Abstract
Epidemiologic evidence has linked chronic exposure to inorganic arsenic (iAs) with an increased prevalence of diabetes mellitus. Laboratory studies have identified several mechanisms by which iAs can impair glucose homeostasis. We have previously shown that micromolar concentrations of arsenite (iAsIII) or its methylated trivalent metabolites, methylarsonite (MAsIII) and dimethylarsinite (DMAsIII), inhibit the insulin-activated signal transduction pathway, resulting in insulin resistance in adipocytes. Our present study examined effects of the trivalent arsenicals on insulin secretion by intact pancreatic islets isolated from C57BL/6 mice. We found that 48-hour exposures to low subtoxic concentrations of iAsIII, MAsIII or DMAsIII inhibited glucose-stimulated insulin secretion (GSIS), but not basal insulin secretion. MAsIII and DMAsIII were more potent than iAsIII as GSIS inhibitors with estimated IC50≤0.1 μM. The exposures had little or no effects on insulin content of the islets or on insulin expression, suggesting that trivalent arsenicals interfere with mechanisms regulating packaging of the insulin transport vesicles or with translocation of these vesicles to the plasma membrane. Notably, the inhibition of GSIS by iAsIII, MAsIII or DMAsIII could be reversed by a 24-hour incubation of the islets in arsenic-free medium. These results suggest that the insulin producing pancreatic β-cells are among the targets for iAs exposure and that the inhibition of GSIS by low concentrations of the methylated metabolites of iAs may be the key mechanism of iAs-induced diabetes.
Keywords: Arsenic, Diabetes, Isolated pancreatic islets, β-Cells, Insulin secretion
Introduction
Arsenic (As) is a naturally occurring toxic metalloid commonly found in ground and surface water reservoirs. Tens of millions of people worldwide are currently exposed to high levels of inorganic arsenic (iAs) in drinking water (BGS and DPHE, 2001; The World Bank, 2005). At least 13 million U.S. residents drink water containing iAs at levels higher than the current EPA maximum contaminant level of 10 μg As/L (ATSDR, 2007). Although the focus of public health and regulatory agencies has traditionally been on the carcinogenic effects of iAs, epidemiological evidence suggests that even greater numbers of people exposed to iAs are at risk of developing non-cancerous diseases, including diabetes mellitus. A panel of experts assembled by the NIEHS National Toxicology Program (NTP) in January 2011 agreed that existing data in humans support an association between diabetes and high exposures to iAs in drinking water (≥150 ppb As); however, the evidence is insufficient to conclude that diabetes is associated with low-to-moderate exposures (<150 ppb As) (Maull et al., 2012). This panel also concluded that the animal and in vitro studies implicate several pathways by which iAs can influence pancreatic β-cell function and insulin sensitivity.
Diabetes associated with iAs exposure has been historically referred to as type 2 diabetes (Longnecker and Daniels, 2001; Maull et al., 2012; Navas-Acien et al., 2006). However, little information has been provided on the mechanisms underlying this disease. We have shown that subtoxic micromolar concentrations of arsenite (iAsIII) and its methylated trivalent metabolites, methylarsonite (MAsIII) and dimethylarsinite (DMAsIII), inhibit the insulin-dependent phosphorylation of PKB/Akt by PDK, thus suppressing the insulin-stimulated glucose uptake in adipocytes (Paul et al., 2007a, 2008; Walton et al., 2004). DMAsIII did not inhibit PKB/Akt phosphorylation, but interfered with insulin signaling downstream from PKB/Akt. While these results are consistent with insulin resistance typically associated with type 2 diabetes, other studies have shown that iAs can also target the mechanisms regulating glucose-stimulated insulin secretion (GSIS) by pancreatic β-cells.
GSIS is a biphasic process (Rorsman et al., 2000). The 1st phase is initiated by the uptake and oxidative metabolism of glucose, resulting in an increased production of ATP and depolarization of plasma membrane, followed by influx of extracellular calcium ions and activation of Ca2+-dependent calmodulin protein kinases that phosphorylate proteins associated with insulin secretory vesicles, the ion channels, and the cytoskeletal structure. The 2nd phase of GSIS involves an ATP-dependent mobilization of storage pool granules to the cell surface to sustain insulin release. Results of previous studies suggest several mechanisms by which iAs could interfere with GSIS: (i) Like glucose, iAsIII is transported across the plasma membrane by GLUT transporters (Liu et al., 2006) and thus, could compete with glucose uptake by β-cells. (ii) iAsIII modulates expression of hexokinase, the enzyme that helps to control flux of glucose-6-phosphate into glycolysis (Pysher et al., 2007). (iii) iAsIII forms a stable complex with dihydrolipoamide, a cofactor of pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, the key enzymes in the oxidative metabolism of glucose (Tsen, 2004). (iv) iAsIII inhibits calpain-10 (Díaz-Villaseñor et al., 2008), the Ca2+-dependent protease that activates SNAP-25 (a member of the insulin secretory machinery) (Turner, 2007). (v) Finally, arsenate (iAsV), the product of iAsIII oxidation, can interfere with ATP synthesis by replacing phosphate in the reactions of oxidative phosphorylation in mitochondria (Gresser, 1981). Another mechanism has been proposed by a recent study using rat insulinoma (INS-1) cells. Here, exposure to iAsIII was shown to provoke an adaptive oxidative stress response that increased antioxidant levels and dampened signaling involving reactive oxygen species that is thought to be essential for regulation of GSIS (Fu et al., 2010).
Notably, all the above studies examined only effects of iAs species, iAsIII and iAsV. In addition, most of these studies used insulinoma cell lines which differ from the insulin secreting pancreatic islets by their morphology, level of differentiation, insulin content and, most importantly by their abnormal response to glucose stimulation. The present study used intact isolated pancreatic islets to compare the effects of iAsIII with effects of its methylated trivalent metabolites, MAsIII and DMAsIII, which are more reactive and more toxic than either iAsIII or iAsV (Styblo et al., 2000, 2002; Thomas et al., 2001). Our results show that both MAsIII and DMAsIII are more potent than iAsIII as inhibitors of GSIS and that the inhibition of GSIS by trivalent arsenicals can be reversed by incubating the islets in As-free medium.
Materials and methods
Isolated pancreatic islets
Pancreatic islets were isolated from adult male C57BL/6 mice (Charles River Laboratories, Wilmington, MA). All procedures involving mice were approved by the University of North Carolina Institutional Animal and Use Committee. Mice were sacrificed by cervical dislocation and pancreas was infused in situ with collagenase P (1 mg/ml, Roche Diagnostics Corp., Indianapolis, IN) via the common bile duct. Pancreas was then removed and digested in the collagenase solution for 12 min at 37 °C. The digestate was washed and islets were purified by centrifugation in a gradient of Ficoll PM 400 (GE Healthcare, Uppsala, Sweden) (Szot et al., 2007).
Treatment
The isolated islets were cultivated overnight at 37 °C with 5% CO2 in RPMI 1640 medium (Mediatech, Manassas, VA) with 10% fetal bovine serum, 10 mM Hepes, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Sigma-Aldrich, St. Louis, MO). The same medium was used in experiments in which the islets were exposed to iAsIII (sodium arsenite; Sigma-Aldrich), MAsIII (methylarsine oxide) or DMAsIII (iododimethylarsine). MAsIII and DMAsIII were provided by Dr. William Culllen (University of British Columbia, Vancouver, Canada). To limit oxidation of trivalent arsenicals, the culture medium was replaced every 24 h with medium containing freshly prepared arsenicals.
Speciation analysis of As
The concentrations and metabolism of arsenicals in the islets were monitored by hydride generation (HG)-cryotrapping (CT)-inductively coupled plasma-mass spectrometry (ICP-MS), using Agilent 7500cx ICP-MS system (Agilent Technologies, Santa Clara, CA) and the HG-CT components and procedures previously described for HG-CT-atomic absorption spectrometry (AAS) technique for speciation analysis of As in biological samples (Hernández-Zavala et al., 2008; Matoušek et al., 2008) (see Supplementary Materials for details).
Static GSIS assay
Islets exposed to arsenicals or control, unexposed islets (15 islets/assay) were transferred into a glucose-free buffer containing 114 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.16 mM MgSO4, 20 mM Hepes, 2.5 mM CaCl2, 0.2% bovine serum albumin, and 25.5 mM NaHCO3 (all from Sigma-Aldrich) for 1 h at 37 °C and 5% CO2, followed by a 1-hour incubation with 2.5 mM glucose (Sigma-Aldrich) and 1-hour incubation with 16.7 mM glucose (Boucher et al., 2004). Medium from each incubation step was frozen and stored for insulin analysis. Insulin concentrations were determined using Rat/Mouse Insulin ELISA kit from Millipore (Billerica, MA). The 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay (Styblo et al., 2002) was used to monitor islet viability.
Quantitative PCR (qPCR)
Islet RNA was extracted using RNEasy Micro kit with on column DNAse treatment (Qiagen, Valencia, CA), and analyzed by a Nanodrop 2000c spectrophotometer (ThermoScientific, Waltham, MA). cDNA was prepared using random primers and Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA). The transcripts of two mouse insulin genes (Ins1 and Ins2) and 18S RNA were quantified using the corresponding Taqman assays with Fast Universal PCR master mix (Applied Biosystems, Foster City, CA) and Lightcycler 480 II instrument and software 1.5.0 (Roche).
Statistical analysis
Data were analyzed by ANOVA and Fisher’s PLSD tests using StatView 5.0.1 software (SAS institute, Cary, NC). Data are represented as mean±standard error (SE). Differences between treatments with p<0.05 were considered statistically significant.
Results and discussion
The iAs-induced diabetes has been referred to as type 2 diabetes because of the adult onset of the disease and absence of ketoacidosis which is typically associated with type 1 diabetes (Haller et al., 2005). The onset and early stages of type 2 diabetes are characterized by insulin resistance, i.e., nonresponsiveness to insulin signaling leading to impaired uptake of glucose in peripheral tissues, and hyperinsulinemia due to a compensatory increase of insulin production by β-cells. The continued insulin resistance ultimately leads to the pancreatic β-cell failure (Prentki and Nolan, 2006). However, results of our recent laboratory and population studies suggest that the phenotype of diabetes associated with iAs exposure may differ from that of type 2 diabetes. Specifically, we have shown that exposure of C57Bl/6 mice to iAs alone or combined with high-fat diet produced diabetes which is characterized by low insulin resistance (HOMA-IR) and impaired insulin production in response to glucose challenge (Paul et al., 2007b, 2011). Similarly, we found negative correlations between iAs exposure and fasting plasma insulin and HOMA-IR among residents of the Zimapan and Lagunera regions in Mexico who drank water containing iAs (up to 215 μg As/L) (Del Razo et al., 2011). Taken together these data point to β-cells as a primary target for the diabetogenic effects of iAs exposure and to an impaired GSIS as the key mechanism of iAs-induced diabetes. The present study used pancreatic islets isolated from C57Bl/6 mice to examine effects of iAs and its toxic methylated trivalent metabolites on GSIS.
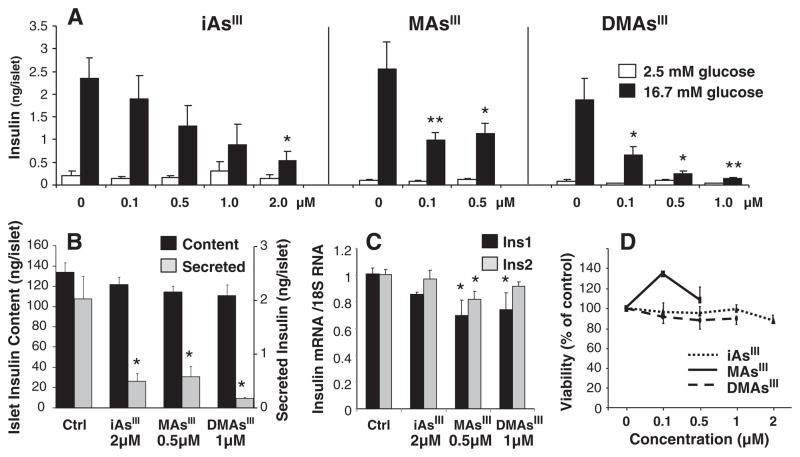
The islets used in this study were fully functional as documented by a strong response to glucose stimulation, i.e., 10–15-fold increase in insulin secretion by control (untreated) islets treated with 16.7 mM glucose (Fig. 1A). Exposures to low sub-micromolar or micromolar concentrations of trivalent arsenicals for 48 h significantly decreased GSIS in islets stimulated with 16.7 mM glucose, but had no effects on basal insulin secretion by islets incubated with 2.5 mM glucose. Notably, MAsIII, and particularly DMAsIII were more potent than iAsIII as GSIS inhibitors, decreasing insulin secretion by >50% at the concentration of 0.1 μM. These exposures, however, did not affect the total insulin content in the culture; i.e., the amount of insulin secreted in response to glucose stimulation plus the amount of insulin remaining in the stimulated islets (Fig. 1B). Results of a previous study using primary rat β-cells have suggested that a 144-hour exposure to cytotoxic 5 μM iAsIII suppresses insulin expression (Díaz-Villaseñor et al., 2006). In the present study, the 48-hour exposure to 2 μM iAsIII that significantly inhibited GSIS had no effects on Ins1 or Ins2 mRNA levels (Fig. 1C). Similarly, 0.5 μM MAsIII and 1 μM DMAsIII decreased Ins1 and/or Ins2 mRNA levels only by small (~20%) margins. The concentrations of arsenicals used in this study did not decrease islet viability (Fig. 1D), indicating that the GSIS impairment was not due to loss of β-cell integrity. Taken together, these results suggest that subchronic exposures to trivalent arsenicals inhibit GSIS by interfering with mechanisms that regulate the assembly and/or secretion of insulin vesicles, but have little effects on de novo insulin synthesis. Consistent with this mode of action were results obtained after treatment of AsIII-exposed and glucose-stimulated islets with KCl. KCl is an insulin secretagogue that depolarizes β-cell membrane and triggers Ca2+ ion influx, thus increasing exocytosis of the insulin-containing secretory granules independent of glucose stimulation or ATP production (Liang and Matschinsky, 1994). In this study, KCl treatment overcame the inhibitory effects of arsenicals in glucose-stimulated islets, resulting in a marked increase in insulin secretion (Fig. 2).
Fig. 1.
Inhibition of glucose stimulated insulin secretion (GSIS) by trivalent arsenicals: A, GSIS by pancreatic islets exposed to iAsIII, MAsIII or DMAsIII for 48 h; mean and standard error (SE) for 4–7 assays with 15 islets per assay are shown (*p<0.05 and **p<0.01 for differences between exposed and unexposed islets incubated with 2.5 mM or 16.7 mM glucose). B, the islet insulin content and amount of secreted insulin in unexposed islet cultures (Ctrl.) and cultures exposed for 48 h to arsenicals and stimulated with 16.7 mM glucose; mean and SE for 5–9 assays with 15 islets per assay are shown (*p<0.05 for differences between exposed and unexposed islets). C, Ins1 and Ins2 mRNA levels in islets exposed to trivalent arsenicals for 48 h and in control islets; mean and SE for 4–8 assays with 15 islets per assay are shown (*p<0.05 for differences between exposed and control islets). D, viability of islets exposed to trivalent arsenicals for 48 h and of control islets measured by MTT assay; mean and SE for 4–9 assays with 15 islets per assay are shown.
Fig. 2.
Effects of trivalent arsenicals on insulin secretion by pancreatic islets stimulated with 16.7 mM glucose in presence or absence of an insulin secretagogue, KCl (35 mM); mean and standard error for 4–10 assays with 15 islets per assay are shown (*p<0.05 for differences between exposed and control (Ctrl), unexposed islets stimulated with 16.7 mM glucose in absence of KCl; †p<0.0001 for differences between insulin secretion by glucose stimulated islets in the presence and absence of KCl; §p<0.02 for difference between the glucose stimulated and KCl-treated islets exposed to 1 μM and 2 μM iAsIII).
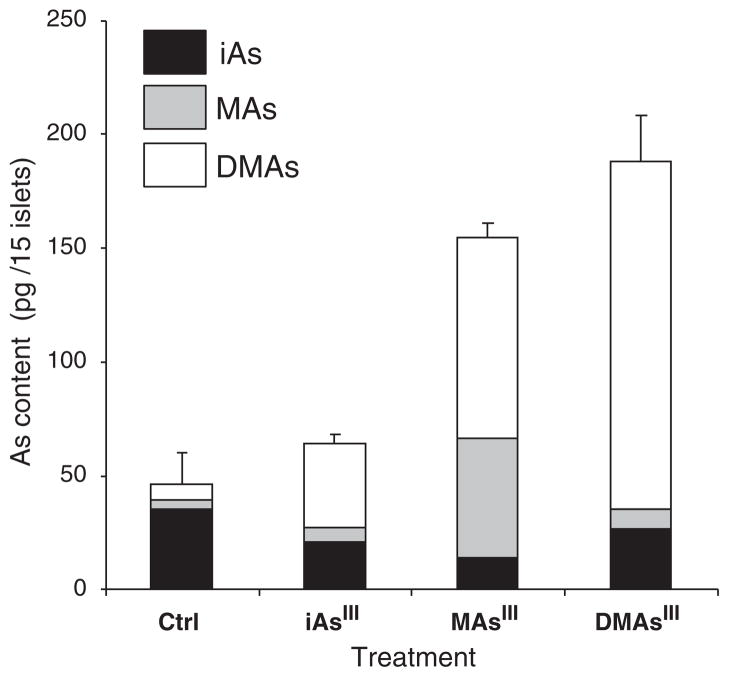
We have previously reported that mice exposed to iAsIII developed diabetes (Paul et al., 2007b, 2008, 2011). HG-CT-AAS analyses showed that significant amounts of iAs and its methylated metabolites, MAs and DMAs, were retained in tissues that regulate glucose homeostasis, including liver, pancreas, skeletal muscle and fat. Liver is thought to be the major site for iAs methylation. However, our whole-mouse experiments could not provide information about the origin of the methylated metabolites found in the pancreas. In this study, we used HG-CT-ICP-MS to identify As species retained in the islets exposed for 48 h to 0.5 μM iAsIII, MAsIII or DMAsIII and in control (unexposed) islets (Fig. 3). We found the total As levels (i.e., sum of As species) to be higher in islets exposed to MAsIII and DMAsIII as compared to islets exposed to iAsIII. This finding is consistent with results of our previous studies (Drobná et al., 2005), suggesting that the methylated trivalent arsenicals are taken up and retained by mammalian cells to a greater extent than iAsIII. The HG-CT-ICP-MS analysis also showed that murine pancreatic islets are capable of methylating iAsIII and MAsIII. Specifically, DMAs accounted for ~57% of As in islets exposed to iAsIII or MAsIII. DMAs was also found in medium in which the islets exposed to iAsIII or MAsIII were maintained (data not shown). Although we did not determine the oxidation state of As, it is possible that the inhibition of GSIS in islets exposed to iAsIII or MAsIII is at least in part due to the formation of DMAsIII. We were able to detect iAs, MAs and DMAs even in control islets. Because the culture medium contained only traces of iAs (≤0.5 nM), the presence of these arsenicals in the islets suggests that mice were exposed in vivo to iAs or methylated arsenicals from diet or the environment. Notably, the control islets contained more iAs but less MAs and DMAs than did the islets exposed to 0.5 μM iAsIII, suggesting again that the methylated arsenicals are responsible for GSIS inhibition. Future studies using pancreatic islets from mice knocked out for As3mt (Hughes et al., 2010), the key enzyme in the methylation pathway for iAs, will help us to better characterize the potentials of individual AsIII species to inhibit GSIS.
Fig. 3.
Arsenic species in pancreatic islets exposed for 48 h to 0.5 μM iAsIII, MAsIII or DMAsIII and in control (Ctrl), unexposed islets; mean and standard error for 4 samples per treatment (15 islets per sample) are shown.
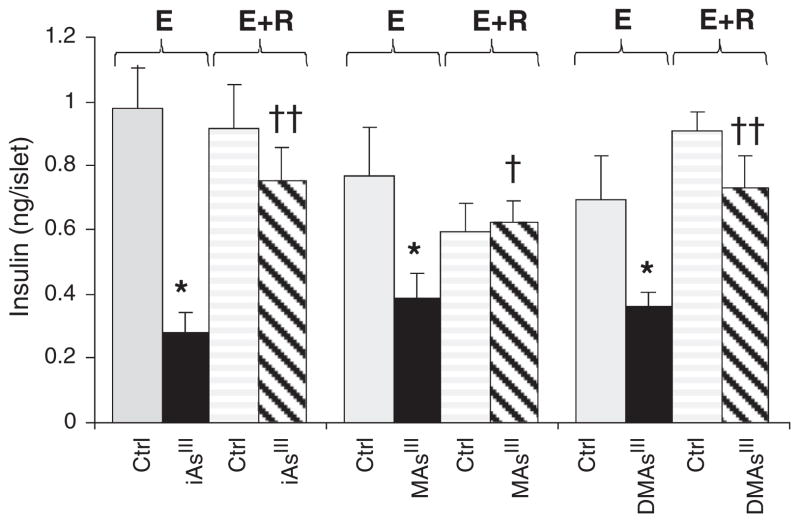
We have recently reported that chronic exposure to iAs in drinking water is associated with an increased risk of diabetes in the Zimapan and Lagunera regions in Mexico (Del Razo et al., 2011). Here, the risk of diabetes was associated only with the current levels of iAs in drinking water, but not with iAs levels recorded in the local water supplies during previous years or with cumulative exposure. These findings indicate that iAs-induced diabetes could be a temporary, possibly reversible condition that lasts only as long as the exposure lasts, i.e., as long as iAs or its metabolites are present in the target tissues. To test the reversibility hypothesis in this study, we examined GSIS by isolated pancreatic islets that were exposed to 2 μM iAsIII, 0.5 μM MAsIII or 0.5 μM DMAsIII for 24 h and then let to recover for additional 24 h in As-free medium (Fig. 4). The 24-hour exposures inhibited GSIS to somewhat lesser extent than the 48-hour exposures described in Fig. 1. Notably, we found that the 24-hour incubation in As-free medium restored GSIS to the levels that were not significantly different from GSIS in control, unexposed islets. Thus, the inhibition of GSIS by trivalent arsenical was reversed when the exposure was discontinued. However, significance of these findings is uncertain. The time of exposure in this study was limited by viability of isolated pancreatic islets, which usually do not survive in culture for longer than 4 days after isolation. It is possible that longer exposures would result in permanent changes and irreversible inhibition of GSIS. Thus, the reversibility of GSIS inhibition by long-term exposures to trivalent arsenicals needs to be examined in laboratory studies using β-cell lines and mice or, preferably, in epidemiologic studies involving human populations chronically exposed to iAs.
Fig. 4.
Reversibility of the inhibition of glucose stimulated insulin secretion by trivalent arsenicals: Glucose-stimulated insulin secretion by control (Ctrl) pancreatic islets and by islets exposed for 24 h to 2 μM iAsIII, 0.5 μM MAsIII or 0.5 μM DMAsIII before (E) and after (E+R) 24-hour recovery in As-free medium; mean and standard error for 4–10 assays with 15 islets per assay are shown (*p<0.001 for differences between exposed and the corresponding control islets; †p<0.01 and ††p<0.0005 for differences between E+R and the corresponding E groups of islets treated with arsenicals).
In summary, our results show that trivalent arsenicals, and specifically the methylated trivalent metabolites MAsIII and DMAsIII are potent inhibitors of GSIS by pancreatic islets. In a broader context, these results provide new evidence that iAs exposure targets pancreatic β-cells and that inhibition of insulin secretion by these cells is at least in part responsible for the diabetogenic effects of iAs exposure.
Supplementary Material
Acknowledgments
Funding
The research presented here was supported by NIH grant no. 5R01 ES015326 to Miroslav Styblo, by a grant from the Ministry of Education, Youth and Sports of the Czech Republic Kontakt II program (project no. LH12040), and by AS CR institutional fund RVO: 68081715. Support was also provided by the UNC Nutrition Obesity Research Center funded by the National Institute of Diabetes and Digestive and Kidney Diseases (grant no. DK056350) and by the Center for Environmental Health and Susceptibility funded by the National Institute of Environmental Health Sciences (grant no. P30ES010126).
The authors thank Dr. William Cullen (University of British Columbia, Vancouver, Canada) for providing MAsIII and DMAsIII for this study.
Abbreviations
- As3mt
arsenic (+3 oxidation state) methyltransferase
- ATP
adenosine tri-phosphate
- DMAs
dimethyl-arsenic
- DMAsIII
dimethylarsinite
- ELISA
enzyme-linked immuno-sorbent assay
- GLUT
glucose transporter
- GSIS
glucose-stimulated insulin secretion
- HG-CT-AAS
hydride generation–cryotrapping–atomic absorption spectrometry
- HG-CT-ICP-MS
hydride generation–cryotrapping–inductively-coupled plasma–mass spectrometry
- iAs
inorganic arsenic
- iAsIII
arsenite
- iAsV
arsenate
- IC50
concentration of an inhibitor at which 50% inhibition of the response is seen
- NIEHS
National Institute of Environmental Health Sciences
- MAs
dimethyl-arsenic
- MAsIII
methylarsonite
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- PCR
polymerase chain reaction
- PKA
protein kinase A
- PKB/Akt
protein kinase B
- PKC
protein kinase C
- SE
standard error to the mean
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.taap.2012.12.007.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- ATSDR (Agency for Toxic Substances, Disease Registry) Toxicological Profile for Arsenic. Vol. 2007. U.S. Department of Health and Human Services, Public Health Service; 2007. Aug, http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=22&tid=3. [Google Scholar]
- BGS, DPHE. Arsenic contamination of groundwater in Bangladesh. Final report. British Geological Survey Report WC/00/19. 2001;2 [Google Scholar]
- Boucher A, Lu D, Burgess SC, Telemaque-Potts S, Jensen MV, Mulder H, Wang MY, Unger RH, Sherry AD, Newgard CB. Biochemical mechanisms of lipid-induced impairment of glucose-stimulated insulin secretion and reversal with a malate analog. J Biol Chem. 2004;279:27,263–27,271. doi: 10.1074/jbc.M401167200. [DOI] [PubMed] [Google Scholar]
- Del Razo LM, García-Vargas GG, Valenzuela OL, Hernandez-Castellanos E, Sánchez-Peña LC, Drobná Z, Loomis D, Stýblo M. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapán and Lagunera regions in Mexico. Environ Health. 2011;10:73. doi: 10.1186/1476-069X-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Villaseñor A, Sánchez-Soto MC, Cebrián ME, Ostrosky-Wegman P, Hiriart M. Sodium arsenite impairs insulin secretion and transcription in pancreatic beta-cells. Toxicol Appl Pharmacol. 2006;214:30–34. doi: 10.1016/j.taap.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Díaz-Villaseñor A, Burns AL, Salazar AM, Sordo M, Hiriart M, Cebrián ME, Ostrosky-Wegman P. Arsenite reduces insulin secretion in rat pancreatic beta-cells by decreasing the calcium-dependent calpain-10 proteolysis of SNAP-25. Toxicol Appl Pharmacol. 2008;231:291–299. doi: 10.1016/j.taap.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Drobná Z, Waters SB, Devesa V, Harmon AW, Thomas DJ, Stýblo M. Metabolism and toxicity of As in human urothelial cells expressing rat arsenic (+3 oxidation state) methyltransferase. Toxicol Appl Pharmacol. 2005;207:147–159. doi: 10.1016/j.taap.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Woods CG, Yehuda-Shnaidman E, Zhang Q, Wong V, Collins S, Sun G, Andersen ME, Pi J. Low-level arsenic impairs glucose-stimulated insulin secretion in pancreatic beta cells: involvement of cellular adaptive response to oxidative stress. Environ Health Perspect. 2010;118:864–870. doi: 10.1289/ehp.0901608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser MJ. ADP-arsenate. Formation by submitochondrial particles under phosphorylating conditions. J Biol Chem. 1981;256:5981–5983. [PubMed] [Google Scholar]
- Haller MJ, Atkinson MA, Schatz D. Type 1 diabetes mellitus: etiology, presentation, and management. Pediatr Clin North Am. 2005;52:1553–1578. doi: 10.1016/j.pcl.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Hernández-Zavala A, Matoušek T, Drobná Z, Adair BM, Dědina J, Thomas DJ, Stýblo M. Speciation of arsenic in biological matrices by automated hydride generation–cryotrapping–atomic absorption spectrometry with multiple microflame quartz tube atomizer (multiatomizer) J Anal At Spectrom. 2008;23:342–351. doi: 10.1039/b706144g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MF, Edward BC, Herbin-Davis KM, Saunders RJ, Styblo M, Thomas DJ. Arsenic (+3 oxidation state) methyltransferase genotype affects steady-state distribution and clearance of arsenic in arsenate-treated mice. Toxicol Appl Pharmacol. 2010;249:217–223. doi: 10.1016/j.taap.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Liang Y, Matschinsky FM. Mechanisms of action of nonglucose insulin secretagogues. Annu Rev Nutr. 1994;14:59–81. doi: 10.1146/annurev.nu.14.070194.000423. [DOI] [PubMed] [Google Scholar]
- Liu Z, Sanchez MA, Jiang X, Boles E, Landfear SM, Rosen BP. Mammalian glucose permease GLUT1 facilitates transport of arsenic trioxide and methylarsonous acid. Biochem Biophys Res Commun. 2006;351:424–430. doi: 10.1016/j.bbrc.2006.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker MP, Daniels JL. Environmental contaminants as etiologic factors for diabetes. Environ Health Perspect. 2001;109:871–876. doi: 10.1289/ehp.01109s6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoušek T, Hernández-Zavala A, Svoboda M, Langerová L, Adair BM, Drobná Z, Thomas DJ, Stýblo M, Dědina J. Oxidation state specific generation of arsines from methylated arsenicals based on L-cysteine treatment in buffered media for speciation analysis by hydride generation–automated cryotrapping–gas chromatography–atomic absorption spectrometry with the multiatomizer. Spetrochim Acta Part B. 2008;63:396–406. doi: 10.1016/j.sab.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, Silbergeld EK, Styblo M, Tseng CH, Thayer KA, Loomis D. Evaluation of the Association between Arsenic and Diabetes: A National Toxicology Program Workshop Review. Environ Health Perspect. 2012;120:1658–1670. doi: 10.1289/ehp.1104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, Guallar E. Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiological evidence. Environ Health Perspect. 2006;114:641–648. doi: 10.1289/ehp.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul DS, Harmon AW, Devesa V, Thomas DJ, Styblo M. Molecular mechanisms of diabetogenic effects of arsenic: inhibition of insulin signaling by arsenite and methylarsonous acid. Environ Health Perspect. 2007a;115:734–742. doi: 10.1289/ehp.9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul DS, Hernández-Zavala A, Walton FS, Adair BM, Dědina J, Matoušek T, Styblo M. Examination of the effects of arsenic on glucose homeostasis in cell culture and animal studies: development of a mouse model for arsenic-induced diabetes. Toxicol Appl Pharmacol. 2007b;222:305–314. doi: 10.1016/j.taap.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul DS, Devesa V, Hernández-Zavala A, Adair BM, Walton FS, Drobná Z, Thomas DJ, Styblo M. Environmental arsenic as a disruptor of insulin signaling. In: Collery P, Maymard I, Theophanides T, Khasanova L, Collery T, editors. Metal Ions in Biology and Medicine. Vol. 10. John Libbey; Eurotext, Paris: 2008. pp. 1–7. [PMC free article] [PubMed] [Google Scholar]
- Paul DS, Walton FS, Saunders RJ, Styblo M. Characterization of the impaired glucose homeostasis produced in C57BL/6 mice by chronic exposure to arsenic and high-fat diet. Environ Health Perspect. 2011;119:1104–1109. doi: 10.1289/ehp.1003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pysher MD, Sollome JJ, Regan S, Cardinal TR, Hoying JB, Brooks HL, Vaillancourt RR. Increased hexokinase II expression in the renal glomerulus of mice in response to arsenic. Toxicol Appl Pharmacol. 2007;224:39–48. doi: 10.1016/j.taap.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P, Eliasson L, Renstrom E, Gromada J, Barg S, Gopel S. The cell physiology of biphasic insulin secretion. News Physiol Sci. 2000;15:72–77. doi: 10.1152/physiologyonline.2000.15.2.72. [DOI] [PubMed] [Google Scholar]
- Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in human cells. Arch Toxicol. 2000;74:289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- Stýblo M, Drobná Z, Jaspers I, Lin S, Thomas DJ. The role of biomethylation in toxicity and carcinogenicity of arsenic: a research update. Environ Health Perspect. 2002;110(Suppl 5):767–771. doi: 10.1289/ehp.110-1241242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot GL, Koudria P, Bluestone JA. Murine pancreatic isolation. J Viz Exp. 2007;(7):255. doi: 10.3791/255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The World Bank. Arsenic contamination of groundwater in Asia. A World Bank and Water and Sanitation Program Report. 2005 Mar;2005 http://siteresources.worldbank.org/INTSAREGTOPWATRES/Resources/ARSENIC_BRIEF.pdf. [Google Scholar]
- Thomas DJ, Styblo M, Lin S. The cellular metabolism and systemic toxicity of arsenic. Toxicol Appl Pharmacol. 2001;176:127–144. doi: 10.1006/taap.2001.9258. [DOI] [PubMed] [Google Scholar]
- Tseng CH. The potential biological mechanisms of arsenic-induced diabetes mellitus. Toxicol Appl Pharmacol. 2004;197:67–83. doi: 10.1016/j.taap.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Turner MD. Coordinated control of both insulin secretion and insulin action through calpain-10-mediated regulation of exocytosis? Mol. Genet Metab. 2007;91:305–307. doi: 10.1016/j.ymgme.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Walton FS, Harmon AW, Paul DS, Drobna Z, Patel YM, Styblo M. Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: possible mechanism of arsenic-induced diabetes. Toxicol Appl Pharmacol. 2004;198:424–433. doi: 10.1016/j.taap.2003.10.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.