Abstract
We previously reported significant body size change in the nematode Caenorhabditis elegans, depending on the food strain of E. coli. Here, we examined this body size change in 11 other nematode species as well, and found that it is common to most of these nematodes. Furthermore, this food-dependent body size change is influenced by sex and growth temperature.
Keywords: body size, nematode, food, sexual dimorphism, temperature-size rule
Introduction
The nematode Caenorhabditis elegans is one of the excellent model animals for biological research. C. elegans is generally grown in the laboratory using an E. coli strain OP50 as the food source.1 However, Avery and Shtonda2 showed that a change among various food bacteria, including E. coli strains DA837 (a derivative of OP50) and HB101, led to a change in the growth rate of the nematode. In addition, we previously showed that E. coli strain HB101 is a better food for the nematode growth than OP50: the worms fed on HB101 are 1.6-fold larger than those fed on OP50.3 Thus, the food has an important role in body size control. The growth temperature also plays an important role in body size control since it is known that organisms incline to be larger when grown at a lower temperature, which is termed the temperature-size rule.4,5 In C. elegans, animals developing at 12°C were much larger than those grown at 24°C.6 Here, we have investigated whether body size control by such environmental factors is common to other nematode species.
Results
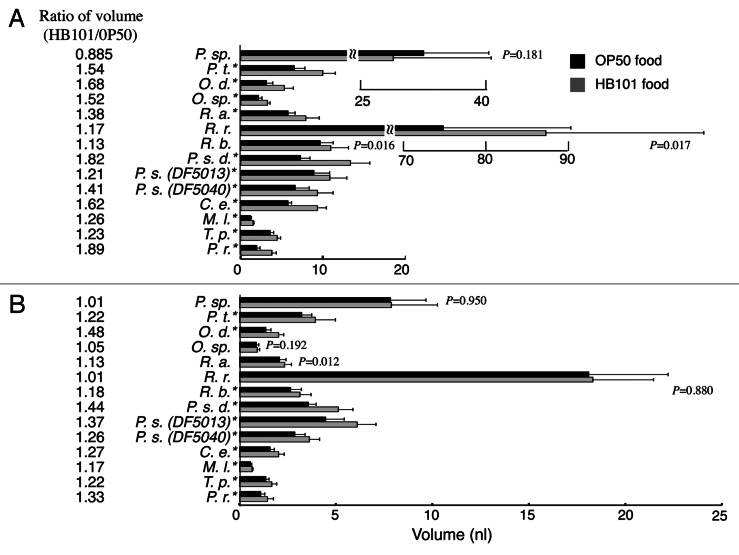
Previously, we designed an automated device to measure body length, diameters and volume of a worm, which for the first time provided precise measurement of the worm body size.7 By using this system, we examined body sizes of 12 nematode species including C. elegans on both foods. Although Flemming et al.8 reported body sizes of several nematode strains, they did not distinguish the two sexes. C. elegans has a self-fertilizing hermaphrodite and a male. Hermaphrodites produce both oocytes and sperms and can reproduce by self-fertilization. The other worms, which we used here, have a male and a female. There is a large difference in the body size between a female/hermaphrodite and a male: a female/hermaphrodite is significantly larger than a male (Fig. 1). In this study, R. regina has the largest body size in both sexes (on OP50 food, a female is 74.6 ± 15 nl and a male is 18.1 ± 4.1 nl; on HB101 food, a female is 87.0 ± 19 nl and a male is 18.3 ± 3.2 nl). The second largest is P. sp (EM434) (on OP50 food, a female is 32.2 ± 7.9 nl and a male is 7.82 ± 1.9 nl; on HB101 food, a female is 28.5 ± 12 nl and a male is 7.86 ± 2.4 nl). These two strains have particularly big body sizes as compared with the other species. In contrast, the smallest one is M. longespiculosa (on OP50 food, a female is 1.18 ± 0.16 nl and a male is 0.557 ± 0.086 nl; on HB101 food, a female is 1.49 ± 0.19 nl and a male is 0.653 ± 0.079 nl).
Figure 1. Body sizes of the nematode species fed on OP50 or HB101. (A) Female or hermaphrodite, and (B) male. The ratios of the body sizes of the worms fed on HB101 over those fed on OP50 are on the left. An asterisk (*) on the species name indicates significant difference (p < 0.01) in the sizes between the two food bacteria. p values larger than 0.01 are indicated in the graph. Error bars on the right indicate standard deviations. Numbers of the worms examined are more than 20 in each case.
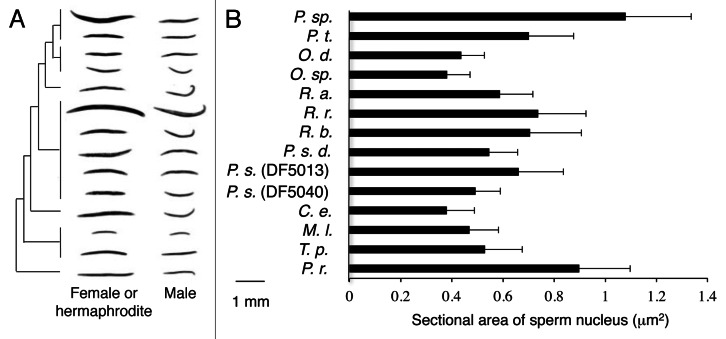
Next we examined the relationship between the body size change among the nematodes examined and their phylogeny (Fig. 2A). The phylogenetic relationships among the nematode species are based on Blaxter et al.9 who constructed the phylogenetic tree of the nematode species using small subunit rDNA sequences. The body sizes seem to have no clear correlation with the genetic relationship among the nematodes. In addition, we determined the nuclear sizes (sectional areas) in the sperms of the 12 species under HB101 food condition. The sperm nuclear sizes seem to show no significant correlation with the body sizes of the nematode species (Fig. 2B).
Figure 2. Phylogenetic tree (A) and sectional areas of the sperm nuclei in the nematode species on HB101 (B). The phylogenetic tree is based on Blaxter et al.9 Error bars on the right indicate standard deviations. Numbers of the worms examined are more than 50.
To investigate the relationship between the growth temperature and the body size, the worms were subjected to 12°C or 25°C as well as 20°C on OP50 or HB101 food (Fig. 3). As compared with the normal growth temperature (20°C), the worms subjected to 25°C reached the final body size faster, about 7–8 d after hatch, and at the lower growth temperature (12°C), the worms grew slowly for about 16–24 d until they reached the maximum size. P. strongyloides (DF5013) and Pellioditis typical tended to grow larger at a lower temperature on HB101 food according to the temperature-size rule (Fig. 3A and B). However, although C. elegans (N2) fed on OP50 grew larger at a lower temperature, C. elegans fed on HB101 grew the largest at 20°C but not at 12°C (Fig. 3C) against the temperature-size rule.
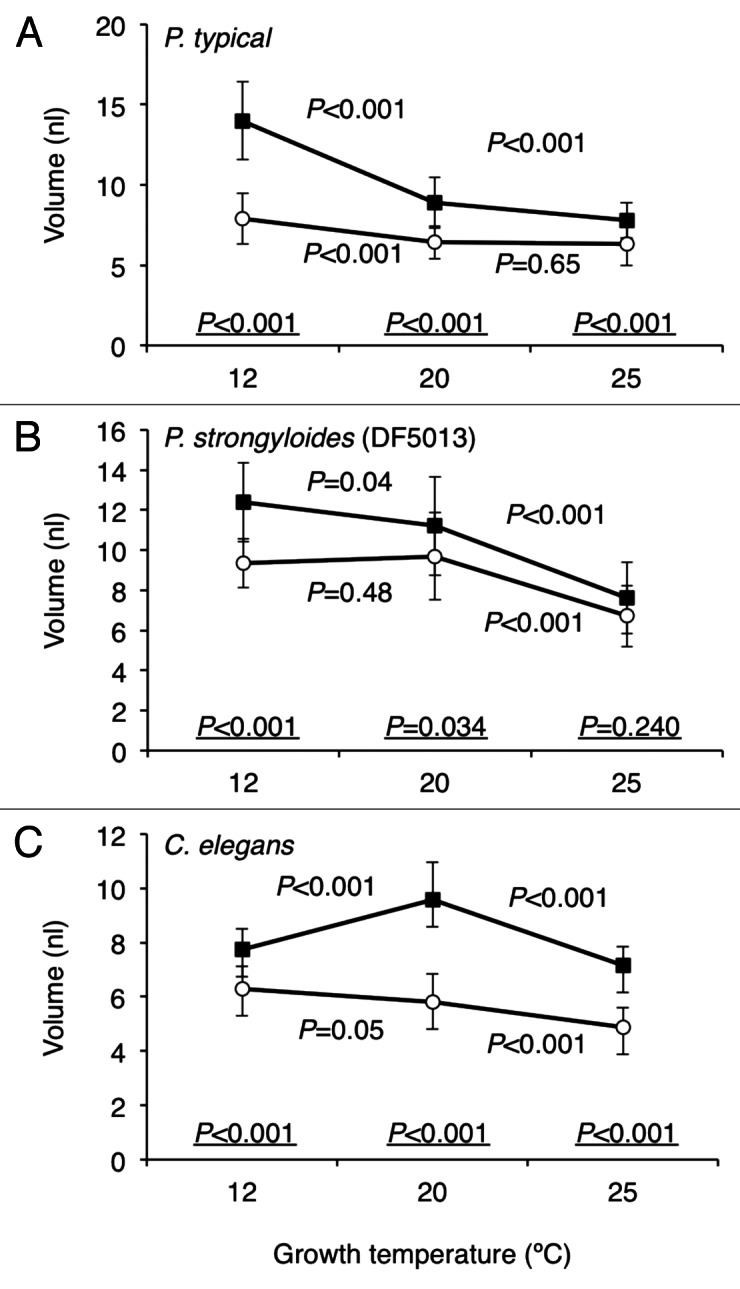
Figure 3. The effects of growth temperature on body sizes of P. typical (A), P. Strongyloides (DF5013) (B) and C. elegans (C) on OP50 food (−○−) or HB101 food (−■−). The maximum sizes of the worms at each growth temperature obtained at the indicated dates after hatch were plotted: P. typical, 12°C, 21 d (OP50) or 24 d (HB101) after hatch; 20°C, 10 d (OP50 and HB101) after hatch; 25°C, 8 d (OP50 and HB101): P. Strongyloides (DF5013), 12°C, 21 d (OP50) or 24 d (HB101); 20°C, 12 d; 25°C, 8 d after hatch (OP50 and HB101): C. elegans, 12°C, 16 d (OP50 and HB101); 20°C, 8 d (OP50 and HB101); 25°C, 7 d (OP50 and HB101). p values obtained by t-tests between the volumes at two consecutive temperatures are shown over or below the connecting line in the graph. p values between the volumes of worms grown on OP50 or HB101 at the same temperature are shown at the bottom of each graph. Error bars indicate standard deviations. Numbers of worms examined at each point are more than 20.
Discussion
In this study, most worm species grew larger when they were fed on HB101 than when fed on OP50 (p < 0.01 in 75% and p < 0.05 in 86% of the 28 data pairs shown in Fig. 1). There was no case in which the worms fed on OP50 grew significantly larger than those fed on HB101, indicating that HB101 is a better food for the growth. However, R. regina males and P. sp (EM434) females and males, which are much larger than the other species, showed no significant difference in the body size between HB101 and OP50 foods (Fig. 1; p = 0.880, 0.181 and 0.950, respectively). This result is consistent with our previous results showing that body sizes of small C. elegans mutants have a tendency to be more susceptible to the change in the food.3 In other words, these larger worms are probably insusceptible to the change in the food. Also, although R. b. females, O. sp (DF5033) males and R. a. males were similar in size to C. elegans, they showed a smaller change in body size between OP50 food and HB101 food (Fig. 1; p = 0.016, 0.192 and 0.012, respectively). These species possibly differ in the metabolic responses to the foods. In C. elegans, metabolism-related genes such as daf-2 encoding an insulin receptor play important roles in the food-dependent body size change and the mutant has almost the same body size between OP50 food and HB101 food, suggesting that the disruption of the food-dependent body size change in the daf-2 mutants is probably caused by abnormality in internal metabolic signals.3
The body size of a female/hermaphrodite has a tendency to be more susceptible to the change in the food compared with that of a male (Fig. 1). This sexual-dimorphism is possibly due to the difference in the energy expenditure. It is recognized that the energy expenditure of a male tends to be higher than that of a female in humans10 and insects.11 The sexual dimorphism of the food-dependent body size change may be affected by the basal metabolic rate. In this report, the sperm nuclear sizes of the nematode species showed no significant correlation with the body sizes. These results agree with the previous report of Flemming et al.8 claiming that the body size does not correlate with the genome size. We previously reported that sectional areas of hypodermal nuclei, intestinal nuclei and neuronal nuclei as a measure of DNA content in C. elegans did not show significant difference between OP50 food and HB101 food, suggesting that the nuclear size does not reflect their body size differences.3 In this paper, the sperm nuclear sizes in the nematode species (Fig. 2B) do not seem to correlate with the ratios of the body sizes of the worms fed on HB101 over those fed on OP50 (Fig. 1A and B).
The thermal plasticity of body size is one of the most widespread rules in biology. It is known that there is a thermal range that is governed by the temperature-size rule.5 For example, an extremely low temperature outside the thermal range of the temperature-size rule leads animals to a reduction in body size. This gives rise to the possibility that C. elegans worms fed on HB101 have a narrower thermal range than those fed on OP50, which may result from an alteration in nutritional status. Although it is unclear that how the effects of temperature on biological processes can give rise to the temperature-size rule, our results suggest that this rule is governed not only by the temperature but also by the food.
In conclusion, the phenomenon that the worm fed on HB101 food grows larger than that fed on OP50 food is mostly conserved in the nematode species examined. Phylogeny and the sperm nuclear size are not correlated with the food-dependent body size change. Although these phenomena are common to both a female/hermaphrodite and a male, the body size change depending on the food shows sexual dimorphism. In addition, growth temperature affects the food-dependent body size change.
Materials and Methods
The full and abbreviated names of the nematode species, sex, food and days after hatch at the maximum body size of each species are as follows: Pellioditis sp. (EM434), P. sp, female; OP50 11 d, HB101 9 d, male; OP50 and HB101 11 d: Pellioditis typical (DF5025), P. t., female; OP50 and HB101 10 d, male; OP50 and HB101 10 d: Oscheius dolichuroides (DF5018), O. d., OP50 female; 11 d, HB101 9 d, male; OP50 and HB101 11 d: Oscheius sp. (DF5033), O. sp, female; OP50 13 d, HB101 15 d, male; OP50 13 d, HB101 15 d: Rhabditella axei (DF5006), R. a., female; OP50 and HB101 12 d, male; OP50 and HB101 12 d: Rhabditoides regina (DF5012), R. r., female; OP50 8 d, HB101 10 d, male; OP50 and HB101 12 d: Rhabditis blumi (DF5010), R. b., female; OP50 13 d, HB101 11 d, male; OP50 and HB101 13 d: Pelodera strongyloides dermatitica (DF5022), P. s. d., female; OP50 9 d, HB101 11 d, male; OP50 9 d, HB101 11 d) Pelodera strongloides (DF5013 and DF5040), P. s. (DF5013) female; OP50 12 d, HB101 10 d, male; OP50 and HB101 12 d: P. s. (DF5040), female; OP50 11 d, HB101 13 d, male; OP50 and HB101 13 d: Caenorhabditis elegans (N2), C. e., hermaphrodite; OP50 and HB101 8 d, male; OP50 and HB101 9 d: Mesorhabditis longespiculosa (DF5017), M. l., female; OP50 13 d, HB101 15 d, male; OP50 15 d, HB101 13 d: Teratorhabditis palmarum (DF5019), T. p., female; OP50 and HB101 15 d, male; OP50 and HB101 15 d: Panagrolaimus rigidus (AF36), P. r., female; OP50 and HB101 15 d, male; OP50 and HB101 15 d.
To synchronize worms, parent worms were allowed to lay eggs for 3–5 h and removed, and the remaining eggs were cultured to adults. Synchronized adult worms were transferred several times to remove their progeny until measurement. Worms were put in 100 mM NaN3 for one hour, and the body volume, body length and diameters of a worm at 100 points along the body axis were measured using Zeiss Axiovert 35 microscope equipped with the automated device Senchu-Gazo-Keisoku-Souchi SVK-3A (Showa Denki Co. Ltd.) as described by Hirose et al.7 To assess whether the worms reached maximum body size, body sizes of the worms were measured every two to four days during several days and the maximum values are used in the figures. The body sizes were statistically compared between the two different food bacteria or growth temperatures by using t-tests, and the resultant p values are shown in Figures 1 and 3.
To measure nuclear sizes of the sperms, adult worms fed on HB101 or OP50 were collected, washed three times in M9 buffer and fixed with Carnoy’s solution (60% v/v ethanol, 30% chloroform, 10% acetic acid) overnight at room temperature. The worms were washed with PBS containing 0.01% Triton X-100 and stained with 2 μg/ml DAPI at 4°C overnight. Then they were washed and suspended in PBS containing 0.01% Triton X-100, 1.1 mg/ml 2-mercaptethylamine and 50% glycerol. The images of sperm nuclei stained with DAPI as above were taken using a Digital Sight DS-2MBWc CCD camera mounted on Nikon ECLIPSE 80i microscope with a 100 × /1.30 oil-immersion objective lens. The sectional areas of the nuclei were analyzed by the image-analyzing software Win ROOF (Mitani Corp.).
Acknowledgments
This work was supported by a grant from Sojo University. Some nematode species were obtained from the Caenorhabditis Genetic Center, which is funded by a grant from the National Institute of Health for Research Resources.
Glossary
Abbreviations:
- DAPI
4′, 6-diamidino-2-phenylindole
- PBS
phosphate buffered saline
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/20175
References
- 1.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avery L, Shtonda BB. Food transport in the C. elegans pharynx. J Exp Biol. 2003;206:2441–57. doi: 10.1242/jeb.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.So S, Miyahara K, Ohshima Y. Control of body size in C. elegans dependent on food and insulin/IGF-1 signal. Genes Cells. 2011;16:639–51. doi: 10.1111/j.1365-2443.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson D. Temperature and organism size—a biological law for ectotherms? Adv Ecol Res. 1994;25:1–58. doi: 10.1016/S0065-2504(08)60212-3. [DOI] [Google Scholar]
- 5.Atkinson D, Ciotti BJ, Montagnes DJ. Protists decrease in size linearly with temperature: ca. 2.5% degrees C(-1) Proc Biol Sci. 2003;270:2605–11. doi: 10.1098/rspb.2003.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kammenga JE, Doroszuk A, Riksen JAG, Hazendonk E, Spiridon L, Petrescu AJ, et al. A Caenorhabditis elegans wild type defies the temperature-size rule owing to a single nucleotide polymorphism in tra-3. PLoS Genet. 2007;3:e34. doi: 10.1371/journal.pgen.0030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirose T, Nakano Y, Nagamatsu Y, Misumi T, Ohta H, Ohshima Y. Cyclic GMP-dependent protein kinase EGL-4 controls body size and lifespan in C elegans. Development. 2003;130:1089–99. doi: 10.1242/dev.00330. [DOI] [PubMed] [Google Scholar]
- 8.Flemming AJ, Shen ZZ, Cunha A, Emmons SW, Leroi AM. Somatic polyploidization and cellular proliferation drive body size evolution in nematodes. Proc Natl Acad Sci U S A. 2000;97:5285–90. doi: 10.1073/pnas.97.10.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, et al. A molecular evolutionary framework for the phylum Nematoda. Nature. 1998;392:71–5. doi: 10.1038/32160. [DOI] [PubMed] [Google Scholar]
- 10.Lovejoy JC, Sainsbury A, Stock Conference 2008 Working Group Sex differences in obesity and the regulation of energy homeostasis. Obes Rev. 2009;10:154–67. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- 11.Rogowitz GL, Chappell MA. Energy metabolism of eucalyptus-boring beetles at rest and during locomotion: gender makes a difference. J Exp Biol. 2000;203:1131–9. doi: 10.1242/jeb.203.7.1131. [DOI] [PubMed] [Google Scholar]