Gillman, Alexandria Nugent, Daniel Guzman, Elizabeth Gordon, and Ariel Ketcherside for technical help and advice. ClockΔ19 mice were a generous gift from Joseph Takahashi.
Footnotes
Conflict of interest The authors report no conflict of interest.
Contributor Information
Angela Renee Ozburn, Email: ozburna@gmail.com, Translational Neuroscience Program, Department of Psychiatry, University of Pittsburgh, 450 Technology Dr. (BSPII Ste 233), Pittsburgh, PA 15219, USA. Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas, TX 75235, USA.
Erin Beth Larson, Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas, TX 75235, USA.
David W. Self, Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas, TX 75235, USA
Colleen A. McClung, Translational Neuroscience Program, Department of Psychiatry, University of Pittsburgh, 450 Technology Dr. (BSPII Ste 233), Pittsburgh, PA 15219, USA. Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas, TX 75235, USA
References
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA, Touriño C, Bonci A, Deisseroth K, de Lecea L. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Beaulé C, Swanstrom A, Leone MJ, Herzog ED. Circadian modulation of gene expression, but not glutamate uptake, in mouse and rat cortical astrocytes. PLoS One. 2009;4:e7476. doi: 10.1371/journal.pone.0007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK. Method for training operant responding and evaluating cocaine self-administration behavior in mutant mice. Psychopharmacology. 1999;147:22–24. doi: 10.1007/s002130051134. [DOI] [PubMed] [Google Scholar]
- Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine. J Neurosci. 2003;23:2488–2493. doi: 10.1523/JNEUROSCI.23-06-02488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coque L, Mukherjee S, Cao JL, Spencer S, Marvin M, Falcon E, Sidor MM, Birnbaum SG, Graham A, Neve RL, Gordon E, Ozburn AR, Goldberg MS, Han MH, Cooper DC, McClung CA. Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockΔ19 mouse model of mania. Neuropsychopharmacology. 2011;36:1478–1488. doi: 10.1038/npp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche V, Caine SB, Heyser CJ, Polis I, Koob GF, Gold LH. Differences in the liability to self-administer intravenous cocaine between C57BL/6×SJL and BALB/cByJ mice. Pharmacol Biochem Behav. 1997;57:429–440. doi: 10.1016/s0091-3057(96)00439-x. [DOI] [PubMed] [Google Scholar]
- Dzirasa K, Coque L, Sidor MM, Kumar S, Dancy EA, Takahashi JS, McClung CA, Nicolelis MA. Lithium ameliorates nucleus accumbens phase-signaling dysfunction in a genetic mouse model of mania. J Neurosci. 2010;30:16314–16323. doi: 10.1523/JNEUROSCI.4289-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcón E, McClung CA. A role for the circadian genes in drug addiction. Neuropharmacology. 2009;56(Suppl 1):91–96. doi: 10.1016/j.neuropharm.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame NJ, Phillips TJ, Burkhart-Kasch Sm, Cunningham CL. Intravenous cocaine self-administration in the C57BL/6J mouse. Pharmacol Biochem Behav. 1995;51:827–834. doi: 10.1016/0091-3057(95)00047-z. [DOI] [PubMed] [Google Scholar]
- Grandin LD, Alloy LB, Abramson LY. The social zeitgeber theory, circadian rhythms, and mood disorders: review and evaluation. Clin Psychol Rev. 2006;26:679–694. doi: 10.1016/j.cpr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Halbout B, Perreau-Lenz S, Dixon CI, Stephens DN, Spanagel R. Per1Brdm1 mice self-administer cocaine and reinstate cocaine-seeking behaviour following extinction. Behav Pharmacol. 2011;22:76–80. doi: 10.1097/FBP.0b013e328341e9ca. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Huang FY, Davies M. Comorbidity between patterns of substance use dependence and psychiatric syndromes. Drug Alcohol Depend. 2001;64:233–241. doi: 10.1016/s0376-8716(01)00126-0. [DOI] [PubMed] [Google Scholar]
- Katada S, Sassone-Corsi P. The histone methyl transferase MLL1 permits the oscillation of circadian gene expression. Nat Struc Mol Biol. 2010;17:1414–1421. doi: 10.1038/nsmb.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Girgenti MJ, Breslin FJ, Newton SS, Taylor JR. Gene profiling the response to repeated cocaine self-administration in dorsal striatum: a focus on circadian genes. Brain Res. 2008;1213:166–177. doi: 10.1016/j.brainres.2008.02.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci. 2000;20(23):8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, Graham A, Gordon E, Enwright JF, 3rd, DiLeone RJ, Birnbaum SG, Cooper DC, McClung CA. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry. 2010;68:503–511. doi: 10.1016/j.biopsych.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn A, McClung C. Ethanol-related behaviors in ClockΔ19 mice. Alcohol Clin Exp Res. 2010;34(95A Supplement) [Google Scholar]
- Perreau-Lenz S, Spanagel R. The effects of drugs of abuse on clock genes. Drug News Perspect. 2008;21:211–217. doi: 10.1358/dnp.2008.21.4.1213350. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluating reinforcing efficacy. Journal of Neuroscience Methods. 1995;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Brebner K, Vincler M, Lynch WJ. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug and Alcohol Dep. 2002;67:291–299. doi: 10.1016/s0376-8716(02)00083-2. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA, Jr, McClung CA. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Durántez E, Hall SK, Steffen C, Self DW. Enhanced acquisition of cocaine self-administration by increasing percentages of C57BL/6J genes in mice with a nonpreferring outbred background. Psychopharmacology (Berl) 2006;186:553–560. doi: 10.1007/s00213-006-0379-2. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Cocaine self-administration under fixed and progressive ratio schedules of reinforcement: comparison of C57BL/6J, 129X1/SvJ, and 129S6/SvEvTac inbred mice. Psychopharmacology (Berl) 2006;184:145–154. doi: 10.1007/s00213-005-0207-0. [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]