Abstract
Arsenic concentration and speciation were determined in benthic fauna collected from the Mid-Atlantic Ridge hydrothermal vents. The shrimp species, Rimicaris exoculata, the vent chimney-dwelling mussel, Bathymodiolus azoricus, Branchipolynoe seepensis, a commensal worm of B. azoricus, and the gastropod Peltospira smaragdina showed variations in As concentration and in stable isotope (δ13C and δ15N) signature between species, suggesting different sources of As uptake. Arsenic speciation showed arsenobetaine to be the dominant species in R. exoculata, whereas in B. azoricus and B. seepensis arsenosugars were most abundant, although arsenobetaine, dimethylarsinate, and inorganic arsenic were also observed, along with several unidentified species. Scrape samples from outside the vent chimneys, covered with microbial mat, which is a presumed food source for many vent organisms, contained high levels of total As, but organic species were not detectable. The formation of arsenosugars in pelagic environments is typically attributed to marine algae, and the pathway to arsenobetaine is still unknown. The occurrence of arsenosugars and arsenobetaine in these deep sea organisms, where primary production is chemolithoautotrophic and stable isotope analyses indicate food sources are of vent origin, suggests that organic arsenicals can occur in a food web without algae or other photosynthetic life.
Introduction
In seawater, As is present at trace levels, predominantly as inorganic As(V), whereas in marine organisms, high concentrations of As can be found, with arsenobetaine (AB) as the major species, regardless of trophic level.[1] While the pathways of As accumulation and transformation have not clearly been deciphered, marine algae have often been considered to play an important role in As cycling. Algae can contain high concentrations of As, and have been shown to transform As(V) to As(III), monomethyl arsenic (MA) and dimethyl arsenic (DMA)[2–4] A significant portion of total As in algae is present in the form of arsenosugars, which are also metabolites,[5] and recently trace amounts of AB have also been reported in algae.[6] It follows that algae have been considered a primary source of these organic As compounds to the marine foodweb, yet high levels of organic As have also been reported in mangrove ecosystems where algae is not the primary producer,[7] and intriguingly, in two specimens of organisms from deep ocean hydrothermal vents, where photosynthetic life cannot exist.[8] These findings suggest the presence of a non-algal source of organic As in marine environments.
The Mid-Atlantic Ridge hydrothermal vents provide habitat to an unusual array of benthic fauna, which live in the turbulent mixing zone between the hot hydrothermal fluids and cold deep-ocean waters. In this environment, the dominant primary production is autochthonous and chemosynthetic by bacteria[9] and symbiotrophy with these bacteria is also a major source of energy for benthic fauna inhabiting the vents.[10] Vent organisms can be exposed to high metal concentrations from the vent fluid waters,[11, 12] although mixing can be rapid, causing high spatial and temporal variation in metal concentrations.[12] In the high pressure and temperature conditions of the Mid-Atlantic Ridge, the composition of vent fluids are controlled by phase separation processes, where shallower sites are more gas-enriched than deeper sites, which are metal-enriched.[13] Bedrock, which varies from ultramafic to basaltic, is also thought to influence metal concentration.[14] Because of this variance in exposure, as well as having unique sources of nutrient uptake, these organisms provide an important opportunity to study metal accumulation and metabolism.
Several studies have addressed metal accumulation in vent organisms, but most of these studies have focused on cationic species such as Cd, Cu, Fe, Zn.[12, 15, 16] Vent organisms have been shown to have a high tolerance to metal toxicity effects, and organ and body burden vary between sites and are related to local environmental conditions.[15] However, very few studies have focused on the uptake of As to biota, despite this element being elevated in sulfidic vent fluids.[17–19] Concentration of As in hydrothermal fluid is controlled by phase separation determined by thermodynamic behavior near the critical curve; high temperature causes gas enrichment, which supports high concentrations of the volatile H3AsO30 species, the dominant form of As in the vent environment.[19]
One previous study of As concentration and speciation in vent organisms[8] showed concentrations in two vent invertebrates to be similar in magnitude to corresponding shallow-dwelling species, and AB and arsenosugars were identified as the major As species in the vent organisms. In oxic marine environments, AB is the major species in most marine animals, but pathways of accumulation and biotransformation are not well understood.[20–22] Furthermore, while the formation of arsenosugars by algae has been well-documented, bacteria are not known to biosynthesize arsenosugars. The presence of these As species in vent organisms, where photosynthetic algae cannot survive, suggests another source of AB and arsenosugars.
At Mid-Atlantic Ridge hydrothermal vents, two dominant organisms are the shrimp, Rimicaris exoculata, and the mussel Bathymodiolus spp. (B. azoricus are present at the northern vents).[23] B. azoricus primarily acquires energy from a symbiotic relationship with thiotrophic and methanotrophic bacteria living in their gills,[24] but they also have a fully functioning gut with which they can metabolize particulate and dissolved organic matter.[25–27] The shrimp, R. exoculata, have also been shown to fix carbon chemolithoautotrophically, by endosymbionts inhabiting their branchial cavity,[28] but can also graze on organic matter around the vents. Because of the unique feeding mechanisms and food sources of these organisms, pathways of As uptake and metabolism are likely different from those in oxic marine environments, and thus they could provide novel insights into the cycling of this potentially toxic element. These two hydrothermal organisms, collected from three different Mid-Atlantic Ridge vents, were the subjects of this study of As speciation. This study was intended to verify the occurrence of organic As species in this deep ocean ecosystem, and to relate differences in As concentration and speciation across sites and species to differences in food sources using stable isotope analysis.
Materials and methods
Sample collection
Samples were collected from the mid-Atlantic hydrothermal vents using Jason, a Deep Submergence Vehicle, during a cruise in July, 2008. Samples were collected from three sites along the Mid-Atlantic Ridge: TAG, the deepest and most southern vent site visited (26°N, 44°W, 3600m depth), Rainbow (36°N, 34°W, 2300m depth) and Lucky Strike (37°N, 32°W, 1700m depth). Aquatic species collected were the vent shrimp, Rimicaris exoculata, the mussel, Bathymodiolus azoricus, a commensal polychaete found within the mantle of B. azoricus, (Branchipolynoe seepensis), and the gastropod, Peltospira smaragdina. All of these species are associated with active edifices. The same organisms were collected across sites where possible; the mussel B. azoricus is not present at TAG,[29] and the shrimp and snail samples were only found at two sites (Table 1).
Table 1.
Total As (mean ± standard deviation) in vent organisms
| Vent | Species | Body part | N | Total As (mg/kg) |
|---|---|---|---|---|
| Rainbow | R. exoculata | Tail | 3 | 3.3 ± 0.8 |
| TAG | R. exoculata | Tail | 3 | 27.6 ± 2.2 |
| TAG | R. exoculata | whole | 3 | 29.8 ± 3.9 |
| Rainbow | B. azoricus | whole | 3 | 9.9 ± 1.7 |
| Lucky Strike | B. azoricus | whole | 12 | 11.6 ± 2.4 |
| Lucky Strike | B.seepensis | whole | 16 a | 18.2 ± 3.8 |
| Lucky Strike | P.smaragdina | whole | 10 b | 67.8 |
| TAG | P.smaragdina | whole | 20 c | 14.0 ± 5.8c |
5 samples were analyzed as composites of n=2; B.seepensis were collected from B. azoricus samples from Lucky Strike
Analyzed as 1 (Lucky Strike) or 2( TAG) composite samples of n=10
Mean ± relative difference (standard deviation could not be calculated for n=2).
Vent organisms were depurated in seawater on board the research vessel after collection, then frozen for the duration of the cruise, and shipped to Dartmouth College for analysis. Samples were then freeze-dried, removed from their shells, and homogenized in Teflon vials using a ball mill (Retsch MM301, Haan, Germany) with 15mm Teflon-coated grinding balls (McMaster Carr, Aurora, OH, USA). Both wet and dry weights were recorded for each organism. Samples were analyzed as whole individual organisms, with the exception of shrimp, for some of which only the tails were analyzed; and for the polychaete and snail samples, which were composited to give adequate sample mass (see Table 1).
Scrape samples of the vent exteriors were also collected at the three vent sites. These samples are a combination of microbial mat and sulfide materials scraped from the outside of the active vent, and were collected to determine whether the microbial mat is a potential source of As species to the vent organisms. These biomass/sulfide mixtures were frozen immediately, and stored frozen until analysis.
δ13C and δ15N determination
Samples of homogenized, freeze-dried vent organism were analyzed for 13C/12C and 15N/14N at Dartmouth College. Samples were combusted in a Carlo Erba (Lakewood, NJ, USA) elemental analyzer and the gases produced were sent via Conflo (Thermo Finnigan, Bremen, Germany) to a Delta+ Advantage mass spectrometer (Thermo Finnigan). Two in-house standards were used for 13C/12C and 15N/14N calibration, along with USGS 25 (IAEA, Vienna, Austria) for 15N/14N. δ13C and δ15N values were expressed relative to Pee Dee Belemnite and atmospheric N2, respectively.
Total As determination
Aliquots of 0.05g of the freeze dried vent organisms were weighed into 7 mL PFA micro-vials (CEM, Matthews, NC, USA), and 3mL of concentrated HNO3 were added (Fisher Optima; Fisher Scientific, Pittsburgh, PA, USA). Two microvials were then placed in a HP 500 Plus high-pressure microwave vessel (CEM) containing a spacer and 10 mL of deionized water. Vessels were placed in a MARS high pressure microwave system (CEM) with a temperature sensor in one vessel; temperature was ramped to 150°C over 20 min., then held at150°C for 10 min. Digests were allowed to cool to room temperature, then transferred to pre-weighed 7 mL PTFE tubes (Sarstedt, Nümbrecht, Germany). Samples were diluted to 5 mL with ultrapure water (>18.2MΩ cm−1) produced by Purelab Pluswater purifier (US Filter, MA, USA). From the solution an aliquot of 0.3 g was weighed into a second 7 mL PTFE tube (Sarstedt), and diluted to 6 mL. Three standard reference materials were prepared and analyzed along with the vent samples as an assessment of analytical accuracy. Certified values for NIST 1566b oyster tissue (NIST, Gaithersburg, USA), NIST 2976 mussel tissue and DORM-3 Fish Protein (NRCC, Ottawa, Canada)) were 7.65, 13.3 and 6.88 mg/kg As, respectively; we obtained 6.41 (n=1), 13.4 (n=1), and (5.71, 6.47 mg/kg (n=2)). Three samples were also digested in duplicate, and relative percent difference was determined to be 10%, 2% and 1%.
Portions of the microbial mat/sulfide samples (0.05g wet weight) were weighed wet into 60 mL PTFE tubes (Sarstedt), along with 5 mL HNO3 (Fisher Optima). Concentrations of As in these samples were attained on wet weights, and dry weight was determined on a separate sample to express concentrations on a dry weight basis. Samples were digested by an open-vessel microwave method, where a temperature probe was placed in one sample, and the heat was ramped to 95°C over 15 min, then held at that temperature for 45 min. After cooling, samples were brought up to 50 mL volume with ultrapure water, and then a portion (0.5mL) was diluted 10 times by weight into a 7 mL PTFE tube (Sarstedt). A standard reference material (NIST 2711 Montana Soil, certified As – 107 mg/kg ± 5) was prepared along with the samples and an As concentration of 100 and 102 mg/kg (n=2) were obtained. Concentrations determined on duplicate samples had a relative percent difference of 6%.
Total As determination was performed by inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7500C; Agilent Technologies, Santa Clara, CA) with He as a collision gas. Digested samples were introduced via a Micromist nebulizer (Glass Expansion, Pocasset, MA, USA) with a flow rate of 0.2 mL min−1. The instrument was operated with an Rf power of 1550 W, a carrier gas flow rate of 0.9 L min−1, and a collision gas (He) flow rate of 0.45 mL min−1.
Extraction of As species
Due to limited sample availability, a smaller subset of samples was analyzed for As speciation. There was insufficient sample mass of the P. smaragdina samples for speciation analysis. Complete recovery of multiple As species from tissue without significant species transformation is a major challenge for liquid chromatography (LC) methods.[30] Ultrapure H2O was chosen as the extractant, as it has been suggested as a suitable reagent for As species, most of which are polar and water-soluble.[31] Vent organisms were extracted for As speciation by weighing 0.5 g freeze-dried sample into a 20mL PTFE tube (Sarstedt), and adding 10 mL ultrapure H2O. Samples were shaken for an hour, sonicated for an hour, and then left overnight. Sample extracts were then filtered through 0.2μm syringe-tip filters into 2mL HPLC vials with Teflon lined septa caps (Fisher).
For extraction of microbial mat samples, 0.5g aliquot of the wet sample was weighed into a 20mL PTFE tube (Sarstedt) and diluted 10mL with 1% HNO3. A weak acid digest with heating[32] was used due to poor As recoveries (<1% total As) by the H2O extraction method outlined above. This is likely due to total As concentrations being dominated by the geological portion of the sample, which was a sulfide material, in some cases with Fe oxide crust; samples were determined to contain only 0.02–0.08% C. Acidic extracts were placed in an open vessel microwave and sequentially heated to 55°C for 15 min., 75°C for 15 min., and then 95°C for 30 min. Samples were filtered through 0.2μm syringe tip filters prior to analysis. An aliquot of each sample extract was diluted 10 times in 1% HNO3 and analyzed for total As, to determine extraction efficiency (as As in the extract divided by As in the total digest).
As speciation
Speciation was performed by using an Agilent LC 1120 system (Agilent Technologies) for liquid chromatography coupled with an Agilent 7500C or Agilent 7700 ICP-MS. The two systems were configured by connecting the column outlet of the LC to a Seaspray nebulizer (Glass Expansion, Pocasset, MA, USA) on the ICP-MS via a length of PEEK tubing. The ICP-MS was operated with an Rf power of 1550 W, a nebulizer gas flow rate of 1.1 mL min−1, and without the use of the collision cell, to maximize sensitivity.
Standards for As speciation were prepared from dilution of As(III) and As(V) stocks (Inorganic Ventures, Christianburg, VA); methylarsonate (MA) and dimethylarsinate (DMA) were prepared from salts of monosodium methylarsonate (Chem Services, West Chester, PA, USA) and dimethylarsinic acid (Sigma Aldrich). Arsenobetaine (AB) was prepared from a BCR standard (Sigma Aldrich, St. Louis, MO, USA). Each single species standard solution was analyzed for total As to attain an accurate concentration. A well-characterized extract of brown algae[33] was used as a standard for four arsenosugars.
Due to the diversity of properties of As species, separation of all analytes of interest is not easily attainable with a single set of chromatographic conditions, and the use of multiple separation schemes has become commonplace in studies of arsenic speciation in marine foodwebs. In this study quantification of As species was determined by two chromatographic methods:
-
1)
Anion exchange using a PRP-X100 Hamilton column (250 × 4.1 mm, 10 μm; Hamilton Co., Reno, Nevada, USA) at 40°C with a mobile phase of 20 mM NH4H2PO4 at pH 6, with a flow rate of 1.5 mL min−1[34] was used for determination of As(III), As(V), DMA, MA, and arsenosugars.
-
2)
Cation exchange using a Supelcosil LC-SCX column (250 × 4.6 mm, 5 μm, Supelco, Bellefonte, USA) at 40°C with a mobile phase of 20mM pyridine at pH 2, with a flow rate of 1.5 mL min−1;[35] was used for determination of AB.
Further investigation of the arsenic species present in the deep vent samples was carried out by another two procedures:
-
3)
Aliquots of a subset of water-extracted samples (2 shrimp, 4 mussel and 2 polychaete samples) were oxidized by addition of H2O2 to 10% v/v, then re-analyzed by anion exchange chromatography (method 1). This procedure oxidized thio-arsenosugars to oxo-arsenosugars, allowing quantification of the thio-arsenosugars by difference.[36] Oxidation of As(III) to As(V) occurrs simultaneously by this method.
-
4)
Reverse phase chromatography using a Shisheido Capcell PAK C18 MGII (4.6 mm × 250 mm, 5 μm, Shiseido Co., Ltd., Tokyo, Japan) with a mobile phase of 10 mM sodium 1-butansulfonate, 4 mM tetramethylammonium hydroxide, 4 mM malonic acid, 0.5% methanol at pH 3, with a flow rate of 1.0 mL min−1 [37] was used for separation of co-eluting unknown organic arsenic species.
Species were identified by their retention times compared with standard compounds and by performing spiking experiments. Concentrations were determined by comparing peak areas to known standards; in the case of unknown peak, peak area was compared with As(V) (anion exchange) or AB (cation exchange) to approximate concentration. The percent of As identified was reported as the sum of concentration of known species divided by the total concentration of As in the extract. Column recoveries for each method were calculated as the sum of the species (including unknowns) divided by the total concentration of water-extracted As in the sample. A standard reference material (Dorm 2, NRC, Ottawa, Canada) was analyzed along with the samples; the certified value for AB was 16.4 ± 1.1 As mg/kg; we obtained 16.6 ± 1.3 As mg/kg (n=2). A single mussel sample (B. azoricus from Lucky Strike) was also analyzed for total As and speciation at the Karl-Franzens University in Graz, to provide further quality assurance (see Accessory Publication). Both analyses identified As-PO4 as the major species, and while extraction efficiency in the Dartmouth sub-sample (75% of Total As) was greater than the one analyzed at Graz (54% of Total As), abundances of AB, DMA, and inorganic As relative to % extracted As were in good agreement (relative difference < 5%).
Statistical Analyses
Statistical analyses were carried out with Stata 11.0 Statistical/Data Analysis package. Distribution of the data was assessed by graphical analyses, and skewness and kurtosis tests of normality. Due to small sample sizes and non-normality (which was not corrected by log transformation), non-parametric analyses were chosen. Differences in As concentrations and individual As species (as absolute concentration and normalized to extractable As) between vent organisms and site locations were analyzed by the Wilcoxon rank sum test (also referred to as the Mann-Whitney U test), and correlations were assessed using Spearman's rho. Principal component analysis (PCA), using covariance matrices, was employed to investigate differences in speciation between groups. A statistical significance criterion was established as p=0.05.
Results and Discussion
Total As and stable isotope ratios
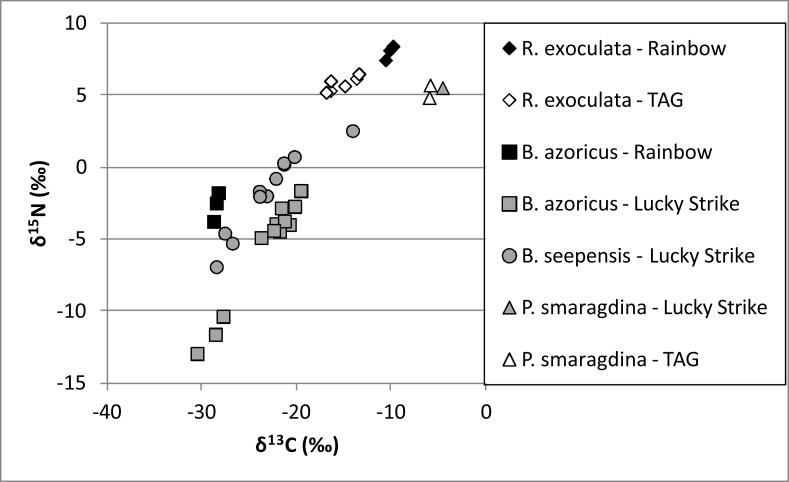
Total As concentrations are given in Table 1, and stable isotope ratios (δ13C vs. δ15N) are plotted in Figure 1. There were no significant correlations between As concentration or stable isotope ratios with sample weight, for any organism (Spearman's rho, p>0.05).
Fig. 1.
δ15N vs. δ13C for vent organisms from three sites along the Mid Atlantic Ridge indicating differing chemosynthetic carbon sources for each species.
For TAG, the deepest and most southern site, tails and whole organisms of R. exoculata were found to have similar total As concentrations (p=0.28) and stable isotope concentrations (p>0.5), indicating tail compositions were representative of whole body burden, and these data were pooled. Concentrations of total As in R. exoculata from TAG (29±2 mg/kg) were higher than those reported by Larsen et al.,(1997) in two samples from the same site (21 mg/kg and 4 mg/kg).[8]
Shrimp have been shown to be the high-end members of δ13C vs. δ15N plots of vent organisms, across Mid-Atlantic Ridge sites,[29] and in this study had the highest values of δ15N. δ13C is considered an indicator of consumed substrate, which in vent organisms in symbiotic relationships with chemoautotrophs can be lower or higher than in other deep sea fauna; δ15N is an indicator of trophic level, but is often more negative in vent organisms than in the pelagic food web.[29, 38] R. exoculata ingest photosynthetic material during their pelagic juvenile phase, then metamorphose and colonize the vents, where they are considered to be isolated from pelagic organic matter.[39] Consumption during their juvenile life influences the shrimps' δ13C signature, which along with δ15N ratio, increases during their adult stage.[39] Feeding during this juvenile phase may also affect As body burden. Once in their adult form, R. exoculata are thought to feed primarily on epibiotic bacteria that colonize their mouthparts,[39, 40] making this a potential pathway for As uptake in the vents. Demina et al., (2010) found the organs with highest As concentration in vent shrimp were maxillipeds,[17] suggesting epibionts are an important source of As. These shrimp also have a functioning gut and ingest particulate matter, which may also be a source of As, although studies of 13C isotope patterns suggest this mechanism is responsible for a small fraction of food uptake.[39, 40]
Two composite samples of the gastropod, P. smaragdina, also collected from TAG (Table 1) were found to have lower As concentrations (20 and 8 mg/kg) than R. exoculata. There are no previous reports of metal concentrations or stable isotope ratios in this species. Samples of P. smaragdina were found to have higher 13C ratios (δ13C= −6.0, −5.9) relative to R. exoculata (δ13C= −15.3±0.6), although δ15N signatures were similar between the two species (P. smaragdina: δ15N= 4.8, 5.7; R. exoculata: δ15N=5.8±0.2), and also similar to oceanic plankton.[39] Gastropods from hydrothermal vents are assumed to be grazers feeding on free-living bacteria on the vents surfaces,[41] although little is known about this particular species. Based on their enriched C isotope ratios, P. smaragdina have a distinct food source (and source of As) from vent shrimp at this site.
Two species were collected from the Rainbow site: R. exoculata and B. azoricus, both common species along the Mid-Atlantic Ridge. Mussels from Rainbow had significantly higher As concentrations than shrimp (p < 0.05), and are the low-end members of the δ13C vs. δ15N plot. The depleted δ13C and δ15N signatures for mussels suggest they are obtaining N and organic C from local vent fluid sources via symbiosis with chemoautotrophic bacteria.[29] While shrimp can be grazers and scavengers, their main food source is also thought to be bacteria, in which case the bacteria must have a significantly different 13C ratio than those associated with mussels,[29] also suggesting a different source of As.
Concentrations of total As in B. azoricus from Lucky Strike ranged from 6 to 16 mg/kg. A single specimen of Bathymodiolus puteoserpentis, another dual symbiont containing mussel species endemic to the Mid-Atlantic Ridge, was reported to have similar As concentration in mantle tissue (11.4 mg/kg) as the B. azoricus samples in this study, and relatively high As in the gill (70.6 mg/kg).[8] Augmented concentrations of As,[42] as well as other metals,[17] have been reported in B. azoricus gills, which are enriched in endosymbionts, and are also the point of suspended particle intake.
Colaco et al. (2002) found two different groups of mussels living at the Lucky Strike site, one which had δ13C signatures of −30 ‰ and δ15N values of −10 ‰, and another with δ13C ratios of −20 ‰ and δ15N ratios of −3.[29] Vent mussels receive nutrition from a symbiotic relationship with sulfide- and methane-oxidizing bacteria.[24],[26] which can affect their δ13C and δ15N signatures. Lower δ13C values (~ −30 ‰) have been attributed to a dominance of thiotrophic bacteria in mussels, whereas higher values (~ −20 ‰) have been associated with methanotrophic bacteria,[43] although distinct C isotope ratios in vent fluids have also been correlated to mussel δ13C.[11] Mussels from Lucky Strike in this study also ranged in stable isotope ratios: three samples had depleted isotope ratios similar to the first group in the Colaco study (δ13C= −28.9±1.2‰; δ15N= −11.7±1.1‰), and the rest had signatures similar to the second group (δ13C= −21.5±1.2 ‰; δ15N= −3.6±1.0 ‰). No difference in As concentration (p=0.08) was evident between these two groups, suggesting that the pathway of symbiotrophy does not affect As uptake.
The polychaete worm samples, B. seepensis, which live commensally with B. azoricus and are presumed to feed upon mucus, pseudofaeces or tissue from the mussel, were collected from both groups of mussels at the Lucky Strike site. B. seepensis had higher As concentrations than its host (p = 0.0005), suggesting As accumulates into the worm. The polychaetes had a large range of 13C (−28 to −14 ‰) and 15N (−7 to 3 ‰) ratios, but did not form two distinct groups like their host mussels. The polychaetes had similar range of δ13C ratios, and slightly enriched δ15N relative to the mussels (p=0.023); this was also observed in other studies.[25, 29]
One sample of P. smaragdina was also collected from Lucky Strike, and had a much higher As concentration than the mussels (68 mg/kg). Stable isotope ratios, which are enriched in both δ13C and δ15N relative to the mussels, indicated different food sources between these species at Lucky Strike.
Differences in As availability may exist between sample sites along the Mid-Atlantic Ridge. Important factors influencing metal availability are bedrock type, and pH and temperature of vent fluids. The bedrock at the Rainbow site is ultramafic, which may predict higher heavy metal concentrations than the other two sites, which are basaltic.[14] However, fluids at both TAG and Rainbow, have Fe, S, Mn- enriched brines relative to seawater, and high levels of chloride,[14] which are associated with low As concentration.[19] TAG and Rainbow also have high mineral particle flux compared to the shallower Lucky Strike site.[13] While data is scarce, water As concentration at TAG, Lucky Strike and Rainbow were reported to be <11 nmol L−1, 200±96 nmol L−1 [19] and 2050±300 nmol L−1,[42] respectively. Availability of metals in vent fluids may be associated with partitioning between the dissolved and suspended particulate fractions, which may also vary greatly within sites due to large ranges of pH and temperature in the mixing zones.[44]
R. exoculata collected at the Rainbow site had significantly lower As concentrations than from the TAG site (p = 0.02), and also lower concentrations than in any other organism in this study. Bedrock type and reported concentrations of As in vent waters suggest As availability is higher at the Rainbow vent, however, the lower As concentration in the shrimp from Rainbow may be due to differences in microenvironments from which the organisms were sampled. Shrimp from the Rainbow site had slightly enriched δ13C (−10.2±0.3) and δ15N (8.0±0.4) than those from TAG (p<0.05) suggesting differences in food substrate; Colaco et al.(2002) found δ13C in shrimp from Rainbow to be higher than those from six other sites.[29] It cannot be ruled out that differences in δ13C and δ15N ratio between sites are due to different in ages of the shrimp.[39] Gebruk et al. (2002) found no difference in stable isotope signatures in R. exoculata from different vents, but that δ13C and δ15N were significantly enriched in adult vs. juvenile phases across sites, and that δ13C increased with age of the adult, corresponding to bacterial growth in their mouthparts.[39] If the depleted δ13C and δ15N in shrimp from TAG are due to these organisms being younger than those from Rainbow, the higher As concentrations could be attributed to As storage from their pelagic juvenile phase, suggesting As decreases once entering the vents.
Mussels from Lucky Strike had similar total As concentration to those from Rainbow (p=0.06) despite potential differences in exposure at these sites due to differences in bedrock type, sediment flux[13] and reported concentrations of As in water.[19, 42] Differences in stable isotope ratios between sites were also insignificant (p>0.05). B. azoricus inhabit a range of microhabitats within each vent site, from vent walls to the chimney bases and surrounding areas.[13] Variation in microhabitat and feeding strategy within site have been shown to explain significant spatial variation in stable isotope signatures in B. azoricus.[25] The mussels feed symbiotrophically,[25, 29] but also behave as filter feeders requiring a constant flow of fluid over their gills,[13] and as such are also exposed to temporal variation in vent fluid composition. The heterogeneity in food source for this organism may make geographic differences in As uptake difficult to decipher.
As speciation
Results of As speciation analyses in R. exoculata, B. azoricus and B. seepensis are given in Table 2 and the Accessory Publication. The dominant As species in R. exoculata from Rainbow (n=1) and TAG (n=3) was AB, representing 64–81% of the water-extractable As. Inorganic As(V) was also present in all samples and ranged from 3–41% of extracted As, but As(III) was not detectable. By cation exchange chromatography, four unknown As species were also observed in the shrimp samples, occurring as peaks between 1.5 and 3.5 min., but these peaks made up only a minor (<10%) portion of total As (Accessory Publication). An earlier study of As speciation in vent organisms by Larsen et al. (2007) first showed the presence of AB in two specimens of R. exoculata from TAG, at concentrations of 18.4 and 3 mg/kg.[8] In this study, shrimp from TAG had AB concentrations of 12.3–15.4 mg/kg. In the Larsen study, AB was reported to be ~100% of total As in the shrimp, with trace amounts of As(III), As(V) and DMA (<2% of extractable As) in one of the two samples. By comparison, As(V) is relatively much higher (3–41%) in this study than in the Larsen samples. Neither this study nor the Larsen study detected arsenosugars in this organism. Studies of As speciation in marine organisms from near-surface environments have reported concentrations of total As in shrimp similar to those found in R. exoculata in this study, and have also reported AB as the dominant As species [7, 45, 46] despite the drastically different food sources of shrimp in photic environments.
Table 2.
Concentration of As species (mean ± standard deviation(mg/kg)) in Mid-Atlantic vent organisms
| Site | species | n | As Total (mg/kg) | Extraction efficiency | Extractable As identifieda | AB (mg/kg) | DMA (mg/kg) | As(III) (mg/kg) | As(V) (mg/kg) | Oxo-As-SugPO4 (mg/kg) | Thio-As-SugPO4 (mg/kg)b | Unknown cation (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Rainbow | R.exoculata | 1 | 3.9 | 39% | 93% | 1.2 | <0.004 | <0.002 | 0.2 | <0.007 | <0.007 | <0.001 |
| TAG | R.exoculata | 3 | 29.8 ± 3.9 | 67 ± 3% | 98% | 14.0 ± 1.3 | <0.004 | <0.002 | 5.5 ± 3.5 | <0.007 | <0.007 | <0.001 |
| Rainbow | B. azoricus | 3 | 9.9 ± 1.7 | 67 ± 3% | 19% | 0.3 ± 0.1 | 0.05 ± 0.01 | 0.31 ± 0.26 | 0.3 ± 0.1 | 0.4 ± 0.1 | n/a | 0.3 ± 0.2 |
| Lucky Strike | B. azoricus | 12 | 11.6 ± 2.4 | 70 ± 9% | 33% | 0.5 ± 0.3 | 0.05 ± 0.03 | 0.55 ± 0.18 | 0.6 ± 0.5 | 0.9 ± 0.8 | 1.5 ± 1 | 0.4 ± 0.3 |
| Lucky Strike | B. seepensis | 4a | 16.9 ± 2.3 | 89 ± 6% | 85% | 1.9 ± 0.6 | 0.37 ± 0.17 | 2.4 ± 0.5 | 1.8 ± 0.6 | 6.9 ± 1.5 | <0.007 | 2.1 ± 0.5 |
% of extracted species; column recoveries were 24–96% for anionic species and 14–103% for cationic species (see Accessory Publications)
Each sample was a composite of 2 organisms.
A subset of samples were analyzed for thio-As-SugPO4: R. exoculata (Rainbow n=1; TAG n=1); B. Azoricus (Lucky Strike n=4), B. Seepensis (Lucky Strike n=2).
n/a = not available
Multiple As species were determined in extracts from the mussels, B. azoricus, from Rainbow and Lucky Strike, as well as their commensals, B. seepensis. Water-extractable As ranged from 49 to 90% of total As in the mussels, whereas recoveries were consistently high in the commensal polychaete samples (85–93% of total As). This resulted in only 10–44% of total As in mussels being identified, whereas 65–85% of As in the polychaete samples was speciated. Arsenosugars were the dominant As species identified in both B. azoricus and B. seepensis; the oxo-arsenosugar-PO4 comprised 4– 31% of the extractable As in the mussels and 37–52% of extractable As in their commensals. The Larsen study also found oxo-arsenosugar-PO4 was the major component (54–58%) of extractable As in the adductor mantle and gill of B. puteoserpentis, a mussel species from the Snake Pit vent site, also on the Mid-Atlantic Ridge. Arsenosugar-glycol was also observed in the B. puteoserpentis specimen,[8] but was not present in either species analyzed in our study. Rather, we found that thio-arsenosugar-PO4, analyzed in a subset of samples, comprised 14–31% of extractable As in three of the mussel samples, but was absent from one mussel sample and two polychaete samples. The absence of the thio-arsenosugar-PO4 in some samples may be due oxidation in the freeze-dried sample during storage, [36, 47] or in the sample extracts which were stored up to 4 days prior to analysis. The potential oxidation of thio-arsenosugars during storage makes precise quantitation of this As species problematic, however, the formation of thio-arsenosugars during storage has been shown to be unlikely,[48] confirming the observed presence of this species is not an artifact. In mussels from surface environments, the presence of thio-arsenosugars have been reported, and are thought to be ingested from algae or formed in vivo.[36, 49]
In both the vent mussels and their commensals, AB and inorganic As were present as significant portions of extractable As, along with trace amounts of DMA and MA in some samples. In B. azoricus and B. seepensis, AB comprised 3–13% and 8–19% of extractable As, respectively. Similarly, AB constituted 16% of extractable As in B. puteoserpentis in the Larsen study.[8] Inorganic As was present in B. azoricus as 6–13% of extractable As, with As(III) comprising 28–70% of inorganic As; whereas the B. puteoserpentis sample contained 8–14% inorganic As, which was predominantly (90%) As(III).[8] Larsen et al. also reported no detectable DMA or MA in the vent mussel, [8] however DMA was a minor As species (≤ 1% extractable As) and in all but two mussel samples in this study as well as in the polychaetes (< 4% extractable As). A few samples of both species also contained trace amounts of MA. These differences between studies may be due to differences in detection limits between analyses, to small sample sizes, or to differences between the mussel species. The As speciation found in these mussels was similar to what is found in near surface mussels, where arsenosugars (As-sug-PO4 and As-sug-glycerol), AB, DMA and inorganic As are found in similar proportions. [50]
A number of unidentified As species were apparent in both the mussels and polychaetes. By anion exchange chromatography, a broad, late-eluting peak (retention time = 19 min) was evident in several of the mussel samples. Treatment of sample extracts with H2O2 caused this peak to disappear while simultaneously causing an increase in the oxo-arsenosugar-PO4 peak, providing further evidence for the presence of sulfur analog of the oxo-arsenosugar-PO4.[6, 36] An unknown cationic As species with a retention time of 4.2 min. represented a significant portion of the water-extractable As in the mussel (3–14%) and worm (9–17%) samples (labeled “unknown cation” in Table 2). By reverse phase chromatography, two unknown peaks were also evident in the mussel and worm samples (Accessory Publication).
No significant difference in concentrations of individual As species were observed between mussels from the Rainbow and Lucky Strike (p>0.05), or by multivariate analysis (PCA). For the 12 mussel samples from Lucky Strike, there was no significant correlation between concentrations of As species and stable isotope signatures (Spearman's rho, p> 0.05), giving no insight into differences in As uptake within species. Polychaetes from Lucky Strike had significantly higher extractable As than mussels from the same site (p=0.008), and concentrations of oxo-As-sugar-PO4, AB, DMA and As(III), normalized to extractable As, than their hosts (p<0.05), whereas normalized concentrations of As(V) and thio-As-sugar-PO4 were similar between the species (p>0.05). The polychaetes, which also had higher concentrations of total As, were from a higher trophic level than their hosts, indicated by their δ15N signature.
A major difference in As speciation was observed between shrimp (from Rainbow and TAG sites), where AB and As(V) were the major species identified, and mussels and their commensals (from Rainbow and Lucky Strike sites) in which arsenosugars and methylated As species, as well as AB and inorganic As were observed. Based on their stable isotope signatures, these species also have a different food sources in the vents, though both are thought to feed primarily via symbiotic relationships with bacteria.
Microbial mats
Concentrations of As species, extraction efficiencies and total As in samples scraped from exterior of the vent chimneys (Table 3 and Accessory Publication) were likely controlled by the amount of biomass vs. geological material in each individual sample. High concentrations of total As (8–193 mg/kg) were found in the vent materials, significantly higher than crustal abundance of ~ 2 mg/kg.[51] The extraction efficiency from these materials was low (0.02–0.08%). It is expected that under the applied extraction conditions (1% HNO3 at 95°C) the As species were efficiently recovered from the biomass fraction of the sample[32] but not from the sulfidic/ferrous chimney material. A report of metal concentrations from the Lost City Mid-Atlantic Ridge vent site found concentrations of As in porous carbonates covered with microbial mats from the Lost City vent site were only slightly elevated relative to porous carbonates free of mats,[42] also suggesting most of the As present in these samples is bound in the geological fraction. In this study, only inorganic As species were present in the microbial mat at measurable concentrations, providing no clue to the source of organic arsenicals to vent organisms. It remains a possibility that organic As is present at ultratrace levels in the vent microbial community, and are biomagnified into the primary consumers of the food web.
Table 3.
As speciation and total As in scrape samples of sulfide and microbial mat from outside the vent chimney
| Site | n | Total As(mg/kg) | Extraction efficiency | Column recovery | As(III) (mg/kg) | As(V) (mg/kg) |
|---|---|---|---|---|---|---|
|
| ||||||
| Rainbow | 3 | 95 ± 61 | 0.2% | 24% | 0.006 ± 0.003 | 0.006 ± 0.003 |
| Lucky strike | 6 | 136 ± 37 | 3.7% | 48% | 1.3 ± 1.1 | 0.6 ± 0.8 |
| TAG | 3 | 68 ± 25 | 2.4% | 43% | 0.5 ± 0.6 | 0.5 ± 0.6 |
Arsenic cycling in deep sea vents
The speciation of As in organisms from deep sea vents is of particular interest as the food web is drastically different than in the photic zone.[8] In the photic environment, seawater contains primarily inorganic As(V), and in algae, the primary producer in surface environments, most As is present as arsenosugars.[52] Marine algae has been shown to preconcentrate and biotransform As(V) to methylated As and arsenosugars, [1, 5, 53, 54] whereas bacteria in photic environments are not thought to biosynthesize arsenosugars.[53] In vent organisms, the source of arsenosugars is unclear, as no algae are present. While chemolithoautotrophy has been shown to be the major source of nutrients to vent shrimp and mussels, both species may also consume organic matter by grazing/filtering, some of which may be of pelagic origin.[25] An alternative explanation, suggested by the Larsen study, is that there may be a pathway of arsenosugar formation in deep sea autotrophic bacteria.[8] In that case the different symbiotrophic relationships between the shrimp and mussel species may explain the high abundances of arsenosugars in the mussel, while this species was not detectable in the shrimp.
The proportions of AB in vent organisms were similar to those in corresponding species from photic environments. While there has been no strong evidence for biosynthesis of AB ab initio in pelagic marine organisms, there has been recent evidence that some algae contain significant amounts of AB,[6, 20, 54] which may be efficiently accumulated by primary consumers.[1] Uptake of AB has been demonstrated by shrimp, Crangon crangon,[46] and mussels, Mytilus edulis,[55, 56] although AB concentrations in marine flora and seawater are low to undetectable. Uptake and transformation of tri-methylated arsenosugars to AB in vivo has been observed[57] but not at sufficient rates to explain body burden.[46] Studies of As species in a tropical[7] and temperate[58] mangrove ecosystem, where mangrove trees, rather than algae, were the primary source of organic matter for primary consumers, showed AB to be the major As species in crustaceans, despite mangrove leaves having low As concentrations with no observed AB. In tropical mangroves, arsenosugar-PO4 was the dominant species in bivalves, with AB also present, which was attributed to phytoplankton being a major dietary source.[7] Similarly, a study of salt marshes where faunal As concentrations were low, and arsenosugar constituents were minor, also found organisms contained high concentrations of AB.[59] The presence of AB in vent organisms also suggests algae are not the sole source of this As species to fauna. The high abundance of AB in R. exoculata, in which methylated species and arsenosugars were undetectable, suggests that if the shrimp synthesize AB in vivo then arsenosugars are not intermediates. In mussels, arsenosugars, the dominant As species, may be a precursor to AB, although this pathway has not been observed in pelagic species.[56] Vent shrimp and mussels feed primarily by symbiotrophy with bacteria, and stable isotope signatures indicated food substrates were largely of vent origin, suggesting a bacterial source of AB in the vents. In this case, production of AB appears to occur in both mussel and shrimp symbionts. While the pathway of AB formation in marine environments is not widely agreed upon, the presence of this compound in a foodweb based on chemoautolithotrophy provides evidence that accumulation from algae is not the only source of this metabolite.
In summary, we present evidence from stable isotope signatures and As speciation that the vent organisms R. exoculata, B. azoricus, B. seepensis and P. smaragdina take up As from different dietary sources. The presence of arsenobetaine and other organic arsenicals in primary consumer organisms of the Mid-Atlantic Ridge, suggests the occurrence of either uptake from a non-photosynthetic (bacterial) food source, or in vivo synthesis of these compounds by the primary consumers.
Supplementary Material
Acknowledgements
The authors would like to Kate Buckman for comments which significantly improved the manuscript. VFT and BPJ are supported by NIH grant P42 ES007373. We thank Anna-Louise Reysenbach, Breea Govenar, and the crew of the R/V Roger Revelle and the DSROV Jason II for their assistance in obtaining the samples.
Footnotes
Environmental Context Arsenic occurs in marine organisms at high levels and in many chemical forms. A common explanation of this phenomenon is that algae play the central role in accumulating arsenic by producing arsenic-containing sugars that are then the source for simpler organic arsenic compounds found in fish and other marine animals. We show that life in deep-sea vent eco-systems, which are uninhabited by algae, contains the same organic arsenic compounds as do pelagic animals, indicating that algae are not the only source of these compounds.
References
- 1.Francesconi KA, Edmonds JS. Arsenic and marine organisms. Advances in Inorganic Chemistry, Vol. 44. 1997;44:147. [Google Scholar]
- 2.Hellweger FL. Dynamics of arsenic speciation in surface waters: As(III) production by algae. Applied Organometallic Chemistry. 2005;19:727. [Google Scholar]
- 3.Hellweger FL, Lall U. Modeling the effect of algal dynamics on arsenic speciation in Lake Biwa. Environmental Science & Technology. 2004;38:6716. doi: 10.1021/es049660k. [DOI] [PubMed] [Google Scholar]
- 4.Andreae MO. Arsenic Speciation in Seawater and Interstitial Waters - Influence of Biological-Chemical Interactions on the Chemistry of a Trace-Element. Limnology and Oceanography. 1979;24:440. [Google Scholar]
- 5.Geiszinger A, Goessler W, Pedersen SN, Francesconi KA. Arsenic biotransformation by the brown macroalga Fucus serratus. Environmental Toxicology and Chemistry. 2001;20:2255. doi: 10.1897/1551-5028(2001)020<2255:abbtbm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Nischwitz V, Pergantis SA. First report on the detection and quantification of arsenobetaine in extracts of marine algae using HPLC-ES-MS/MS. Analyst. 2005;130:1348. doi: 10.1039/b509547f. [DOI] [PubMed] [Google Scholar]
- 7.Khokiattiwong S, Kornkanitnan N, Goessler W, Kokarnig S, Francesconi KA. Arsenic compounds in tropical marine ecosystems: similarities between mangrove forest and coral reef. Environmental Chemistry. 2009;6:226. [Google Scholar]
- 8.Larsen EH, Quetel CR, Munoz R, FialaMedioni A, Donard OFX. Arsenic speciation in shrimp and mussel from the Mid-Atlantic hydrothermal vents. Marine Chemistry. 1997;57:341. [Google Scholar]
- 9.Tarasov VG, Gebruk AV, Mironov AN, Moskalev LI. Deep-sea and shallow-water hydrothermal vent communities: Two different phenomena? Chemical Geology. 2005;224:5. [Google Scholar]
- 10.Goffredi SK. Indigenous ectosymbiotic bacteria associated with diverse hydrothermal vent invertebrates. Environmental Microbiology Reports. 2010;2:479. doi: 10.1111/j.1758-2229.2010.00136.x. [DOI] [PubMed] [Google Scholar]
- 11.Von Damm KL, Bray AM, Buttermore LG, Oosting SE. The geochemical controls on vent fluids from the Lucky Strike vent field, Mid-Atlantic Ridge. Earth and Planetary Science Letters. 1998;160:521. [Google Scholar]
- 12.Cosson RP, Thiebaut E, Company R, Castrec-Rouelle M, Colaco A, Martins I, Sarradin PM, Bebianno MJ. Spatial variation of metal bioaccumulation in the hydrothermal vent mussel Bathymodiolus azoricus. Marine Environmental Research. 2008;65:405. doi: 10.1016/j.marenvres.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Desbruyeres D, Almeida A, Biscoito M, Comtet T, Khripounoff A, Le Bris N, Sarradin PM, Segonzac M. A review of the distribution of hydrothermal vent communities along the northern Mid-Atlantic Ridge: dispersal vs. environmental controls. Hydrobiologia. 2000;440:201. [Google Scholar]
- 14.Douville E, Charlou JL, Oelkers EH, Bienvenu P, Colon CFJ, Donval JP, Fouquet Y, Prieur D, Appriou P. The rainbow vent fluids (36 degrees 14 ' N, MAR): the influence of ultramafic rocks and phase separation on trace metal content in Mid-Atlantic Ridge hydrothermal fluids. Chemical Geology. 2002;184:37. [Google Scholar]
- 15.Martins I, Cosson RP, Riou V, Sarradin PM, Sarrazin J, Santos RS, Colaco A. Relationship between metal levels in the vent mussel Bathymodiolus azoricus and local microhabitat chemical characteristics of Eiffel Tower (Lucky Strike) Deep-Sea Research Part I-Oceanographic Research Papers. 2011;58:306. [Google Scholar]
- 16.Rousse N, Boulegue J, Cosson RP, Fiala-Medioni A. Bioaccumulation of metals within the hydrothermal mytilidae Bathymodiolus sp. from the Mid-Atlantic Ridge. Oceanologica Acta. 1998;21:597. [Google Scholar]
- 17.Demina LL, Galkin SV. On the role of abiogenic factors in the bioaccumulation of heavy metals by the hydrothermal fauna of the Mid-Atlantic Ridge. Oceanology. 2008;48:784. [Google Scholar]
- 18.Cave RR, German CR, Thomson J, Nesbitt RW. Fluxes to sediments underlying the Rainbow hydrothermal plume at 36 degrees 14 ' N on the Mid-Atlantic Ridge. Geochimica Et Cosmochimica Acta. 2002;66:1905. [Google Scholar]
- 19.Douville E, Charlou JL, Donval JP, Hureau D, Appriou P. As and Sb behaviour in fluids from various deep-sea hydrothermal systems. Comptes Rendus De L Academie Des Sciences Serie Ii Fascicule a-Sciences De La Terre Et Des Planetes. 1999;328:97. [Google Scholar]
- 20.Grotti M, Soggia F, Lagomarsino C, Goessler W, Francesconi KA. Arsenobetaine is a significant arsenical constituent of the red Antarctic alga Phyllophora antarctica. Environmental Chemistry. 2008;5:171. [Google Scholar]
- 21.Francesconi KA. Current perspectives in arsenic environmental and biological research. Environmental Chemistry. 2005;2:141. [Google Scholar]
- 22.Francesconi KA. Arsenic species in seafood: Origin and human health implications. Pure and Applied Chemistry. 2010;82:373. [Google Scholar]
- 23.Von Cosel R, Comtet T, Krylova EM. Bathymodiolus (Bivalvia: Mytilidae) from hydrothermal vents on the Azores Triple Junction and the Logatchev hydrothermal field, Mid-Atlantic Ridge. Veliger. 1999;42:218. [Google Scholar]
- 24.Riou V, Halary S, Duperron S, Bouillon S, Elskens M, Bettencourt R, Santos RS, Dehairs F, Colaco A. Influence of CH4 and H2S availability on symbiont distribution, carbon assimilation and transfer in the dual symbiotic vent mussel Bathymodiolus azoricus. Biogeosciences. 2008;5:1681. [Google Scholar]
- 25.De Busserolles F, Sarrazin J, Gauthier O, Gelinas Y, Fabri MC, Sarradin PM, Desbruyeres D. Are spatial variations in the diets of hydrothermal fauna linked to local environmental conditions? Deep-Sea Research Part Ii-Topical Studies in Oceanography. 2009;56:1649. [Google Scholar]
- 26.Martins I, Colaco A, Dando PR, Martins I, Desbruyeres D, Sarradin PM, Marques JC, Serrao-Santos R. Size-dependent variations on the nutritional pathway of Bathymodiolus azoricus demonstrated by a C-flux model. Ecological Modelling. 2008;217:59. [Google Scholar]
- 27.Riou V, Duperron S, Halary S, Dehairs F, Bouillon S, Martins I, Colaco A, Santos RS. Variation in physiological indicators in Bathymodiolus azoricus (Bivalvia: Mytilidae) at the Menez Gwen Mid-Atlantic Ridge deep-sea hydrothermal vent site within a year. Marine Environmental Research. 2010;70:264. doi: 10.1016/j.marenvres.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Wirsen CO, Jannasch HW, Molyneaux SJ. Chemosynthetic Microbial Activity at Mid-Atlantic Ridge Hydrothermal Vent Sites. Journal of Geophysical Research-Solid Earth. 1993;98:9693. [Google Scholar]
- 29.Colaco A, Dehairs F, Desbruyeres D. Nutritional relations of deep-sea hydrothermal fields at the Mid-Atlantic Ridge: a stable isotope approach. Deep-Sea Research Part I Oceanographic Research Papers. 2002;49:395. [Google Scholar]
- 30.Francesconi KA. Complete extraction of arsenic species: a worthwhile goal? Applied Organometallic Chemistry. 2003;17:682. [Google Scholar]
- 31.Francesconi KA, Kuehnelt D. Determination of arsenic species: A critical review of methods and applications, 2000–2003. Analyst. 2004;129:373. doi: 10.1039/b401321m. [DOI] [PubMed] [Google Scholar]
- 32.Foster S, Maher W, Krikowa F, Apte S. A microwave-assisted sequential extraction of water and dilute acid soluble arsenic species from marine plant and animal tissues. Talanta. 2007;71:537. doi: 10.1016/j.talanta.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 33.Madsen AD, Goessler W, Pedersen SN, Francesconi KA. Characterization of an algal extract by HPLC-ICP-MS and LC-electrospray MS for use in arsenosugar speciation studies. Journal of Analytical Atomic Spectrometry. 2000;15:657. [Google Scholar]
- 34.Raber G, Francesconi KA, Irgolic KJ, Goessler W. Determination of `arsenosugars' in algae with anion-exchange chromatography and an inductively coupled plasma mass spectrometer as element-specific detector. Fresenius Journal of Analytical Chemistry. 2000;367:181. doi: 10.1007/s002160051621. [DOI] [PubMed] [Google Scholar]
- 35.Kuehnelt D, Goessler W, Irgolic KJ. Arsenic compounds in terrestrial organisms.2. Arsenocholine in the mushroom Amanita muscaria. Applied Organometallic Chemistry. 1997;11:459. [Google Scholar]
- 36.Schmeisser E, Raml R, Francesconi KA, Kuehnelt D, Lindberg AL, Soros C, Goessler W. Thio arsenosugars identified as natural constituents of mussels by liquid chromatography mass spectrometry. Chemical Communications. 2004;1824 doi: 10.1039/b406917j. [DOI] [PubMed] [Google Scholar]
- 37.Miyashita S, Shimoya M, Kamidate Y, Kuroiwa T, Shikino O, Fujiwara S, Francesconi KA, Kaise T. Rapid determination of arsenic species in freshwater organisms from the arsenic-rich Hayakawa River in Japan using HPLC-ICP-MS. Chemosphere. 2009;75:1065. doi: 10.1016/j.chemosphere.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 38.Fisher CR. Toward an appreciation of hydrothermal vent animals: their environment, physiological ecology, and tissue stable isotope values. Geophysical Monographs. 1995;91:297. [Google Scholar]
- 39.Gebruk AV, Southward EC, Kennedy H, Southward AJ. Food sources, behaviour, and distribution of hydrothermal vent shrimps at the Mid-Atlantic Ridge. Journal of the Marine Biological Association of the United Kingdom. 2000;80:485. [Google Scholar]
- 40.Polz MF, Robinson JJ, Cavanaugh CM, Van Dover CL. Trophic ecology of massive shrimp aggregations at a Mid-Atlantic Ridge hydrothermal vent site. Limnology and Oceanography. 1998;43:1631. [Google Scholar]
- 41.Bergquist DC, Eckner JT, Urcuyo IA, Cordes EE, Hourdez S, Macko SA, Fisher CR. Using stable isotopes and quantitative community characteristics to determine a local hydrothermal vent food web. Marine Ecology-Progress Series. 2007;330:49. [Google Scholar]
- 42.Demina LL, Holm NG, Galkin SV, Lein AY. Concentration function of the deep-sea vent benthic organisms. Cahiers De Biologie Marine. 2010;51:369. [Google Scholar]
- 43.Vandover CL, Fry B. Stable Isotopic Compositions of Hydrothermal Vent Organisms. Marine Biology. 1989;102:257. [Google Scholar]
- 44.Geret F, Riso R, Sarradin PM, Caprais JC, Cosson RP. Metal bioaccumulation and storage forms in the shrimp, Rimicaris exoculata, from the Rainbow hydrothermal field (Mid-Atlantic Ridge); preliminary approach to the fluid-organism relationship. Cahiers De Biologie Marine. 2002;43:43. [Google Scholar]
- 45.Larsen EH, Pritzl G, Hansen SH. Arsenic Speciation in Seafood Samples with Emphasis on Minor Constituents - an Investigation Using High-Performance Liquid-Chromatography with Detection by Inductively-Coupled Plasma-Mass Spectrometry. Journal of Analytical Atomic Spectrometry. 1993;8:1075. [Google Scholar]
- 46.Francesconi KA, Hunter DA, Bachmann B, Raber G, Goessler W. Uptake and transformation of arsenosugars in the shrimp Crangon crangon. Applied Organometallic Chemistry. 1999;13:669. [Google Scholar]
- 47.Meier J, Kienzl N, Goessler W, Francesconi KA. The occurrence of thio-arsenosugars in some samples of marine algae. Environmental Chemistry. 2005;2:304. [Google Scholar]
- 48.Nischwitz V, Kanaki K, Pergantis SA. Mass spectrometric identification of novel arsinothioyl-sugars in marine bivalves and algae. Journal of Analytical Atomic Spectrometry. 2006;21:33. [Google Scholar]
- 49.Soeroes C, Goessler W, Francesconi KA, Schmeisser E, Raml R, Kienzl N, Kahn M, Fodor P, Kuehnelt D. Thio arsenosugars in freshwater mussels from the Danube in Hungary. Journal of Environmental Monitoring. 2005;7:688. doi: 10.1039/b503897a. [DOI] [PubMed] [Google Scholar]
- 50.Schaeffer R, Francesconi KA, Kienzl N, Soeroes C, Fodor P, Varadi L, Raml R, Goessler W, Kuehnelt D. Arsenic speciation in freshwater organisms from the river Danube in Hungary. Talanta. 2006;69:856. doi: 10.1016/j.talanta.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 51.Winter M. WebElements. 2011. [Google Scholar]
- 52.Francesconi KA, Edmonds JS. Arsenic species in marine samples. Croatica Chemica Acta. 1998;71:343. [Google Scholar]
- 53.Murray LA, Raab A, Marr IL, Feldmann J. Biotransfonnation of arsenate to arsenosugars by Chlorella vulgaris. Applied Organometallic Chemistry. 2003;17:669. [Google Scholar]
- 54.Llorente-Mirandes T, Ruiz-Chancho MJ, Barbero M, Rubio R, Lopez-Sanchez JF. Measurement of arsenic compounds in littoral zone algae from the Western Mediterranean Sea Occurrence of arsenobetaine. Chemosphere. 2010;81:867. doi: 10.1016/j.chemosphere.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Clowes LA, Francesconi KA. Uptake and elimination of arsenobetaine by the mussel Mytilus edulis is related to salinity. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology. 2004;137:35. doi: 10.1016/j.cca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Francesconi KA, Gailer J, Edmonds JS, Goessler W, Irgolic KJ. Uptake of arsenic-betaines by the mussel Mytilus edulis. Comparative Biochemistry and Physiology C-Pharmacology Toxicology & Endocrinology. 1999;122:131. doi: 10.1016/s0742-8413(98)10095-6. [DOI] [PubMed] [Google Scholar]
- 57.Francesconi KA, Goessler W, Panutrakul S, Irgolic KJ. A novel arsenic containing riboside (arsenosugar) in three species of gastropod. Science of the Total Environment. 1998;221:139. [Google Scholar]
- 58.Kirby J, Maher W, Chariton A, Krikowa F. Arsenic concentrations and speciation in a temperate mangrove ecosystem, NSW, Australia. Applied Organometallic Chemistry. 2002;16:192. [Google Scholar]
- 59.Foster S, Maher W, Taylor A, Krikowa F, Telford K. Distribution and speciation of arsenic in temperate marine saltmarsh ecosystems. Environmental Chemistry. 2005;2:177. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.