Abstract
Animals have evolved multiple strategies for coping with the presence of pathogenic microbes. The best characterized is the immune response where animals activate their physical and cellular defenses to respond to invading microorganisms. However, behavioral changes can also be triggered by exposure to microbes and play an important role in defending many species, including humans, from pathogen attack. In our recent study we demonstrate that, following infection, C. elegans uses the same G-protein signaling pathway in neurons and epithelial cells to coordinate avoidance behaviors and immune responses. Coordination of these responses allows animals to mount an immune response to the immediate threat while simultaneously taking action to remove the pathogen, however, the complicated nature of the mammalian brain and immune system has made it difficult to identify the molecular mechanisms mediating these interactions. With its simple, well described, nervous system and a rapidly growing understanding of its immune system, C. elegans has emerged as an excellent model to study the mechanisms by which animals recognize pathogens and coordinate behavioral and immune responses to infection.
Keywords : infection, behavior, immune response, neuronal function, G-protein, Rho GTPase
G Protein Signaling is Required for Pathogen Avoidance
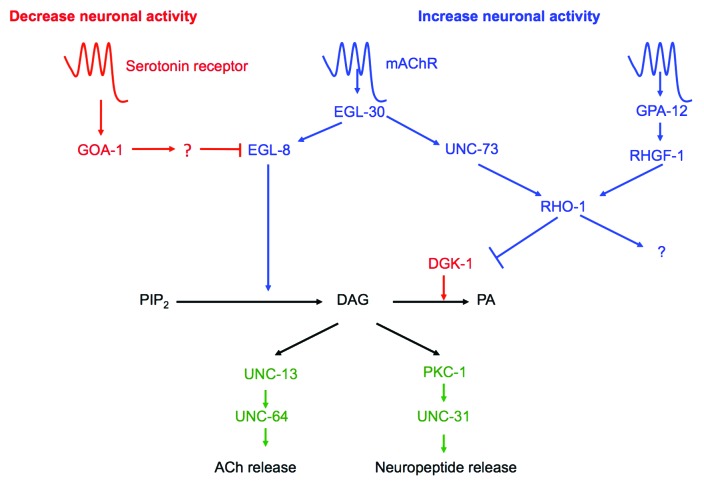
In its natural environment the C. elegans nervous system is constantly sensing and responding to attractive and aversive signals by altering its locomotion. Work from several labs has defined a network of G-protein signaling pathways that modulate release of the neurotransmitter acetylcholine (ACh) in the motor neurons to alter the worm’s locomotion11 (Fig. 1). However, the signals which act upon G-protein coupled receptors (GPCRs) to regulate G-protein signaling, modulating the activity of cholinergic motor neurons and altering locomotion are less well understood. Our data demonstrates that one of these signals is the presence of pathogenic microbes.2
Figure 1. A G-protein network in the cholinergic motor neurons mediates neurotransmitter release. A model for G-protein networks acting within C. elegans cholinergic motor neurons to regulate neuronal activity. Shown in blue are those proteins that increase neuronal activity (as defined by increases in locomotion and/or ACh release), whereas those in red decrease neuronal activity. Not shown is Gαs, whose role in control of cholinergic motorneuron activity remains unclear. Modified from McMullan and Nurrish1
C. elegans can be easily infected with a variety of natural and clinically-relevant pathogens.3 Previous studies by the Ewbank,4 Kim5 and Hodgkin6 labs have demonstrated that animals avoid some of these pathogens. Aversive cues produced by microbes are directly sensed by chemosensory neurons located in the animal’s head.4 In the case of the pathogen Serratia marcescens, mutant library screening has identified the secreted surfactant peptide, serrawettin W2, as the aversive cue sensed by C. elegans to trigger aversive behavior to this microbe.4 The receptors for these pathogen-associated cues are unknown, however, the neuropeptide GPCR NPR-1 is required to mediate avoidance of Pseudomonas aeruginosa.7,8 Furthermore the G-protein ODR-3 and the TAX-2/4 cGMP gated channel are required to mediate avoidance to S. marcescens4 and TAX-2/4 is also required for animals to avoid Microbacterium nematophilum and P. aeruginosa6 implicating GPCRs in the early stages of the response to at least some of these pathogens.
We now show that avoidance of the naturally-occurring pathogen M. nematophilum requires increased release of ACh from the cholinergic motor neurons and changes in locomotion behavior that are dependant on the activation of a EGL-30 (Gαq) – UNC-73 (Trio Rho GEF) – RHO-1 pathway.2 Thus, in response to a pathogen attack on C. elegans, G-protein signaling is activated in the nervous system by internally generated signals and acts at a late step in the avoidance response to infection. C. elegans avoids a number of other pathogens and the role of cholinergic Gαq signaling in avoiding these remains to be determined, although it is tempting to speculate that this late step in the avoidance response is common to all pathogen infections.
What are the signals that activate GPCRs in the cholinergic neurons to mediate aversive behavior? Identification of the GPCR and its ligand that act upstream of EGL-30 will be a significant step toward understanding these behaviors. It is possible that following ingestion of microbes the intestine or rectal epithelium produces secreted ligands that activate neuronal GPCRs. These tissues express a number of antimicrobial peptides following infection9,10 which could, in principle, act as ligands for neuronal GPCRs. Alternatively these ligands could be generated by the nervous system in response to the sensing of aversive cues by chemosensory neurons. This would allow animals to respond to pathogen attack before they become infected. Consistent with this hypothesis the cGMP-gated channel TAX-2/4, which acts in the chemosensory neurons, is required for avoidance of M. nematophilum6 and our data does not exclude a role for Gαq signaling in both the early and late stages of the avoidance response. Indeed EGL-30 is required in the sensory neurons to modulate ASH-mediated aversive responses.11
If the ligand for the cholinergic Gαq-coupled GPCR is neuronal it should be possible to use mutants defective in neuronal function and avoidance behaviors together with previously described tools such as the calcium sensor cameleon to investigate a complete neuronal circuit that begins with recognition of microbial cues by the chemosensory neurons and ends with activation of the cholinergic motor neurons resulting in movement. These types of studies are already being used to describe the neural circuits required for responses to food and touch12 and understanding how the nervous system integrates these different behaviors as the worm responds to its environment will be an exciting challenge for the future. In this context, it is interesting that C. elegans appears to be able to modify its behavior following exposure to pathogens, learning to avoid pathogenic bacteria while becoming attracted to non-pathogenic food.13
G Proteins Mediate the C. Elegans Immune Response to Infection
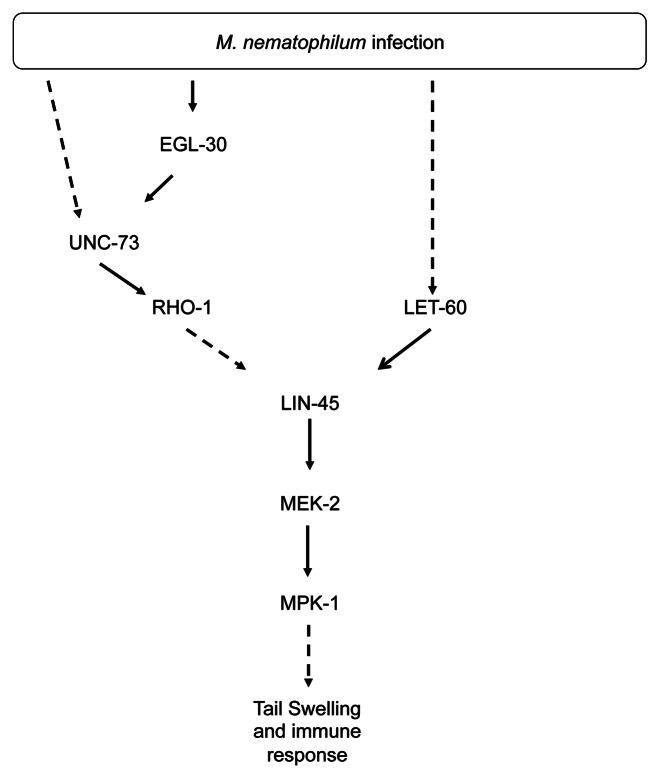
While the neuronal EGL-30 (Gαq)–UNC-73 (Trio Rho GEF)–RHO-1 pathway is required for avoidance of M. nematophilum, the same pathway is required in non-neuronal cells to mediate the immune response.2 Upon M. nematophilum infection of wild-type C. elegans, the pathogen adheres to the cuticle surrounding the rectal opening giving rise to swelling around this opening known as the deformed anal region (dar) phenotype. This mitigates the effects of infection and forms part of the innate immune response.14 While performing behavioral assays on M. nematophilum infected egl-30 and unc-73 mutants we noticed that the dar phenotype was significantly decreased. Unlike avoidance behavior, which requires G-protein signaling in the cholinergic motor neurons, the EGL-30 (Gαq) – UNC-73 (Trio Rho GEF) – RHO-1 pathway acts in the rectal epithelium altering the shape and size of these cells to cause the dar phenotype and mediate the immune response (Fig. 2).2 M. nematophilum infection can also trigger changes in the expression of antimicrobial peptides including CLEC-60 and F53A9.8.9 Using a heat shock inducible constitutively active version of RHO-115 we observe an increase in the expression of F53A9.8 but not CLEC-60 (A. Anderson and R. McMullan unpublished observation) suggesting that the RHO-1 pathway may be required for other components of the innate immune response.
Figure 2. Gαq-Rho GEF Trio-Rho Signaling and Ras converge on Raf to regulate morphology during the immune response to infection. Following pathogen infection RHO-1 is activated in the rectal epithelial cells by multiple upstream regulators including EGL-30 (Gαq) and UNC-73 (Trio). Together with Ras, Rho signaling converges on Raf to activate the MAPK pathway. Activation of these pathways, together with at least one other, in the rectal epithelial cells leads to the changes in morphology that occur as part of the immune response.2
EGL-30 (Gαq) is also required in intestinal cells for the immune response to P. aeruginosa.16 During P. aeruginosa infection EGL-30 (Gαq) acts via the phospholipaseC-β EGL-8.16 EGL-8 mutants were also isolated by the Hodgkin lab in a screen for animals that fails to develop the dar phenotype following M. nematophilum infection.6 Our data places EGL-8 downstream of EGL-30 in this response. In addition we have shown that Rho signaling acts downstream of EGL-30 to trigger the immune response to M. nematophilum. This is the first demonstration of a role for RHO-1 signaling in the C. elegans immune response, however, the RHO-1 mammalian ortholog RhoA is a key regulator of mammalian immune responses acting to regulate Toll receptor signaling, leukocyte migration, and phagocytosis of pathogens17 suggesting further parallels between mammalian and C. elegans innate immunity.
In the motor neurons, neurotransmission is modulated by a G-protein signaling network1 (Fig. 1). Does the rectal epithelium use the same network to regulate the innate immune response? Other G-proteins are required for immune responses to different pathogens. During P. aeruginosa infection, GOA-1 (Gαo) signaling in the nervous system regulates the release of insulin-like neuropeptides to alter expression of immunity-related genes in the intestine.18 GOA-1 (Gαo) also influences the expression of the antimicrobial peptide NLP-29 and expression of this peptide in response to infection by D. coniospora requires the G-protein GPA-12 (Gα12).19 It will be interesting to test whether these G-protein networks play a role in modulating the dar phenotype following M. nematophilum infection.
Regardless of whether EGL-30 (Gαq) acts alone or as part of a G-protein network the Gq-coupled GPCRs that trigger the dar phenotype remain unidentified. GPCRs and their ligands are of significant interest in drug discovery and using C. elegans to identify the GPCRs and ligands required for immune responses to clinically-relevant pathogens may define new therapeutic targets. Expression of EGL-30 in the cholinergic motor neurons rescues the behavioral, but not immune, response to infection in an egl-30 mutant whereas EGL-30 expression in the rectal epithelium of egl-30(ad805) animals is sufficient to almost fully rescue this immune response, but has no effect on avoidance behavior.2 Therefore, our results demonstrate separate sites of action for the behavioral and immune responses to infection and argue against a model where activation of the rectal epithelial cells by a ligand produced as a result of Gαq signaling in another cell is sufficient to trigger the dar phenotype. Thus, Gq-coupled GPCRs must be present on the rectal epithelial cells. Animals lacking these GPCRs will fail to trigger the dar response following M. nematophilum infection. Only two GPCRs have so far been associated with the C. elegans immune response. The single C. elegans leucine-rich-repeat (LRR)-containing GPCR, FSHR-1, is required in the intestine for the immune response to P.aeruginosa,20 while the catecholamine receptor OCTR-1 is required in the nervous system to suppress this response.21 Even more elusive are the ligands that activate GPCRs during the immune response. Identifying the ligands that activate the receptors coupled to EGL-30 (Gαq) will provide important clues as to how C. elegans recognizes that it has been infected. It is possible that GPCRs act as pathogen receptors recognizing pathogens directly or pathogen-produced virulence factors to trigger immune responses. To do this, GPCRs must be expressed on tissues such as the intestine or the hypodermis that directly contact infectious microbes. The apical surface of the rectal epithelium is protected from the environment by the cuticle, thus it is unclear whether GPCRs expressed on the surface of these cells can directly contact M. nematophilum. One alternative hypothesis is that GPCRs expressed on the rectal epithelium recognize ligands produced internally by C. elegans in response to infection. These ligands may be produced by the hypodermal cells themselves or by other tissues such as the nervous system, which senses the presence of infection via its chemosensory neurons. This provides a mechanism for coordinating behavioral and immune responses to infection and is discussed in more detail below.
Immune Responses are Harder to Trigger than Behavioral Responses
During neurotransmission the EGL-30 (Gαq)–UNC-73 (Trio Rho GEF)–RHO-1 pathway acts via a Diacylglycerol (DAG) kinase to localize the DAG-binding protein UNC-13 and regulate ACh release,15 however UNC-13 mutants remain dar following M. nematophilum infection (A. Anderson, unpublished observation) suggesting that this signaling pathway has different downstream targets in the nervous system and rectal epithelium. We show that in rectal epithelial cells the EGL-30 (Gαq)–UNC-73 (Trio Rho GEF)–RHO-1 acts together with LET-60 (Ras) to converge on LIN-45 (Raf) (Fig. 2).2 The requirement for convergent RhoA and Ras signaling to activate Raf has also been observed in mammalian cells, where dominant negative forms of RhoA blocked the ability of Ras to activate Raf, indicating that Rho signaling is required for Raf activation, although the mechanism is unknown.22 Alternative interactions between Rho and Ras also exist. During C. elegans vulval formation RHO-1 appears to act upstream of LET-60 (Ras)23 suggesting that the Rho and Ras signaling pathways can either act in parallel or in series depending on the cell type. Interactions between Rho and Ras pathways appear to be essential during cellular transformation and co-activation of RhoA and Ras signaling can lead to different responses from those signaled by either pathway alone.24 It will be important to identify the cell specific factors that control the interactions between the Rho and Ras signaling pathways. Infection of C. elegans with M. nematophilum provides a starting point for genetic screens to identify the molecular mechanisms by which RhoA and Ras act together to activate Raf. The factors that allow this co-operation are likely to be critical in C. elegans and mammals for signaling involved in innate immunity and oncogenesis.
In contrast to neurotransmission, where chromosomal gain-of-function EGL-30 (Gαq) mutations are sufficient to increase ACh release and alter behavior,25 the same chromosomal gain-of-function EGL-30 (Gαq) mutations did not trigger the dar phenotype suggesting that higher levels of EGL-30 (Gαq) signaling are required for the immune response than the behavioral response.2 In addition the dar response requires coincident EGL-30 (Gαq) and LET-60 (Ras) signaling that is not required for the behavioral response.2 Thus immune responses appear harder to trigger than behavioral responses. Perhaps the consequences of inappropriate activation of the innate immune response are more severe than inappropriate activation of the behavioral response. For example increases in locomotion and ACh release can be short-term and reversible, whereas we have not observed reversal of the dar response once triggered, suggesting the decision to trigger dar, once made, is probably irreversible. The coincident activation of multiple pathways could also allow C. elegans to generate specific responses to different pathogens; activation of either the EGL-30 (Gαq) or LET-60 (Ras) pathways is not sufficient to trigger a dar response, thus only pathogen infections that activate both pathways will cause a dar phenotype.
C. Elegans as a Model to Study Coordinated Behavioral and Immune Responses to Infection
Although the behavioral changes following infection is less well studied than the immune response, they play an important role in defending many species, including humans, from pathogen attack. The complicated nature of the mammalian immune and nervous systems makes dissection of the relationships between these two systems difficult. A growing interest in the C. elegans immune response together with its simple, well described, nervous system make C. elegans a powerful model system for dissecting these interactions.
Our data suggests a mechanism by which behavioral and immune responses can be coordinated to allow animals to simultaneously respond to the immediate threat by mounting an immune response as well as taking steps to avoid the pathogen. A single signal, produced in response to infection, activates Gq-coupled GPCRs present on both the cholinergic motor neurons and the rectal epithelial cells. Depending on the location of these GPCRs activation will trigger either behavioral or immune responses.2
Our results demonstrate separate sites of action for the EGL-30 (Gαq) signaling pathway in behavioral and immune responses to infection and argue against a model in which EGL-30 (Gαq) signaling acts in a single cell to produce further secreted signals that are sufficient to trigger the behavioral and immune responses to infection. Although we cannot exclude the possibility that neuronal signals are able to modulate the immune response or that feedback circuits exist that allow the immune response to signal to the nervous system our current data also argues against a model whereby the dar response triggers neuronal changes that alter behavior and vice versa. This is in contrast to previously published findings from the Tan,26 Ewbank27 and Aballay8 labs that have identified roles for the nervous system in triggering the immune response. During P. aeruginosa infection, release of the insulin-like peptide, INS-7, from the C. elegans nervous system is increased. INS-7 acts in a neuroendocrine fashion to activate the DAF-2 signaling pathway and inhibit DAF-16-mediated expression of antimicrobial genes in the intestine.26 P. aeruginosa is able to suppress this neuroendocrine modulation of immune function by inducing INS-7 expression, highlighting the importance of this regulation.28 Because the release of neuropeptides, such as INS-7, from dense core vesicles is modulated by G-proteins it is tempting to speculate that, following M. nematophilum infection, altered neuronal Gαq signaling may lead to the release of neuroendocrine factors that regulate the epithelial immune response. Neuroendocrine regulation of immune responses is not restricted to the intestine. Neuronally-derived DBL-1 is required to regulate the expression of epithelial antimicrobial genes during infection by D. coniospora.27
Communication between the nervous and immune systems is not restricted to C. elegans. In mammals, signaling between these two systems is bi-directional with immune cells producing neuropeptides and cytokines capable of influencing the nervous system and the nervous system releasing factors, such as serotonin, that are sensed by receptors expressed on immune cells.29 In humans, stress is associated with immunosuppression29 and in the future, C. elegans studies will play an important role in a more systematic investigation of the interplay between neuronal and immune systems, which should provide novel therapeutic approaches.
Acknowledgments
R. McMullan and A. Anderson are funded by a Wellcome Trust Career Development Fellowship awarded to R. McMullan.
Glossary
Abbreviations:
- ACh
Acetylcholine
- GPCR
G-protein coupled receptor
- LRR
Leucine rich repeat
- DAG
Diacylglycerol
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/20466
References
- 1.McMullan R, Nurrish SJ. Rho deep in thought. Genes Dev. 2007;21:2677–82. doi: 10.1101/gad.1615807. [DOI] [PubMed] [Google Scholar]
- 2.McMullan R, Anderson A, Nurrish S. Behavioral and immune responses to infection require Gαq- RhoA signaling in C. elegans. PLoS Pathog. 2012;8:e1002530. doi: 10.1371/journal.ppat.1002530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gravato-Nobre MJ, Hodgkin J. Caenorhabditis elegans as a model for innate immunity to pathogens. Cell Microbiol. 2005;7:741–51. doi: 10.1111/j.1462-5822.2005.00523.x. [DOI] [PubMed] [Google Scholar]
- 4.Pradel E, Zhang Y, Pujol N, Matsuyama T, Bargmann CI, Ewbank JJ. Detection and avoidance of a natural product from the pathogenic bacterium Serratia marcescens by Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104:2295–300. doi: 10.1073/pnas.0610281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shivers RP, Kooistra T, Chu SW, Pagano DJ, Kim DH. Tissue-specific activities of an immune signaling module regulate physiological responses to pathogenic and nutritional bacteria in C. elegans. Cell Host Microbe. 2009;6:321–30. doi: 10.1016/j.chom.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yook K, Hodgkin J. Mos1 mutagenesis reveals a diversity of mechanisms affecting response of Caenorhabditis elegans to the bacterial pathogen Microbacterium nematophilum. Genetics. 2007;175:681–97. doi: 10.1534/genetics.106.060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy KC, Andersen EC, Kruglyak L, Kim DH. A polymorphism in npr-1 is a behavioral determinant of pathogen susceptibility in C. elegans. Science. 2009;323:382–4. doi: 10.1126/science.1166527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Styer KL, Singh V, Macosko E, Steele SE, Bargmann CI, Aballay A. Innate immunity in Caenorhabditis elegans is regulated by neurons expressing NPR-1/GPCR. Science. 2008;322:460–4. doi: 10.1126/science.1163673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Rourke D, Baban D, Demidova M, Mott R, Hodgkin J. Genomic clusters, putative pathogen recognition molecules, and antimicrobial genes are induced by infection of C. elegans with M. nematophilum. Genome Res. 2006;16:1005–16. doi: 10.1101/gr.50823006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troemel ER, Chu SW, Reinke V, Lee SS, Ausubel FM, Kim DH. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2006;2:e183. doi: 10.1371/journal.pgen.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris G, Mills H, Wragg R, Hapiak V, Castelletto M, Korchnak A, et al. The monoaminergic modulation of sensory-mediated aversive responses in Caenorhabditis elegans requires glutamatergic/peptidergic cotransmission. J Neurosci. 2010;30:7889–99. doi: 10.1523/JNEUROSCI.0497-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki H, Kerr R, Bianchi L, Frøkjaer-Jensen C, Slone D, Xue J, et al. In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron. 2003;39:1005–17. doi: 10.1016/j.neuron.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–84. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]
- 14.Hodgkin J, Kuwabara PE, Corneliussen B. A novel bacterial pathogen, Microbacterium nematophilum, induces morphological change in the nematode C. elegans. Curr Biol. 2000;10:1615–8. doi: 10.1016/S0960-9822(00)00867-8. [DOI] [PubMed] [Google Scholar]
- 15.McMullan R, Hiley E, Morrison P, Nurrish SJ. Rho is a presynaptic activator of neurotransmitter release at pre-existing synapses in C. elegans. Genes Dev. 2006;20:65–76. doi: 10.1101/gad.359706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawli T, Wu C, Tan MW. Systemic and cell intrinsic roles of Gqalpha signaling in the regulation of innate immunity, oxidative stress, and longevity in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2010;107:13788–93. doi: 10.1073/pnas.0914715107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bokoch GM. Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 2005;15:163–71. doi: 10.1016/j.tcb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Kawli T, Tan MW. Neuroendocrine signals modulate the innate immunity of Caenorhabditis elegans through insulin signaling. Nat Immunol. 2008;9:1415–24. doi: 10.1038/ni.1672. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler K, Kurz CL, Cypowyj S, Couillault C, Pophillat M, Pujol N, et al. Antifungal innate immunity in C. elegans: PKCdelta links G protein signaling and a conserved p38 MAPK cascade. Cell Host Microbe. 2009;5:341–52. doi: 10.1016/j.chom.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Powell JR, Kim DH, Ausubel FM. The G protein-coupled receptor FSHR-1 is required for the Caenorhabditis elegans innate immune response. Proc Natl Acad Sci U S A. 2009;106:2782–7. doi: 10.1073/pnas.0813048106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J, Singh V, Kajino-Sakamoto R, Aballay A. Neuronal GPCR controls innate immunity by regulating noncanonical unfolded protein response genes. Science. 2011;332:729–32. doi: 10.1126/science.1203411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Chong H, Guan KL. Function of the Rho family GTPases in Ras-stimulated Raf activation. J Biol Chem. 2001;276:34728–37. doi: 10.1074/jbc.M103496200. [DOI] [PubMed] [Google Scholar]
- 23.Canevascini S, Marti M, Fröhli E, Hajnal A. The Caenorhabditis elegans homologue of the proto-oncogene ect-2 positively regulates RAS signalling during vulval development. EMBO Rep. 2005;6:1169–75. doi: 10.1038/sj.embor.7400574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bar-Sagi D, Hall A. Ras and Rho GTPases: a family reunion. Cell. 2000;103:227–38. doi: 10.1016/S0092-8674(00)00115-X. [DOI] [PubMed] [Google Scholar]
- 25.Lackner MR, Nurrish SJ, Kaplan JM. Facilitation of synaptic transmission by EGL-30 Gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron. 1999;24:335–46. doi: 10.1016/S0896-6273(00)80848-X. [DOI] [PubMed] [Google Scholar]
- 26.Kawli T, Tan MW. Neuroendocrine signals modulate the innate immunity of Caenorhabditis elegans through insulin signaling. Nat Immunol. 2008;9:1415–24. doi: 10.1038/ni.1672. [DOI] [PubMed] [Google Scholar]
- 27.Zugasti O, Ewbank JJ. Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-beta signaling pathway in Caenorhabditis elegans epidermis. Nat Immunol. 2009;10:249–56. doi: 10.1038/ni.1700. [DOI] [PubMed] [Google Scholar]
- 28.Evans EA, Kawli T, Tan M-W. Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. PLoS Pathog. 2008;4:e1000175. doi: 10.1371/journal.ppat.1000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serafeim A, Gordon J. The immune system gets nervous. Curr Opin Pharmacol. 2001;1:398–403. doi: 10.1016/S1471-4892(01)00069-8. [DOI] [PubMed] [Google Scholar]